Abstract
Objective
To study the effect of telomerase activity (TA) in human luteinised granulosa cells (GCs) on the outcome of in vitro fertilisation treatment.
Methods
Fifty-six women, aged 23 to 39 years, were enrolled and divided into four groups according to their levels of TA.
Results
Seventeen cases in group A exhibited nondetectable TA, 16 cases in group B expressed low levels of TA (between 0.1 and 0.65 OD × mm), 14 cases in group C expressed moderate TA levels (between 0.66 and 1.00 OD × mm) and 9 cases in group D expressed high levels of TA (more than 1.00 OD × mm). The level of total serum testosterone (T) was significantly higher in groups C and D than in group A (1.43 ± 0.10 vs. 1.08 ± 0.11 nmol/L, P < 0.030 and 1.56 ± 0.08 vs. 1.08 ± 0.11 nmol/L, P < 0.005, respectively). The TA level was positively correlated with T (r = 0.291, P < 0.011). No obvious differences were observed in rates of fertilisation, cleavage, mature oocyte formation or good-quality embryos among the groups. The patients in group D exhibited the highest rates of embryo implantation and clinical pregnancy (with rates of 52.63% and 77.78%, respectively, compared with 18.92% and 29.41% in group A, 25.71% and 37.50% in group B and 48% and 50% in group C, with P < 0.018 and P = 0.112, respectively). The patients in group D also had a greater likelihood of becoming pregnant than those in group A (OR: 9.703, P < 0.023), group B (OR: 14.765, P < 0.009) or group C (OR: 5.560, P = 0.103).
Conclusions
Luteinised GCs have a certain potential for proliferation and TA of luteinised GCs may predict the clinical outcomes of IVF treatment. Some unknown regulatory mechanisms between TA and T should be studied in further trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Telomerase is a ribonucleoprotein complex that consists of a protein catalytic component and an RNA component and contains a complementary repeat sequence, which can add hexameric (TTAGGG), telomeric DNA repeat sequences to the 3’ends of eukaryotic chromosomes [1–4]. Most somatic cells do not contain detectable levels of TA; therefore, their telomeres become shorter after each cell division. Once the telomeric DNA reaches a critically short length [5], the cell stops dividing and, eventually, undergoes apoptosis and/or chromosomal fusions [5, 6]. However, TA is observed in cells of highly proliferative and periodically or continuously renewing tissues, such as cells of the haematopoietic system [7], epidermis [8] and tumours [9, 10], and it enables these cells to escape senescence and to proliferate at a high rate. Published reports concerning the expression and role of telomerase in ovarian follicles, and especially in human ovarian GCs, are limited. Kim et al. [11] first reported that TA can be detected in normal ovarian and testicular tissues. Further research [12] indicated that the detectable activity of telomerase in the ovary originates from GCs, which arise from a population of stem cells, rather than from the eggs themselves.
GCs are one of the major somatic cell components of the ovarian follicle [13]. They undergo profound morphological and physiological changes during the processes of follicular proliferation, differentiation, ovulation, luteinisation and atresia [14]. More than any other cell type, GCs determine the final size of the preovulatory follicle. Russo et al. [15] found that the reverse transcriptase subunit (TERT), which is responsible for the enzymatic activity of telomerase, was found in the GCs in all of the developmental stages of pig ovarian follicles, from primordial to antral follicles. Accordingly, experiments have demonstrated that oocyte development is related to the TA of GCs, which reside in the periphery of oocytes. Yoshiaki et al. [16] determined that the TA of GCs in follicles with atretic changes was significantly lower than that of normally developed follicles and small follicles. These findings suggested that the telomerase in GCs may play an important role in the life of a healthy follicle and that the loss of its activity may be associated with follicular atresia, although all of the above studies analysed TA in animal GCs, instead of in human ovarian GCs. Only one study reported detectable levels of TA in human luteinised GCs and that TA decreased as age and basal FSH levels increased [17]. This study was limited to an initial exploration of the correlations between the TA of luteinised GCs and ovarian function. They did not further explore the association between TA and IVF-ET.
Therefore, the aim of this study was to confirm whether ovarian GCs possess TA, to examine TA levels in infertile women who underwent in vitro fertilisation (IVF) treatment and to investigate whether altered levels of TA correlated with different IVF outcomes.
Methods
Patients
A total of 56 women, aged 23 to 39 years, who underwent in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment at the Reproductive Medicine Centre of Sun Yat-sen Memorial Hospital between September 10, 2009 and December 8, 2009 were prospectively enrolled in this study and gave their informed consent for these researchers to use their follicular fluid.
