Abstract
Purpose
This prospective study was designed to investigate whether anti-Müllerian hormone (AMH) levels at basal and ovulation triggering day are associated with ovarian response and pregnancy outcome for in vitro fertilization (IVF).
Method
60 infertility women undergoing IVF were prospectively studied. On day 3 of the menstrual cycle (D3), measurements of AMH, inhibin B, FSH, LH, and E2 and ultrasound evaluation of antral follicle count (AFC) were performed. Serum AMH and inhibin B levels were remeasured on the day of hCG administration (DhCG). The outcome measures were the number of retrieved oocytes and clinical pregnancy.
Results
Number of retrieved oocytes was statistically significant and correlated with D3 AMH, AFC, DhCG AMH, DhCG inhibin B, FSH, and age (r = 0.885, 0.874, 0.742, 0.732, −0.521, −0.385, respectively). Statistically significant differences were found between pregnant and non-pregnant women regarding D3 AMH and AFC. Multiple regression analysis for prediction of pregnancy showed D3 AMH to be a good predictor of clinical pregnancy.
Conclusion
AMH correlates better than age, FSH, and inhibin B with the number of retrieved oocytes. Serum basal AMH may offer a better prognostic value for clinical pregnancy than other currently available markers of IVF outcome in our preliminary study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessment of “ovarian reserve” is important before in vitro fertilization (IVF) treatment is undertaken. Identification of both low and high responders prior to ovulation induction allows physicians to optimize stimulation protocols to decrease cycle cancellation rate and side effects, such as ovarian hyperstimulation syndrome (OHSS).Traditionally, day 3 follicle-stimulating hormone (FSH), estradiol (E2), and inhibin B levels have been used as indicators of ovarian reserve. However, their predictive values remain somewhat controversial and require specific menstrual days for accurate analysis [1–3]. Furthermore, several investigators have reported the usefulness of ovarian volume [4, 5] and antral follicle count (AFC) [6, 7] in predicting ovarian response to hormone stimulation. Nonetheless, ultrasonography is subjective, and the interpretation of the observations may not be consistent [8]. Recently, a new endocrine marker, anti-Müllerian hormone (AMH), has been evaluated by several groups as a marker of ovarian response [9–16].
Anti-Müllerian hormone (AMH), also called Müllerian inhibiting substance, belongs to the transforming growth factor-β (TGF-β) superfamily, and it is considered a local growth factor and a cellular differentiation factor [17]. In women, AMH is exclusively produced in the ovary by the granulosa cells surrounding preantral and small antral follicles [18, 19]. Hence, it is thought that serum AMH levels are a reflection of the size of the growing cohort of small follicles [11, 20], which in turn reflects the number of residual primordial follicles, or the ovarian reserve.
Growing evidence indicates that serum AMH levels have showed greater sensitivity to ovarian aging [20], a stronger relationship with the number of early antral follicles [10], and better cycle-to-cycle reproducibility [21] compared with FSH, E2 and inhibin B levels. Recent results show AMH to be a predictor for success rates in ART [22–24]; however, others have not found it predictive of pregnancy outcome [16, 25, 26]. Furthermore, there were few reports addressing the clinical significance of AMH levels measured at late follicular phase during ovarian stimulation. Our prospective study was designed to investigate whether AMH levels at basal and ovulation triggering day compared with FSH, LH, E2, inhibin B, and AFC are associated with ovarian response and pregnancy outcome for stimulated IVF cycles.
Materials and methods
Subjects
A total of 60 infertility women enrolled in our IVF program were recruited for the prospective study between January 2007 and December 2007. The inclusion criteria were: (1) first cycle of ovarian stimulation, (2) <40 years of age, (3) both ovaries present on transvaginal ultrasound scan, (4) no previous history of ovarian surgery, and (5) no evidence of endocrinological disorders (normal testosterone, prolactin, thyroid stimulating hormone), (6) absence of any hormonal therapy in the past 3 months.
The study was approved by the Institutional Review Board (IRB) and the committee on ethics for research involving human subjects of Changhua Christian Hospital Medical Center (Republic of China). All couples participating in the study signed informed consent.
Blood sampling and hormones assays
On day 3 (D3) of the menstrual cycle before treatment, blood samples for assay of FSH, inhibin B, AMH, luteinizing hormone (LH) and estradiol (E2) were collected, about 5 ml, by venipuncture and divided into 2 plain tubes. All samples were immediately centrifuged to separate the serum and stored in aliquots at −70°C. One tube was used for the FSH, LH, and E2 assays, and the other was for inhibin B and AMH. AMH was measured by using the ultrasensitive ELISA (Bechman-Caulter, France) and inhibin B was measured by a double antibody ELISA (Serotec, Varilhes, France). All samples were assayed at the same time to minimize intra-assay variation. Serum levels of FSH, LH and E2 were determined by using RIA kit.
