Abstract
Purpose
Successful embryo implantation depends on trophoblast proliferation, migration and, lastly, invasion of the endometrium (to anchor the trophoblast to the uterus). This invasion is mediated by locally produced soluble factors. Of these, vascular endothelial growth factor (VEGF) is the best characterized regulator of angiogenesis. Here, we investigate the association between the VEGF + 405 C/G genotype and the recurrence of embryo implantation failure in women undergoing in vitro fertilization (IVF) program with intracytoplasmic sperm injection (ICSI).
Methods
Forty women with recurrent implantation failure defined by absence of pregnancy after transfer of more than 10 embryos and 131 women control, with at least one live birth after the transfer of fewer than 10 embryos were included. Genomic DNA was analysed with an allele-specific polymerase chain reaction and a Chi-2 test was used to compare the respective VEGF + 405 C/G genotype frequencies in cases and controls.
Results
The frequency of the VEGF +405C/C genotype was higher in women with recurrent implantation failure after ICSI-embryo transfer than in controls (17.5 % and 5.3 %, respectively, p = 0.01).
Conclusion
The VEGF +405 G/C polymorphism may influence embryo implantation and VEGF + 405 C/C genotype may predispose to recurrent implantation failure after ICSI-ET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent implantation failure (RIF) following embryo transfer (ET) is a major factor in the lack of a clinical pregnancy after several IVF/ICSI attempts. The causes of RIF are still poorly known. A variety of aetiologies have been suggested: decreased endometrial receptivity, embryonic defects and multifactorial causes. Various uterine pathologies (such as a thin endometrium and the altered expression of cell adhesion molecules and immune factors) may decrease endometrial receptivity, whereas male or female genetic abnormalities, sperm defects, embryonic aneuploidy and zona hardening are cited among the embryonic causes of implantation failure. Endometriosis and hydrosalpinx may influence both endometrial receptivity and implantation [27]. Furthermore, it has been suggested that the abnormal maternal expression of genes associated with angiogenesis, immunity and apoptosis interferes with successful implantation [13,30,37].
Embryo implantation is a multifactorial event that depends on interplay between the blastocyst and the receptive endometrium. The process consists of molecular signalling by the embryo, followed by apposition and attachment to the endometrium. After the formation of a foetal–maternal interface [41], the critical second step involves invasion of the endometrium by the embryo. This invasion is associated with endometrial angiogenesis, which is promoted by numerous inducers and growth factors. Of these, vascular endothelial growth factor (VEGF) increases vascular permeability and induces endothelial cell proliferation, migration and differentiation and capillary formation [8].
The VEGF gene is located on chromosome 6p21.3 and comprises eight exons. Several single nucleotide polymorphisms (SNPs) have been found in the VEGF gene. Some SNPs (such as VEGF −2578 A/C (in the promoter region), −1154 G/A (also in the promoter region), +405 G/C (in the 5′-untranslated region) and 936 C/T (in the 3′-untranslated region)) are associated with altered VEGF expression [4,22,33,42]. Dysregulation of VEGF expression is implicated in many disease situations in which angiogenesis may be critical, such as breast cancer progression [17], cardiovascular disease [34], essential hypertension [14] and psoriasis [44].
In the field of reproduction, researchers have examined the role of VEGF polymorphisms on embryo implantation. An association between VEGF −1154 G/A polymorphism and RIF after IVF-ET has been evidenced [13,30]. Furthermore, the C allele of +405 G/C has been proposed as a candidate SNP associated with the pathogenesis of several female reproductive tract diseases, such as pre-eclampsia [2], endometriosis [11], recurrent pregnancy loss [7] and ovarian hyperstimulation syndrome following controlled ovarian hyperstimulation [15].
Given the important role of angiogenesis in embryo implantation, we decided to examine the association between VEGF polymorphisms (+450 G/C) and the susceptibility to RIF by comparing women who became pregnant after transfer of fewer than 10 embryos in an ICSI program (controls) with women with RIF.
Materials and methods
Patients
The study was approved by an independent ethics committee and was performed in accordance with the Declaration of Helsinki. All the women provided their prior, written, informed consent to participation.