Patients reported the characteristics of their menstrual cycles on a self-administered questionnaire, and all of the following criteria were met: (1) normal ovulatory function, according to mid-luteal serum P concentration and regular menses (every 24 to 33 days); (2) a normal serum level of basal follicle-stimulating hormone (FSH) of ≤8.78 IU/L during the early follicular phase; (3) undergoing conventional IVF or ICSI treatment using the GnRH agonist long protocol; (4) no history of ovarian surgery in the three months before the treatment to ensure unimpacted ovarian function; and (5) no family history or personal history of chromosomal diseases. The infertility factors for these patients included male factor (n = 12), tubal or pelvic factor (n = 11) and a combination of male and female factors (n = 33). Patients with a diagnosis of polycystic ovary syndrome (PCOS) or a self-reported history of irregular menstrual cycles and a primary diagnosis of ovulatory infertility, hyperprolactinemia, endometriosis cysts, any kind of functional or organic ovarian cysts or uterine myoma were excluded from this study. Patients were classified and divided into four groups as follows: negative telomerase activity (group A), a low level of telomerase activity (group B (between 0.1 and 0.65 OD × mm)), a moderate level of telomerase activity (group C (between 0.66 and 1.00 OD × mm)) and a high level of telomerase activity (group D (more than 1.00 OD × mm)).
Ovarian stimulation protocol
The IVF or ICSI treatment regimen using a GnRH agonist long protocol cycle was as follows: pituitary downregulation, accomplished by an intramuscular injection of 0.75 to 1.25 mg of triptorelin acetate (Diphereline; Ipsen Pharma Biotech, France), was commenced in the midluteal phase of the menstrual cycle prior to IVF or ICSI treatment. Downregulation was then confirmed by ultrasonography and endocrinology (hormone levels): if the ovaries were inactive, the basal serum FSH would be <5 IU/L, luteinising hormone (LH) would be <5 IU/L and E2 levels would be <50 ng/L. Once this result was confirmed, controlled ovarian hyperstimulation (COH) was initiated using recombinant follicle-stimulating hormone (FSH; Gonal-F; Merck Serono, Geneva, Switzerland or Puregon, Organon, Barcelona, Spain), and/or highly purified human menopausal gonadotropin (hMG; Livzon, Zhuhai, China) and/or highly purified luteinising hormone (LH; Luveris, Merck Serono, Geneva, Switzerland) in doses that varied from 75 to 300 IU/day from cycle days 2 to 13. Depending on age, menstrual cycles, basal hormones, antral follicle count, follicular growth pattern, BMI and response to any previous COHs, the number of injections was determined. Gonadotropin dose was adjusted according to ovarian response monitored by transvaginal ultrasound assessments performed on a ultrasound machine (ALOKA 3500, Japan) and by measuring serum estradiol (E2) levels from the fifth day of stimulation. Human chorionic gonadotropin (HCG, Livzon, Zhuhai, China or Ovidrel, Serono) was used in doses of 4,000 to 10,000 IU to induce oocyte maturation; the dose depended on the mean diameter and the number of leading follicles, together with the serum E2 level, and the administration of gonadotropins was discontinued on that day. After 36 h, aspiration of the oocytes was performed transvaginally under ultrasound guidance with a single-channel needle.
Embryo culture and transfer
Fertilisation and survival were assessed 16 to 18 h after IVF or ICSI, and embryo cleavage was determined 24 h thereafter. Good-quality embryos were 1) grade 1 embryos, with two to four blastomeres of equal sizes and less than 20% fragmentation [18] on day 2; 2) grade 1 embryos with more than six blastomeres of equal sizes and less than 20% fragmentation on day 3; 3) compact/morula embryos or early blastocysts on day 4. Embryo transfer was performed 2, 3 or 5 days after oocyte retrieval, with a maximum of three embryos for patients above the age of 35 or undergoing repeated cycles and two embryos for patients under the age of 35 undergoing their first treatment cycle. A pregnancy test was performed 14 days after embryo transfer with a quantitative serum value for β-hCG of >5 IU/L considered positive, and a primary transvaginal ultrasound examination was performed 3 weeks later to check for the number of embryonic sacs.
According to the age and BMI of the patient, luteal phase support was provided by an intramuscular injection of either progesterone (40 mg/day) (Progesterone Injection, Xian Ju Pharmaceutical Co., Zhejiang, China) or HCG (2,000 IU every 3 days) (Livzon, Zhuhai, China) for a total of four times from the day of ovum retrieval and continuing at least until the result of the transvaginal ultrasound examination was determined.