Another blood samples on the day of administration of hCG (DhCG) were also collected and measured in the same way.
Stimulation protocol
Before starting treatment, the total number of antral follicles measuring 2–10 mm in diameter was counted by transvaginal ultrasound. All patients underwent IVF treatment using GnRH antagonist protocol and the COH protocol was as previously described [27]. In brief, ovarian stimulation was initiated with exogenous gonadotropins in the form of recombinant FSH (Gonal-F, Serono) from day 3 of cycle. The starting daily dose was decided according to the age and the baseline FSH levels. GnRH antagonist (cetrorelix, Serono) 0.25 mg subcutaneous injection daily was given since day 8 of cycle for preventing premature LH surge. On the same day, a changing dosage of gonadotropin was given according to sequential transvaginal ultrasonography and serum E2. When at least two or more follicles of ≥18 mm in diameter were detected; hCG (5,000 IU, Pregnyl, Organon) was administered. Transvaginal ultrasound-guided oocyte retrieval was performed 36 h after hCG injection. The number of retrieval oocytes was recorded. The oocytes were then fertilized in the laboratory with her partner's sperm. Fertilization was assessed using an established pronuclei scoring system. Then embryos were transferred 2 days later and vaginal progesterone gels (crinone 90 mg daily) were used to support luteal phase till the day of serum pregnancy test. The number of transferred embryo was decided to the wish of the couple and the number of embryos available. A positive pregnancy test was defined by >50 IU/L of plasma β-hCG on day 14 after embryo transfer. Two weeks later, a transvaginal ultrasound was done to confirm a clinical pregnancy.
The study group was divided into two subgroups according to the number of oocytes retrieved. Patients with an oocyte count of four or less were considered poor responders, and patients with more than four as normal responders.
Statistical analysis
All analyses were performed using SPSS software version 15.0 (SPSS Inc., Chicago, IL). Fisher's exact test was used to examine differences with categorical variables. Values are presented as mean (±SD). We used Mann-Whitney U test, Kruskal Wallis test and Jonckheere-Terpstra test to compare the different groups. Pearson (r) correlation coefficients were calculated to explore the relationships between the measured parameters. Multivariate logistic regression analyses were used to test the association between poor response (oocyte No.≦ 4) or pregnancy with the measured parameters. For all statistical analyses, P < 0.05 was considered to be statistically significant.
Results
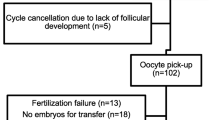
Of the 60 women tested, 14 had ≦ 4 oocytes (poor responders), and 46 had ≧ 5 oocytes (normal responders). Table 1 shows patient and ovarian reserve test characteristics of poor and normal responders. Statistically significant differences existed between poor and normal responders in D3 FSH, LH, inhibin B, AFC, and AMH levels The main parameter of the study, D3 AMH, was found to be considerably higher in normal responders; mean level was 4.4 ± 2.2 ng/mL. The AMH level was 0.7 ± 0.8 ng/mL in poor responders (P < 0.01)
There were statistically significant positive correlations between the number of retrieved oocytes and D3 AMH (r = 0.885, P < 0.001), followed by AFC (r = 0.874, P < 0.001), DhCG AMH (r = 0.742, P < 0.001), and DhCG inhibin B (r = 0.730, P < 0.001) (Table 2). Statistically significant inverse correlations between the number of retrieved oocytes and D3 FSH (r = −0.521, P < 0.001), and age (r = −0.385, P = 0.002) were also observed. No correlation was identified between the number of retrieved oocytes and D3 inhibin B (P = 0.776) or LH (P = 0.616). Overall, D3 AMH had the strongest statistically significant correlation with the number of oocytes that were retrieved. D3 AMH levels were correlated with AFC (r = 0.836 ; P < 0.001).
The clinical pregnancy rate per started cycle was 43.3% (26/60). Data for pregnant and nonpregnant women are presented in Table 3. D3 AMH and AFC were significantly different between pregnant and nonpregnant women. Women who achieved pregnancy had higher AMH levels (4.3 ± 2.6 ng/mL vs. 3.4 ± 2.4 ng/mL; P = 0.011), but similar FSH levels (5.6 ± 1.2 IU/L vs. 6.0 ± 1.7 IU/L; P = 0.31).When multiple regression analysis was used for prediction of clinical pregnancy, D3 AMH levels were the only independent predictors of pregnancy (β coefficient [±SE], 0.635 ± 0.325; P = 0.049) (Table 4). Neither AFC nor D3 FSH proved to be an independent predictor.