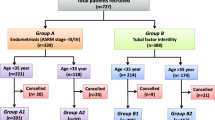
A total of 171 patients (Table 1), ongoing ICSI-ET programme for male infertility or IVF failure without ICSI, were included and divided in two populations, according to their embryo implantation history. The condition of ovarian stimulation and embryo transfer were similar for all patients.
Forty women with a history of RIF after an ICSI-ET programme (defined in this study as a failure to achieve a pregnancy following 2 to 6 IVF cycles, in which more than 10 high-grade embryos were transferred to the uterus [39]. The control group comprised 131 women with at least one live birth after the transfer of fewer than 10 embryos. Women with at least one live birth after the transfer of more than 10 high grade embryos or those who failed to achieve pregnancy after the transfer of fewer than 10 embryos were not considered.
Before the ICSI procedure, all patients had been extensively evaluated in terms of their personal and family medical history, clinical and serological status, hysterosalpingography, day-three hormone profile (FSH, luteinizing hormone and oestrogen) and karyotype. The study's exclusion criteria included prior chemotherapy, unilateral ovariectomy, maternal diethylstilbestrol treatment, an abnormal karyotype, any identified genetic abnormalities, fewer than 10 embryos transferred (for unpregnant women) and more than 10 embryos transferred (for pregnant women).
DNA preparation
A blood sample for DNA analysis was collected from each patient. Genomic DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Southampton, UK), according to the manufacturer’s protocol. Genotyping was performed from IVF procedures had been completed.
Primer design
Primer pairs were designed using Primer3 online software (www.ncbi.nlm), as follows: forward primer 1, CTCACTTTGCCCCTGTCG; forward primer 2, CTCACTTTGCCCCTGTCC; reverse primer, GAGGCGCAGCGGTTAG. The allele-specific forward primers 1 and 2 were synthesized by Eurogentec (Seraing, Belgium) and marked with 6-carboxyfluorescein (FAM, blue colour) and hexachlorocarboxyfluorescein (HEX, green colour), respectively. On receipt, primers were diluted to an appropriate concentration for polymerase chain reaction (PCR) assays in 10 mM Tris buffer and stored at - 20 °C until use.
Allele-specific PCR amplification
The 20 μl PCR reaction mixture (QIAGEN Hilden, Germany) contained 2 μl of extracted DNA and 0.2 μM of each primer. A Silver 96-Well GeneAmp 2700 PCR system (Applied Biosystems, Foster City, California, USA) was used for DNA amplification. The initial denaturation step (15 min at 95 °C) was followed by 24 PCR cycles (denaturation: 30 s at 94 °C; annealing: 90 s at 65 °C; extension: 60 s at 72 °C) and a final extension phase (30 min at 60 °C). All experiments were repeated twice.
Genotyping
Polymerase chain reaction products were diluted 1:10 in sterile water and 1 μL of this dilution was added to 19.5 μL formamide (Applied Biosystems) and 0.5 μl ROX 500 size marker (Applied Biosystems) in 96-well PCR plates. The samples were denatured for 3 min at 95 °C and analyzed by capillary electrophoresis using an ABI Prism 3100 genetic analyzer (Applied Biosystems). Allele interpretation was performed with Genemapper® v.3.1 software (Applied Biosystems) (Fig. 1).
Statistics
The association between groups and genotypes was analyzed using odds ratio (OR) with 95 % confidence intervals (95 % CI). Statistical tests (two-tailed) with a less than 5 % P-value were considered as significant. The Mann–Whitney U-test was used to analyze patients characteristics. Deviations from the Hardy-Weinberg equilibrium were assessed by means of a Chi-2 test.
Results
The participants' mean age and hormone levels prior to the first ICSI attempt in our centre are described in Table 1, and men sperm characteristics summarized in Table 2.
In Table 1, the estradiol level was significantly higher in group of women with RIF compared to the control group (58.0 ± 24.3 UI/L vs 40.3 ± 21.6 UI/L, respectively, p = 0.002), but this value was in the standards. In Table 2, sperm characteristics were statistically different, sperm numeration and normal morphology rate were higher in RIF group.