Collection of GCs
A pooled collection of follicular fluid (FF) from each patient was collected into a 50-ml centrifuge tube (Corning Costar, New York, USA) from the operating room and transported to the laboratory in a thermal container filled with water at a constant temperature of 37°C immediately after finishing the ovum retrieval and isolation of the cumulus-oocyte complexes. GCs were harvested from the pooled FF by 50% density gradient centrifugation with Ficoll solution (Lymphoprep™, Axis-Shield, Oslo, Norway) at 2,000 rpm for 15 min at room temperature (RT). The interphase layer of cells was extracted and diluted 1:1 with phosphate-buffered saline solution (PBS; SAGE, USA) and then centrifuged (at 1,000 rpm for 10 min at RT). After finishing this step, the supernatant was discarded, and the pellet of cells was suspended in 1 to 3 ml of red blood cell lysis buffer (Beyotime, Haimen, China) according to the pellet’s volume. It was gently retropipetted two to three times to obtain a homogeneous mixture and incubated at RT for one to two min, then centrifuged at 500 g for 5 min at 4°C. If the red blood cells were not lysed satisfactorily, the previous steps were selectively repeated. This purified, luteinised preparation of GCs was then washed and suspended in an appropriate volume of PBS and counted in a haemocytometer. The viability of the GCs in all cases was approximately 75%, as assessed by trypan blue staining (Sigma, Saint Louis, USA), and computed as 2 × 105 cells per patient. Each sample was centrifuged at 3,000 g for 10 min at 4°C in a refrigerated centrifuge (Eppendorf 5415R, Hamburg, Germany). Next, the supernatant was carefully removed, the pellet of cells from each patient was suspended in PBS and centrifugation was repeated as described above. Finally, the samples were collected and stored at −80°C until needed for TRAP analysis.
Hormone testing
A blood sample was obtained from each patient prior to initiating an IVF cycle, which was, ideally, between cycle days one and five. The levels of serum sex hormones (including prolactin, luteinising hormone, follicle-stimulating hormone, estradiol and total testosterone) were measured automatically by chemiluminescence using the Beckman Coulter UniCel DxI 800 (Beckman Coulter, Los Angeles, USA),. Serum specimens were stored at −80°C if they were not assayed immediately.
Telomerase activity assay
A modified protocol based on a commercial kit (the TRAPeze Telomerase Detection Kit, Roche Applied Science, Germany) was applied to evaluate telomerase activity by utilising the telomeric repeat amplification protocol (TRAP), based on a non-isotopic method described by Wen JM et al. [53]. A six-nucleotide, repeat sequence TTAGGG was added to the template by telomerase, which resulted in the formation of an electrophoretic ladder.
Preparation of extracts from GCs
Each cryopreserved GC sample was first thawed on ice, resuspended in 200 μl pre-cooled lysis reagent supplied with the kit and lysed after a 30-min incubation on ice. Next, the lysate was centrifuged at 16,000 g for 20 min at 4°C in a refrigerated centrifuge (Eppendorf 5415R). The supernatant (175 μl) was collected to ensure that no cellular debris from the pelleted cells was transferred, and protein concentration was measured using the BCA kit (Shenergy Biocolor Bioscience & Technology Company, Shanghai, China). Finally, aliquots of the cell extract were shock-frozen in liquid nitrogen and stored at −80°C until the TRAP reaction was performed, as described below.
Telomeric repeat amplification protocol (TRAP reaction)
Heat treatment of a cellular extract for 10 min at 85°C prior to the TRAP reaction was used to inactivate the telomerase protein to prevent the production of a negative control, and an extract of the human telomerase-positive embryonic kidney cell line 293 provided in the kit was used as a positive control for telomerase activity. For each sample and the controls, 14.63 μg total protein was transferred into a 25-μl reaction mixture (which included Tris buffer, telomerase substrate, primers, nucleotides, Taq polymerase, one-step telomerase-mediated primer elongation, biotin-labelled P1-TS primer and P2 primer provided by the kit), as well as DNA/RNA-free sterile water (Promega, Madison, Wisconsin, USA), which was added to a final volume of 50 μl per assay for PCR amplification (all the above pipetting steps were performed on ice). Each TRAP reaction mixture was then incubated in a Biometra Tgradient PCR thermocycler (Biometra, Germany) at 37°C for 30 min to elongate the primer and 94°C for 5 min to inactivate the telomerase, followed by 33 cycles of 94°C for 30 s, 50°C for 30 s and 72°C for 90 s; followed by one cycle of 72°C for 10 min. The TRAP products were stored at −20°C.
Non-denaturing rolyacrylamide gel electrophoresis (native PAGE)
To visualise and analyse the products obtained by the TRAP reaction, 10 μl of each DNA sample was completely mixed with 2 μl of 6 × DNA loading buffer (Takara, Kyoto, Japan) and separated by electrophoresis with a constant electric power of 30 w at 4°C for three and a half hours after pre-electrophoresis under the same conditions for 1 h on a 15% polyacrylamide gel (PAG) using an SSCP gel apparatus (model JY-CX2B, JunYi., Inc., Beijing, China; gel size: 35 × 45 cm; 60 lanes). At the end of the electrophoretic migration, silver staining was performed on the PAG to identify TA. Briefly, the PAG was first fixed with 0.5% glacial acetic acid and 10% alcohol for 10 min and then incubated in 0.2% silver nitrate for 25 min. After several changes of double-distilled H2O, the PAG was developed using 0.75% sodium hydroxide with 0.165% formaldehyde; development was terminated with acetic acid until the bands could be visualised. TA was considered positive if some DNA ladders that had intervals of 6 bp appeared on the PAG; otherwise, TA was considered negative. Quantity One 4.6.2 software (Bio-Rad, California, USA) was used for the semi-quantitative analysis of bands. TA was defined as weakly positive (+) when the mean optical density of the area under the curve was ≤0.65 OD × mm, while moderately positive (++) TA was defined as 0.65 to 1.00 OD × mm, and highly positive (+++) TA was defined as >1.00 OD × mm.