Discussion
This prospective study was conducted to evaluate the relevance of routine AMH measurements during IVF treatment. Age, FSH-, inhibin B- and AMH-levels and their predictive values for ovarian response and clinical pregnancy rate were compared by discriminant analyses.
Currently, most IVF clinicians determine starting doses of gonadotrophin in the first cycle of IVF based principally on age and basal FSH levels [28]. Our study suggests that AMH and AFC are superior predictors of oocyte yield compared with age and basal FSH. Linear regression analysis shows a significant association between AMH, AFC, and FSH and the number of oocytes collected. In keeping with the recent study [29], AMH correlates better than age, FSH, LH, E2, and inhibin B with the number of retrieved oocytes. Our analysis confirmed that AMH and baseline FSH demonstrate a negative linear relationship and that, as previously noted [30]. Consistent with previous studies [8, 10, 13, 16, 23, 31], our results demonstrated a strong association among AMH and antral follicles and retrieved oocyte count. The performance of AMH in the prediction of poor response from the other studies and the correlation between AMH and retrieved oocyte count, are summarized in Table 5.
Therefore, serum basal AMH levels may reflect the size of antral follicle pool and provide a marker associated with the number of retrieved oocytes after controlled ovarian hyperstimulation (COH). Furthermore, our findings are in agreement with those of the previous studies [32, 33] in that serum AMH at ovulation triggering day has a significant positive correlation with the number of oocytes retrieved.
We observe that D3 AMH levels and AFC were significantly lower in the poor responders than in the normal responding women (Table 1). The performance of AMH in identifying poor responders was very similar to that of AFC in the previous studies [13, 16, 34]. However, an accurate AFC depends on the clinician’s experience and the ultrasound properties. By contrast, AMH levels are obtained by objective measurements performed in laboratory medium and thus are free of interobserver variability and personal comments.
In agreement with other studies [11, 35–37], we found that serum AMH level declined significantly during COH , thus confirming the reported low levels of AMH expression by larger follicles. Although the physiological mechanisms implicated in such a process remain undetermined, these results are in keeping with previous study indicating that AMH is preferentially secreted by pre-antral and early antral follicles [18]. This decrease in AMH concentrations between start of stimulation and the day of hCG administration reflects the reduction in the number of small growing follicles recruited during ovarian stimulation [11, 35].
One of most attractive advantages of AMH is that its levels have been shown to be stable under various influences such as hormonal contraception [38, 39], GnRH agonist [40], pregnancy [41], and the menstrual cycle [42–45]. Therefore, measurements can be made anytime during the menstrual cycle. However, two reports suggested that AMH levels actually fluctuate during the menstrual cycle [46, 47]. Discrepancies between studies might be explained by differences in age of population, size of population, and methodology of the AMH assay. Two commercial AMH ELISA assays (Beckman Coulter and DSL) are available on the market. A recent study showed a close linear relationship between the two methods but the AMH levels were almost 4.6-fold higher with the Beckman Coulter than with the DSL kit [48]. Recently, Streuli et al [49] concluded that the changes in AMH levels after ovulation are slight, and therefore are not clinically relevant as far as AMH measurements for clinical purposes are concerned. In daily practice, AMH therefore can be measured anytime during the menstrual cycle.
Predicting the ovarian reserve in IVF patients is useful in optimizing the stimulation protocol to obtain a good response. However, it remains a challenge to identify young women, with normal ovulatory cycles but low ovarian reserve. Compared to inhibin B and AFC, AMH was more consistently correlated with the clinical degree of follicle pool depletion in young women presenting with elevated FSH levels [50]. A younger woman with a reduced ovarian reserve may then choose to pursue treatment sooner. A further potential application for the prediction of poor response is the augmentation of the starting dose of gonadotrophins in predicted poor responders. It is not certain that this may lead to higher pregnancy rates [51], but randomized prospective data on this issue are still lacking.
Application of AMH, as a predictor of ongoing pregnancy following IVF appears to be limited in view of the fact that they only represent the quantitative aspect of ovarian reserve, whereas pregnancy is also dependent on the oocyte quality, embryo development and endometrium receptivity. Therefore, some studies had shown that the serum level of AMH found to predict oocyte number may not predict the probability of pregnancy [14, 16, 25, 26, 52–55]. However, we found that high serum AMH levels correlated not only with oocyte number but also with pregnancy rates. D3 AMH and AFC were significantly different between pregnant and non-pregnant women (Table 3). When logistic regression analysis was used for prediction of clinical pregnancy, D3 AMH levels were the only independent predictors of pregnancy. Neither AFC nor D3 FSH proved to be an independent predictor. While our results show such an association, the number of pregnant women is very small to make such a correlation. A recent meta-analysis study examining the link between AFC and pregnancy outcome found that AFC was not predictive of pregnancy during IVF treatment [56]. Another recent study also found that AMH was superior in predicting IVF outcomes in comparison with FSH [24]. Moreover, recent reports suggested better predictive capabilities for pregnancy for AMH [12, 13, 22–24, 57]. These results confirm those found by other investigators, whose studies showed that serum AMH levels during COH may reflect oocyte and embryo quality [31, 58]. Up to now, only one study has been published relating serum AMH levels to the live birth rate following IVF [59]. In this prospective study, it was demonstrated that the live birth rate dramatically increases with increasing basal AMH value.