As expected, mean embryos transferred per women was significantly higher in RIF group compared with control (14.1 ± 4.0 vs. 4.5 ± 2.6, respectively, p = 0.0001). At the opposite, mean embryo implanted per women was significantly lower in RIF group compared with control (0 vs. 1.4 ± 0.5, respectively, p = 0.0001).
The respective frequencies of the VEGF +405 G/C polymorphism in women with RIF and control patients are presented in Table 3. There was a statistically significant difference between the two groups. A higher frequency of the VEGF +405 C/C genotype was found for women with RIF, when compared with control women with at least 1 embryo implanted and less than ten embryos transferred (17.5 % and 5.3 %, respectively, OR = 3.72, 95 % CI [1.03 ; 13.42], p = 0.01). Concerning allele frequency, no statistical difference was observed (p = 0.09). These polymorphisms were in Hardy-Weinberg equilibrium, with a 1 % error interval.
Discussion
In the present study, we investigated the potential association between the VEGF +405 G/C gene polymorphism and RIF. The VEGF +405 G/C polymorphism is located within the 5′-untranslated region and leads to a difference in VEGF expression, with the lowest production observed for the C/C genotype [42]. This polymorphism may impact all VEGF mechanisms and it has been associated with susceptibility to many diseases [14,17,34,44].
As IVF program clearly impact embryo implantation, we defined a control group with similar IVF program and not a group of fertile women having at least two live births with no history of IVF intervention. If female characteristics were similar in our two groups, man sperm quality was statistically different, but surprising better in RIF group, excluding an impact of standard sperm characteristics to explain the implantation failure in our series.
Vascular endothelial growth factor is a potent angiogenic factor and is involved in vasodilatation, vascular permeability and anti-apoptosis [3,9]. It also plays a critical role in oocyte maturation, decidualized endometrial vascularization, embryo implantation/development and placenta angiogenesis/vascularization in early gestation [16,24,25,47]. Inadequate angiogenesis is known to be associated with RIF in both animal [40] and human studies [21]. In the present study, women with RIF had a higher frequency of the VEGF +405 C/C genotype (resulting in lower VEGF expression) than control patients did. Our data confirm the importance of VEGF's role in embryo implantation.
Expression of VEGF in the human endometrium can be observed during the mid-secretory phase [38]; VEGF receptor 1 expression increases over the mid-secretory phase and VEGF receptor 2 expression peaks in the late proliferative phase [26]. Expression in the endometrium during the menstrual cycle is regulated by ovarian steroids, including oestrogen and progesterone. The clinical consequences of an impaired VEGF–VEGF-receptor system on assisted reproduction are not clear, although an impact on embryo implantation could legitimately be hypothesized. Given that RIF is a defect of embryo invasion, it could be regarded as a part of a group of diseases associated with abnormal cytotrophoblast invasion (such as pre-eclampsia and pregnancy loss). A higher than average frequency of the VEGF +405 C allele has been observed in these pathologies. This polymorphism is associated with inadequate angiogenesis, which could lead to human embryo implantation failure. Hence, one can hypothesize that VEGF +405 C allele predisposes to RIF.
In IVF, between days 4 and 6 of the luteal phase in a spontaneous menstrual cycle, VEGF expression in endometrium patients is higher in pregnant women compared with non-pregnant ones. This suggests that high levels of endometrial VEGF expression are related to successful implantation [19].
Furthermore, higher serum concentrations of VEGF on the day of oocyte retrieval in an IVF cycle were predictive of subsequent pregnancy [6]. As observed in mice [36], recombinant FSH treatment also seems to reduce serum VEGF concentrations in humans [6].