Statistical analysis
All data were statistically analysed using SPSS software (Statistical Product and Service Solutions, Chicago, USA) and are presented as means ± SEM if they demonstrated a normal distribution or presented as medians (ranges) for non-normal distribution. Statistical comparisons were performed by one-way ANOVA and Kruskal-Wallis tests, where appropriate. The chi-squared test was used to compare implantation rates among four groups. Blastocyst transfer, clinical pregnancy and early pregnancy loss rates were compared by the Fisher’s exact test. Binary logistic regression was performed to quantify the effect of TA on pregnancy between groups. The significance of the model was calculated by the likelihood ratio, and the uncertainty was evaluated and explained by Nagelkerke R2. The effect of TA on pregnancy outcome was expressed through an adjusted odds ratio and a 95% confidence interval (CI). In the regression analysis, the following possible confounding factors were considered: female age, basal serum FSH and LH, total dose of Gn, peak estradiol level and number of oocytes collected, all of which were introduced into the regression equation by the Enter method. Differences with a P value (two-tailed) of <0.05 were considered significant.
Results
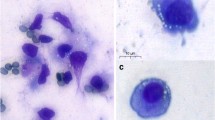
A total of 56 infertile women received GnRH agonist and rFSH/HMG or rFSH/LH stimulation for IVF or ICSI. Thirty-nine (69.64%) women exhibited detectable levels of telomerase activity. There were 17 cases in group A, 16 cases in group B (two cases experienced a cancelled embryo transfer), 14 cases in group C and 9 cases in group D (Fig. 1 depicts the expression of TA in GCs). Overall, the PR was 44.64%, the overall implantation rate was 32.76% and the overall early pregnancy loss rate was 16.70%. As Table 1 indicates, there were no differences among the four groups in any of the following parameters: age, BMI, antral follicle count and basal serum FSH, LH, FSH/LH and E2. These data demonstrated that all the patients who participated in our study had similar baseline characteristics. The serum testosterone (T) level was significantly higher for patients in group C and group D than in group A (1.43 ± 0.10 vs. 1.08 ± 0.11 nmol/L, P < 0.030 and 1.56 ± 0.08 vs. 1.08 ± 0.11 nmol/L, P < 0.005, respectively). No differences were observed in the procedure for controlled ovarian stimulation. With regard to the IVF laboratory parameters, similar numbers of oocytes were retrieved, with the same proportion of metaphase II oocytes among groups when ICSI was performed. The rates of mature oocytes and good-quality embryos tended to increase as TA increased (with 85.45 ± 2.77% and 6.25% for group A vs. 88.89% and 7.18% for group B vs. 93.65% and 15.78 ± 3.69% for group C vs. 80.42 ± 5.02% and 16.53 ± 6.28% for group D, respectively; P > 0.05). No differences were observed in the insemination procedure, fertilisation and cleavage rates, utilisation rates of embryos, day of embryos transferred or mean numbers of embryos transferred among the four groups (Table 2). Patients in group D, whose mean number of good-quality embryos transferred was minimal (with 0.33 ± 0.24 in group D vs. 0.56 ± 0.22 in group A vs. 0.73 ± 0.25 in group B vs. 1.31 ± 0.24 in group C; P > 0.05), exhibited a slightly higher rate of blastocyst transfer (31.57% in group D vs. 5.41% in group A vs. 11.43% in group B vs. 12.00% in group C; P > 0.05). Spearman analysis indicated that telomerase activity was positively correlated with T level in the menstrual phase (r = 0.291, P < 0.011). No significant associations between TA and fertilisation rate (r = 0.077, P = 0.499), cleavage rate (r = 0.186, P = 0.100), number of good-quality embryos produced (r = 0.187, P = 0.095), number of good-quality embryos transferred (r = 0.069, P = 0.546), or blastocysts transferred (r = 0.219, P = 0.054) were found, which indicates a similar embryo status in all groups (Table 3). Subjects in group D had the highest rates of embryo implantation and clinical pregnancy (52.63% and 77.78%, respectively, compared with 18.92% and 29.41% in group A, 25.71% and 37.50% in group B and 48% and 50% in group C [P < 0.018 and P = 0.081, respectively]). Binary logistic regression analysis was performed to evaluate the effect of TA on pregnancy rate. A lowering of pregnancy rate was observed as TA decreased, and this result was expressed by an odds ratio (Exp[B]) with 95% confidence intervals (CI). We found an OR: 8.400 (95% CI: 1.274–55.394, P < 0 .027) for group D when group A was considered a reference, an OR: 5.833 (95% CI: 0.900–37.818, P = 0.064) for group D when group B was considered a reference and an OR: 2.500 (95% CI: 0.357–17.500, P = 0.356) for group D when group C was considered a reference (shown in Table 4; this model did not adjust for confounding factors). Possible confounding factors including age, BMI, basal serum FSH and LH, basal FSH/LH, total testosterone, total dose of Gn, peak estradiol level, number of collected oocytes, good quality embryos, good quality embryos transferred and blastocyst transferred, may interfere with the relationship between TA and pregnancy rate. These factors were introduced into the regression equation by the Enter method. The following conclusions were made after confounding factors were adjusted: women in group D had a greater likelihood of becoming pregnant than those in group A (OR: 9.703, 95% CI: 1.366–68.914, P < 0.023), group B (OR: 14.765, 95% CI: 1.956–111.435, P < 0.009) and group C (OR: 5.560, 95% CI: 0.708–43.667, P = 0.103). (Figure 2 depicts the trend toward higher pregnancy and implantation rates as TA increased.) In addition, PR was negatively correlated with basal FSH (OR: 0.481, 95% CI: 0.279–0.828, P < 0.008) in the adjusted model.
Discussion
Fifty-six patients agreed to participate in this study during the process of sample collection. A non-isotopic telomeric repeat amplification protocol (TRAP) sliver staining for the detection of telomerase activity described previously [19] was used. Electrophoresis images were analysed by using Quantity One 4.6.2 software with the relative quantitative method of volume contour, and the optical density of the area under the curve was automatically calculated.
Levels of TA in Luteinised GCs
The ends of linear chromosomes are capped by specialised nucleoprotein structures known as telomeres, and this telomeric lengthening can be achieved through de novo synthesis catalyzed by telomerase [20], an enzyme with reverse-transcriptase activity, which is detectable and reactive in most tumours [10, 11], stem cells [21], germ cells [22] and a variety of normal tissues, such as the germinal layer of skin cells, intestinal mucosal cells, medullary hematopoietic stem cells, endometrial cells and liver cells. Ovary GCs arise from a population of stem cells [23–26]. Some researchers have found that a small proportion of ovarian GCs that are isolated from antral follicles can divide and form colonies, which demonstrates that some GCs exhibit the growth characteristics of stem cells [23–27]. Furthermore, prior studies have indicated that the success rate of cloning by nuclear transfer from GCs was 2.8% [28], compared with overall success rates of less than 0.4% from mammary epithelial cell lines [29] and 0.9% from cumulus cells [30], respectively. Do GCs possess TA because they originate from stem cells, and what is the level of TA exhibited in GCs during various stages of follicular development? Lavranos et al. [12] discovered that TA was highest in the smallest follicles examined (diameters of 60 to 100 μm), whereas TA decreased significantly as the follicles enlarged. The telomerase RNA component has been found to localise to the GCs of growing follicles but has not been detected in primordial follicles and was found to be expressed either in the middle and antral layers or in the middle and basal layers of antral follicles [12]. R.J. Rodgers et al. interpreted these various telomerase RNA staining patterns as predicting that the younger GCs would migrate in a basal direction if the follicles undergoing rapid antrum expansion or located toward the antrum did not expand or expanded slowly [31]. In the present study, GCs were collected from a pool of FF from each patient; thus, the purified GCs may have been derived from the basal, middle or antral layers of GCs. The detection of TA in freshly isolated, luteinised GCs in this study indicated that TA was present in most patients (approximately 69.64%), which is in line with the results of Liu et al. [17]. This result indicates that luteinised GCs may have a certain potential for replication and that their telomere length can be maintained by TA, thereby protecting their luteal function after ovulation.
Interestingly and unexpectedly, this study found that the TA of luteinised GCs was positively correlated with total testosterone (T) during the menstrual phase (r = 0.291, P < 0.011) There is evidence that androgen level is related to TA [32–34]. Androgens have been shown to exert a negative regulation of TA in the normal prostate [35]. Removal of testosterone by castration results in increased TA in the normal prostate of rat [36] and monkey [35] species. Conversely, prostate cancer cells exhibit high levels of TA, even in the presence of androgens, while deprivation of androgen in androgen-sensitive LNCaP cells leads to a decrease in TA, and the subsequent addition of 5α-dihydrotestosterone (DHT) restores TA [34]. However, why is the normal level of T during the menstrual period (early follicular phase) positively correlated with TA in luteinised GCs? This finding may be related to the proliferation and development of GCs through the enhanced functions of FSH [37], GDF-9 [38], IGF-1 [39, 40] and their receptors after stimulation by T during the early stage of follicular development. It has been widely reported that, at the early stages of follicular development, androgens produced by thecal cells under LH stimulation act as an enhancer of FSH-stimulated follicular differentiation. Androgens not only synergistically enhance the expression of a wide range of FSH-responsive genes but also modulate follicular differentiation by interacting with several protein kinases to promote follicular maturation [41]. FSH-stimulated steroidogenesis, produced by increasing cAMP levels through increasing cAMP production and/or decreasing cAMP catabolism, has been shown to be enhanced by androgens in mammals [42]. Furthermore, androgens synergistically enhance the expression and activity of the tissue-type plasminogen activator in cultured rat GCs, which is stimulated by gonadotropin-releasing hormone (GnRH, an activator of protein kinase C) and epidermal growth factor [43] (EGF, an activator of tyrosine kinase). In vitro studies have suggested that androgens promote the proliferation of ovarian GCs by activating the growth function of GDF-9 and accelerate the development of preantral follicles and early antral follicles [44–46]. Thus, we speculated that increased serum T levels within the normal range during the menstrual period lead to improved development of the follicles and increased GC proliferation during follicular development and, therefore, increased TA in luteinised GCs. However, this study only found a surface phenomenon, and the concrete regulatory mechanism of telomerase is not clear, as it is a special reverse transcriptase. Further study will be needed to elucidate the regulatory mechanism of telomerase.