As VEGF + 405C/C genotype is associated with lower VEGF expression [42], our data suggest that this genotype hampers angiogenesis and embryo invasion and thus predisposes women included in an ICSI program to RIF. Furthermore, in early pregnancy, peripheral blood mononuclear cells (PBMCs) regulate (along with hCG) corpus luteum function after embryo implantation and thus directly affect embryo outgrowth into the endometrium. The intrauterine administration of autologous PBMCs reportedly improves pregnancy rates for patients with RIF after ICSI-ET [29]. The C allele has been reported to affect VEGF production by lowering transcriptional activity in PBMCs. It is not known whether either embryonic or uterine VEGF or factors released by PBMCs are responsible for the higher incidence of RIF, pre-eclampsia and recurrent pregnancy loss.
Vascular endothelial growth factor is also produced by the invading blastocyst and induces vasodilatation and angiogenesis. Both VEGF and its functional receptor (flt-1) are expressed by the trophoblast, especially by the invasive first trimester extravillous cytotrophoblast, suggesting that VEGF helps to regulate trophoblast proliferation, migration/invasion and metabolic activity [5]. Our hypothesis is supported by the observation of a higher frequency of the VEGF +405 C/C genotype in spontaneously aborted foetuses [18]. Given that the blastocyst itself has been shown to be a source of VEGF at the time of implantation, it would be interesting to examine whether the occurrence of RIF correlates with partner genotypes. Indeed, a G/G genotype in the partner could modulate the decrease in endometrial VEGF, since all the embryos will have a G/C genotype. Conversely, an increase in RIF might be seen with C/C genotype partners.
Furthermore, VEGF is expressed by follicular granulosa and theca lutein cells [20]. The production of angiogenic factors by granulosa cells may help (i) maintain the vasculature and yield a healthy pre-ovulatory follicle and (ii) recruit blood vessels into the early corpus luteum [31,46]. However, thecal production of angiogenic factors is probably responsible for the follicular vascularisation that enables the follicle to grow and develop [32].
Ovarian angiogenesis is regulated independently in each follicle [10]. Selected follicles have a more elaborate vasculature [35] and a highest vascular density than other follicles. It has been suggested that a highly developed follicular vasculature is required to deliver hormones and their precursors, oxygen and nutrients and could have an important role in the selection and growth of the dominant follicle [12,32,43,45]. Thus, greater vascularity may be a primary determinant of follicular dominance. Conversely, low vascularity may limit atretic follicles’ access to nutrients, substrates and hormones in vivo [28,32]. These data indicate that an active blood supply seems to be essential for obtaining high- oocytes quality [10]. Indeed, defects in ovarian angiogenesis may contribute to a variety of disorders, including anovulation, polycystic ovary syndrome [1] and ovarian hyperstimulation syndrome [23].
We could hypothesize that low implantation rates could be also due to the dysfunctional ovarian angiogenesis which impaired oocyte quality and finally may be lead to RIF.
In conclusion, our study is the first to show that the VEGF +405 C/C genotype, which has been previously reported to be associated with lower VEGF expression [42], may serve as a predictive factor of RIF after IVF.
References
Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payne NN, Bekis J, Tan SL, Campbell S, Jacobs HS. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod. 1998;13:651–5.
Banyasz I, Szabo S, Bokodi G, Vannay A, Vasarhelyi B, Szabo A, Tulassay T, Rigo Jr J. Genetic polymorphisms of vascular endothelial growth factor in severe pre-eclampsia. Mol Hum Reprod. 2006;12:233–6.
Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997;94:8761–6.
Brogan IJ, Khan N, Isaac K, Hutchinson JA, Pravica V, Hutchinson IV. Novel polymorphisms in the promoter and 5' UTR regions of the human vascular endothelial growth factor gene. Hum Immunol. 1999;60:1245–9.
Daniel Y, Geva E, Lerner-Geva L, Eshed-Englender T, Gamzu R, Lessing JB, Bar-Am A, Amit A. Levels of vascular endothelial growth factor are elevated in patients with ectopic pregnancy: is this a novel marker? Fertil Steril. 1999;72:1013–7.
Dorn C, Reinsberg J, Kupka M, van der Ven H, Schild RL. Leptin, VEGF, IGF-1, and IGFBP-3 concentrations in serum and follicular fluid of women undergoing in vitro fertilization. Arch Gynecol Obstet. 2003;268:187–93.