TA and oocyte/embryo quality
The processes of normal follicular differentiation and oocyte development are dependent on gap junctional intercellular communication between the germinal and somatic compartments. GCs, by means of gap junctions, provide the biosynthetic substrates for approximately 85% of the oocyte’s metabolic needs [47] as it hypertrophies and completes its growth in the preantral follicle. It has generally been recognised that successful maturation, fertilisation and preimplantation embryonic development depends on a regulated programme of oocyte growth and differentiation coordinated with the development and differentiation of the surrounding GCs [48]. Contact communication between GCs and oocytes is essential for oocyte development, and GCs are crucial in regulating the ability of the oocyte to mature through the meiotic process and acquire its full developmental potential. Previous studies have shown that co-culture with GCs could strongly induce the cytoplasmic maturation of oocytes. Jane E [49] found that germinal vesicle (GV)-stage oocytes cultured with autologous cumulus cells matured at a significantly higher rate than did GV oocytes cultured without cumulus cells. In addition, Boon Chin Heng et al. [50] found that co-cultured porcine GCs exhibited a beneficial effect on the meiotic maturation of oocytes. These results demonstrated that GCs play an important role in the maturation of oocytes and that they are closely correlated with the quality of oocytes. This study found that TA was detectable in luteinised GCs obtained from IVF/ICSI cycles, which suggested that telomerase may participate in the regulation of luteinised GCs, thereby indirectly influencing the activation of oocytes. Our results show that the rates of oocyte maturation and good-quality embryos increased as TA levels increased but that these differences were not statistically significant (as presented in Table 3), and no apparent correlation between TA and rates of oocyte maturation and good-quality embryos were observed by Spearman analysis. One possible cause of this phenomenon could be a limited sample size, which may have obscured notable differences in the quality of oocytes and embryos among the four groups.
TA and IVF/ICSI outcome
This study found that women in Group D had a greater likelihood of becoming pregnant than those in Groups A and B after adjusting possible confounding factors, such as age, basal serum FSH and LH, total dose of Gn, peak estradiol level and number of oocytes collected, which may interfere with the relationship between TA and clinical pregnancy rate. In addition, the clinical pregnancy rate was negatively correlated with basal FSH. This result suggested that TA in luteinised GCs was positively correlated with clinical pregnancy rate, while basal FSH had an adverse effect.
Possible explanations for these findings could be that the TA of luteinised GCs is related to luteal function and endometrial receptivity. A previous study [51] has reported that an E2/P ratio between 1.0 and 5.0 on the day of hCG administration was associated with an improved endometrial receptivity, especially ratios between 3.01 and 4.0. While other factors were not significantly different, women whose E2/P ratio was in the range of 3.01 to 4.0 exhibited significantly higher pregnancy rates and single-embryo implantation rates [51]. Thus, an E2/P ratio between 3.01 and 4.0 appears most beneficial for the improvement of endometrial receptivity and embryo implantation. In this study, the E2/P ratio on hCG day gradually increased as the TA of luteinised GCs increased, and Group D, with a ratio that fit exactly into the above range of 3.01 to 4.0, was the highest (at 3.04 ± 0.60). As expected, patients in Group D also exhibited significantly higher implantation rates (58.33%) and clinical pregnancy rates (77.78%), which were consistent with the above study. This result indicated that the higher the TA of luteinised GCs, the better the luteal function after ovulation; thus, the better the endometrial receptivity and the more conducive to embryo implantation. However, the exact mechanisms remain to be explored. The TA of luteinised GCs may be related to the potentiality of embryonic development. As shown above, patients in group D, whose mean number of good-quality embryos transferred was minimal, had a slightly higher rate of blastocysts transferred than those in the other groups when the all patients’ characteristics were comparable. We demonstrated that patients with a higher TA of luteinised GCs might have a greater potential for embryonic development. Therefore, the levels of TA in luteinised GCs may indirectly reflect the quality and developmental potential of the patient’s embryos and may benefit the existing system of embryo quality evaluation.
To our knowledge, embryos can successfully implant into the endometrium and, ultimately, pregnancy may ensue due to the efforts of various factors, but not a simple agent. However, the quality and developmental potential of embryos and endometrial receptivity were the main factors that determined the success of IVF/ICSI treatment. The importance of knowing the concealed correlations between the TA of luteinised GCs and the two crucial factors mentioned above is compelling, and further investigation is needed to clarify what mechanisms are involved.
This research was focused on pooled granulosa cell samples, rather than granulosa cell samples from certain specified follicles; therefore, it may not reflect what happens in specified individual follicles. The sample size was small, which make significant differences difficult to assess. Therefore, the outcomes of this paper were the preliminary findings, and sample size will be calculated in further study, and we plan to distinguish follicles as diameter ≥16 mm and <16 mm before oocytes retrieval procedure, and then puncture them respectively according to above assort. In order that GCs from different sorts of follicles are classified. In addition, utilization of a quantitative assay (TRAP-ELISA) on classified follicles in a larger study population would be ideal to confirm the findings. Predicting the outcome of treatment is useful if it can be done in advance of the treatment itself and this problem may be solved by a blinded study research.
Conclusion
Based on the results of this trial, we found that the majority of patients who underwent controlled ovarian hyperstimulation had the detectable TA in their luteinised GCs, which suggested that GCs had a certain potential for proliferation and TA may participate in the regulation of the function of luteinised GCs, thereby TA may indirectly influence the activation of oocytes.
We found that women with high level of TA had a greater likelihood of becoming pregnant than those with nondetectable TA or low levels of TA, while basal FSH was negatively correlated with the clinical pregnancy rate. This mean TA of luteinised GCs was positively correlated with clinical pregnancy rate, while basal FSH had an adverse effect. It indicated that TA of ovarian luteinised GCs could help us predict the clinical outcomes of IVF treatment.
In this study, we also found that the TA of luteinised GCs was positively correlated with total T during the menstrual phase. It suggested that there would be some regulatory mechanisms between TA and T, and further study should be needed to elucidate this problem.
References
Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–7.
Lingner J, Cech TR. Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc Natl Acad Sci USA. 1996;93:10712–7.
Lendvay TS, Morris DK, Sah J, Balasubramanian B, Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144:1399–412.
Feng J, Funk WD, Wang SS, et al. The RNA component of human telomerase. Science. 1995;269:1236–41.
Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6.
Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36.
Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci. 1995;92:9082–6.
Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci. 1996;93:6476–81.
Kim NW, PiatyszekMA PKR, Harley CB, WestMD Ho PL, Coviello GM, Wright WE, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91.
Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5.
Lavranos TC, Mathis JM, Latham SE, Kalionis B, Shay JW, Rodgers RJ. Evidence for ovarian granulosa stem cells: telomerase activity and localisation of the telomerase RNA component in bovine ovarian follicles. Biol Reprod. 1999;61:358–66.
Orly J. Molecular events defining follicular developments and steroidogenesis in the ovary. In: Shupnik MA, editor. Gene engineering in endocrinology. Totowa: Humana Press Inc; 2001. p. 239–76.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55.
Russo V, Berardinelli P, Capacchietti G, et al. Localization of the telomerase catalytic subunit (TERT) in pig ovarian follicles. Vet Res Commun. 2003;27 Suppl 1:623–6.
Yoshiaki Y, Yasuhiko N, Kenji U, et al. Changes in telomerase activity in experimentally induced atretic follicles of immature rats. Endocrinology. 2002;49:589–95.
Liu W, Zhu GJ. Expression of telomerase in human ovarian luteinized granulosa cells and its relationship to ovarian function. Zhonghua Fu Chan Ke Za Zhi. 2003;38:402–4.
Van de Velde H, Nagy ZP, Joris H, De Vos A, Van Steirteghem AC. Effects of different hyaluronidase concentrations and mechanical procedures for cumulus cell removal on the outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:2246–50.
Wen JM, Sun LB, Zhang M, Zheng MH. A non-isotopic method for the detection of telomerase activity in tumour tissues: TRAP-silver staining assay. Mol Pathol. 1998;51(2):110–2.
Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–65.
Greenwood MJ, Lansdorp PM. Telomeres, telomerase, and hematopoietic stem cell biology. Arch Med Res. 2003;34:489–95.
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–9.
Lavranos TC, Rodgers HF, Bertoncello I, Rodgers RJ. Anchorage-independent culture of bovine granulosa cells: the effects of basic fibroblast growth factor and dibutyryl cAMP on cell division and differentiation. Exp Cell Res. 1994;211:245–51.
Lavranos TC, O’Leary PC, Rodgers RJ. Effects of insulin-like growth factors and binding protein-1 on bovine granulosa cell division in anchorage-independent culture. J Reprod Fertil. 1996;106:221–8.