Eller AG, Branch DW, Nelson L, Porter TF, Silver RM. Vascular endothelial growth factor-A gene polymorphisms in women with recurrent pregnancy loss. J Reprod Immunol. 2011;88:48–52.
Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9 Suppl 1:2–10.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18.
Gentilini D, Somigliana E, Vigano P, Vignali M, Busacca M, Di Blasio AM. The vascular endothelial growth factor +405 G > C polymorphism in endometriosis. Hum Reprod. 2008;23:211–5.
Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril. 2000;74:429–38.
Goodman C, Jeyendran RS, Coulam CB. Vascular endothelial growth factor gene polymorphism and implantation failure. Reprod Biomed Online. 2008;16:720–3.
Hamedian AA, A Esteghamati, S Noshad, M Mozafari, H Moin-Tavakkoli, M Nakhjavani, T Mahmoudi, M Nikzamir, R Safary and A Nikzamir. Vascular endothelial growth factor (VEGF) +405 C/G polymorphism is associated with essential hypertension in a population from Tehran of Iran. Mol Biol Rep. 2011
Hanevik HI, Hilmarsen HT, Skjelbred CF, Tanbo T, Kahn JA. Increased risk of ovarian hyperstimulation syndrome following controlled ovarian hyperstimulation in patients with vascular endothelial growth factor +405 cc genotype. Gynecol Endocrinol. 2012
Jackson MR, Carney EW, Lye SJ, Ritchie JW. Localization of two angiogenic growth factors (PDECGF and VEGF) in human placentae throughout gestation. Placenta. 1994;15:341–53.
Jacobs EJ, Feigelson HS, Bain EB, Brady KA, Rodriguez C, Stevens VL, Patel AV, Thun MJ, Calle EE. Polymorphisms in the vascular endothelial growth factor gene and breast cancer in the Cancer Prevention Study II cohort. Breast Cancer Res. 2006;8:R22.
Jeon YJ, Kim JH, Rah H, Kim SY, Yoon TK, Choi DH, Cha SH, Shim SH, Kim NK. Vascular endothelial growth factor gene polymorphisms in spontaneously aborted fetuses. Am J Reprod Immunol. 2011;66:544–53.
Jinno M, Ozaki T, Iwashita M, Nakamura Y, Kudo A, Hirano H. Measurement of endometrial tissue blood flow: a novel way to assess uterine receptivity for implantation. Fertil Steril. 2001;76:1168–74.
Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol. 1995;146:157–65.
Kapiteijn K, Koolwijk P, van der Weiden RM, van Nieuw Amerongen G, Plaisier M, van Hinsbergh VW, Helmerhorst FM. Human embryo-conditioned medium stimulates in vitro endometrial angiogenesis. Fertil Steril. 2006;85 Suppl 1:1232–9.
Koukourakis MI, Papazoglou D, Giatromanolaki A, Bougioukas G, Maltezos E, Sivridis E. VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer. 2004;46:293–8.
Krasnow JS, Berga SL, Guzick DS, Zeleznik AJ, Yeo KT. Vascular permeability factor and vascular endothelial growth factor in ovarian hyperstimulation syndrome: a preliminary report. Fertil Steril. 1996;65:552–5.
Krussel J, Behr B, Hirchenhain J, Wen Y, Milki AA, Cupisti S, Bielfeld P, Polan ML. Expression of vascular endothelial growth factor mRNA in human preimplantation embryos derived from tripronuclear zygotes. Fertil Steril. 2000;74:1220–6.
Krussel JS, Behr B, Milki AA, Hirchenhain J, Wen Y, Bielfeld P, Lake Polan M. Vascular endothelial growth factor (VEGF) mRNA splice variants are differentially expressed in human blastocysts. Mol Hum Reprod. 2001;7:57–63.
Krussel JS, Bielfeld P, Polan ML, Simon C. Regulation of embryonic implantation. Eur J Obstet Gynecol Reprod Biol. 2003;110 Suppl 1:S2–9.
Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–43.
Moor RM, Seamark RF. Cell signaling, permeability, and microvasculatory changes during antral follicle development in mammals. J Dairy Sci. 1986;69:927–43.
Okitsu O, Kiyokawa M, Oda T, Miyake K, Sato Y, Fujiwara H. Intrauterine administration of autologous peripheral blood mononuclear cells increases clinical pregnancy rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J Reprod Immunol. 2011;92:82–7.
Papazoglou D, Galazios G, Papatheodorou K, Liberis V, Papanas N, Maltezos E, Maroulis GB. Vascular endothelial growth factor gene polymorphisms and idiopathic recurrent pregnancy loss. Fertil Steril. 2005;83:959–63.
Redmer DA, Grazul-Bilska AT, Reynolds LP. Contact-dependent intercellular communication of bovine luteal cells in culture. Endocrinology. 1991;129:2757–66.
Redmer DA, Reynolds LP. Angiogenesis in the ovary. Rev Reprod. 1996;1:182–92.
Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37:443–8.
Rodriguez-Rodriguez L, Garcia-Bermudez M, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Fernandez-Gutierrez B, Llorca J, Martin J, Gonzalez-Gay MA. Vascular endothelial growth factor A and cardiovascular disease in rheumatoid arthritis patients. Tissue Antigens. 2011;77:291–7.
Schams D, Steinberg V, Steffl M, Meyer HH, Berisha B. Expression and possible role of fibroblast growth factor family members in porcine antral follicles during final maturation. Reproduction. 2009;138:141–9.
Sibug RM, Helmerhorst FM, Tijssen AM, de Kloet ER, de Koning J. Gonadotrophin stimulation reduces VEGF(120) expression in the mouse uterus during the peri-implantation period. Hum Reprod. 2002;17:1643–8.
Sipak-Szmigiel O, Ronin-Walknowska E, Miklaszewicz A, Dolubeczko A, Zejmo M, Giedrys-Kalemba S. Association between HLA-DQA1, HLA-DQB1 alleles and risk of early pregnancy loss. Ginekol Pol. 2007;78:792–5.
Sugino N, Kashida S, Karube-Harada A, Takiguchi S, Kato H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction. 2002;123:379–87.
Tan BK, Vandekerckhove P, Kennedy R, Keay SD. Investigation and current management of recurrent IVF treatment failure in the UK. Bjog. 2005;112:773–80.
Tayade C, Black GP, Fang Y, Croy BA. Differential gene expression in endometrium, endometrial lymphocytes, and trophoblasts during successful and abortive embryo implantation. J Immunol. 2006;176:148–56.
Vinatier D, Monnier JC. The fetal-maternal interface. Description of its elements from an immunologic perspective. J Gynecol Obstet Biol Reprod (Paris). 1990;19:691–700.
Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine. 2000;12:1232–5.
Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143:2797–807.
Zablotna M, Sobjanek M, Nedoszytko B, Lange M, Kozicka D, Glen J, Roszkiewicz J. Association of psoriasis with the VEGF gene polymorphism in the northern Polish population. J Eur Acad Dermatol Venereol. 2011
Zeleznik AJ, Schuler HM, Reichert Jr LE. Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology. 1981;109:356–62.
Zheng J, Redmer DA, Reynolds LP. Vascular development and heparin-binding growth factors in the bovine corpus luteum at several stages of the estrous cycle. Biol Reprod. 1993;49:1177–89.
Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110 Suppl 1:S10–8.
Acknowledgment
We thanks AMMR-PSG and Schering-Ploug for their financial help.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule
Higher frequency of vascular endothelial growth factor (VEGF) +405 C/C polymorphism was observed in women after recurrent implantation failure in an IVF programme.
Rights and permissions
About this article
Cite this article
Boudjenah, R., Molina-Gomes, D., Wainer, R. et al. The vascular endothelial growth factor (VEGF) +405 G/C polymorphism and its relationship with recurrent implantation failure in women in an IVF programme with ICSI. J Assist Reprod Genet 29, 1415–1420 (2012). https://doi.org/10.1007/s10815-012-9878-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9878-8