Rodgers HF, Lavranos TC, Vella CA, Rodgers RJ. Basal lamina and other extracellular matrix produced by bovine granulosa cells in anchorage-independent culture. Cell Tissue Res. 1995;282:463–71.
Rodgers RJ, Vella CA, Rodgers HF, Scott K, Lavranos TC. Production of extracellular matrix, fibronectin and steroidogenic enzymes, and growth of bovine granulosa cells in anchorage-independent culture. Reprod Fertil Dev. 1996;8:249–57.
Lavranos TC, Rodgers RJ. An assay of tritiated thymidine incorporation into DNA by cells cultured under anchorage-independent conditions. Anal Biochem. 1995;223:325–7.
Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999;60:996–1005.
Campbell KHS, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–6.
Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–74.
Rodgers RJ, Irving-Rodgers HF, van Wezel IL, et al. Dynamics of the membrana granulosa during expansion of the ovarian follicular antrum. Mol Cell Endocrinol. 2001;171:41–8.
Bouchal J, Baumforth KR, Svachova M, Murray PG, von Angerer E, Kolar Z. Microarray analysis of bicalutamide action on telomerase activity, p53 pathway and viability of prostate carcinoma cell lines. J Pharm Pharmacol. 2005;57:83–92.
Bouchal J, Kolar Z, Mad’arova J, Hlobilkova A, von Angerer E. The effects of natural ligands of hormone receptors and their antagonists on telomerase activity in the androgen sensitive prostatic cancer cell line LNCaP. Biochem Pharmacol. 2002;63:1177–81.
Soda H, Raymond E, Sharma S, Lawrence R, Davidson K, Oka M, et al. Effects of androgens on telomerase activity in normal and malignant prostate cells in vitro. Prostate. 2000;43:161–8.
Ravindranath N, Ioffe S, Marshall G, Ramaswamy S, Plant T, Dym M. Androgen depletion activates telomerase in the prostate of nonhuman primate, Macaca mulatta. Prostate. 2001;49:79–89.
Meeker A, Sommerfeld H, Coffey D. Telomerase is activated in the prostate and seminal vesicles of the castrated rat. Endocrinology. 1996;137:5743–6.
Weil S et al. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–6.
Hickey TE et al. Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod. 2005;73:825–32.
Vendola K et al. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999;14:2328–32.
Vendola K et al. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61:353–7.
Tetsuka M, Hillier SG. Differential regulation of aromatase and androgen receptor in granulosa cells. J SteroidBiochem Molec Biol. 1997;61:233–9.
Hillier SG, Tetsuka M. Role of androgens in follicle maturation and atresia. Baillieres Clin Obstet Gynaecol. 1997;11:249–60.
Jia XC, Ny T, Hsueh AJW. Synergistic effect of glucocorticoids and androgens on the hormonal induction of tissue plasminogen activator activity and messenger ribonucleic acid levels in granulosa cells. Molec Cell Endocrinol. 1990;68:143–51.
Hillier SG, Ross GT. Effects of exogenous testosterone on ovarian weight, follicular morphology and intraovarian progesterone concentration in estrogen-primed hypophysectomized immature female rats. Biol Reprod. 1979;20:261–8.
Nandedkar TD, Munshi SR. Effect of dihydrotestosterone on follicular development, ovulation and reproductive capacity of mice. J Reprod Fertil. 1981;62:21–4.
Murray AA et al. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113:27–33.
Buccione R, Schoroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod. 1990;43:543–7.
Klinger FG, De Felici M. In vitro development of growing oocytes from fetal mouse oocytes: stage-specific regulation by stem cell factor and granulosa cells. Dev Biol. 2002;244:85–95.
Johnson JE, Lee Higdon III H, Boone WR. Effect of human granulosa cell co-culture using standard culture media on the maturation and fertilization potential of immature human oocytes. Fertil Steril. 2008;90:1674–9.
Boon CH, Tong GQ, Ng SC, et al. Effects of granulosa coculture on in-vitro oocyte meiotic maturation within a putatively less competent murine model. Theriogenology. 2004;62:1066–92.
Niu ZH, Yun F. The clinical study of relationship between serum E2/P ratio on the day of HCG injection and embryo implantation. J Pract Obstet gynecol. 2005;21:370–2.
Acknowledgments
We thank all of the patients and family members for their participation. This study was supported by the Science Research of Population and Family Planning Commission of Guangdong Province in China (Grant No. 2009231), the Science Technology Research and Development Project of Guangdong Province (Grant No. 2010B031600043) and the Science Technology Research Project of Guangdong Province(Grant No. 2008B080701026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Telomerase activity of ovarian luteinised GCs can help us predict the clinical outcomes of IVF treatment
Rights and permissions
About this article
Cite this article
Chen, H., Wang, W., Mo, Y. et al. Women with high telomerase activity in luteinised granulosa cells have a higher pregnancy rate during in vitro fertilisation treatment. J Assist Reprod Genet 28, 797–807 (2011). https://doi.org/10.1007/s10815-011-9600-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9600-2