Recently, a large prospective study performed on 538 patients undergoing ART [60], indicates that a single AMH assay may be used to individualize treatment strategies for IVF. The AMH-based strategy of controlled ovarian stimulation was associated with a significant reduction of excess response to stimulation, reduced cycle cancellation and a trend towards increased clinical efficacy. Although AMH has the potential to guide clinical management in IVF, a number of important questions relating to its clinical implications need to be answered [61].
In conclusion, there are many advantages of using serum AMH over other serum markers. Firstly, serum AMH levels begin to decline before serum FSH and inhinbin B levels become abnormal [20]. Secondly, serum AMH can be measured throughout the cycle, in contrast to the other parameters, which can only be determined in the early follicular phase. Finally, unlike AFC measurements, serum AMH assays are not observer-dependant, resulting in less interobserver variability. In this preliminary study, we found that either ultrasonic (AFC) or endocrine assessment (basal AMH and FSH) could predict ovarian response. Our results may support the assumption that there is an association between AMH and pregnancy rate in IVF based on the small sample size. This issue requires to increase the numbers of subjects in an effort to increase the power of the study.
References
Ravhon A, Lavery S, Michael S, Donaldson M, Margara R, Trew G, et al. Dynamic assays of inhibin B and oestradiol following buserelin acetate administration as predictors of ovarian response in IVF. Hum Reprod. 2000;15:2297–301.
Bancsi LF, Broekmans FJ, Eijkemans MJ, de Jong FH, Habbema JD, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–36.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Updat. 2006;12:685–718.
Lass A, Skull J, McVeigh E, Margara R, Winston RM. Measurement of ovarian volume by transvaginal sonography before ovulation induction with human menopausal gonadotrophin for in-vitro fertilization can predict poor response. Hum Reprod. 1997;12:294–7.
Wallace WH, Kelsey TW. Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod. 2004;19:1612–7.
Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505–10.
Hendriks DJ, Mol BW, Bancsi LF, Te Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83:291–301.
Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384–90.
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–71.
Van Rooij IA, Broekmans FJM, te Velde ER, Fauser BCJM, Bancsi LFJMM, de Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71.
Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–7.
Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82:1323–9.
Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20:3178–83.
Penarrubia J, Fabregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist–gonadotropin treatment. Hum Reprod. 2005;20:915–22.
Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-mullerian hormone as a marker of ovarian reserve. Aust NZ J Obstet Gynaecol. 2005;45:20–4.
Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–6.
Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, et al. Müllerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–6.
Durlinger AL, Visser JA. Themmen AP Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–9.
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83.
De Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Anti-mullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–62.
Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–7.
Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Müllerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14:602–10.
Wunder DM, Guibourdenche J, Birkhäuser MH, Bersinger NA. Anti-Müllerian hormone and inhibin B as predictors of pregnancy after treatment by in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2008;90:2203–10.
Barad DH, Weghofer A, Gleicher N. Comparing anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91:1553–5.
Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Thomas CM, Braat DD. Antimüllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223–6.
Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti-mullerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2008;23:160–7.
Hsieh YY, Chang CC, Tsai HD. Comparisons of different dosages of gonadotropin-releasing hormone (GnRH) antagonist, short-acting form and single, half-dose, long-acting form of GnRH agonist during controlled ovarian hyperstimulation and in vitro fertilization. Taiwan J Obstet Gynecol. 2008;47:66–74.
Borini A, Dal Prato L. Tailoring FSH and LH administration to individual patients. Reprod Biomed Online. 2005;11:283–93.
Riggs RM, Duran EH, Baker MW, Kimble TD, Hobeika E, Yin L, et al. Assessment of ovarian reserve with anti-Müllerian hormone: a comparison of the predictive value of anti-Müllerian hormone, follicle-stimulating hormone, inhibin B, and age. Am J Obstet Gynecol. 2008;199:202.e1–8.
Singer T, Barad DH, Weghofer A, Gleicher N. Correlation of antimüllerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril. 2009;91:2616–9.
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, Laing I. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization.Fertil Steril. 2008 Oct 16. [Epub ahead of print].
Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159–63.
Jee BC, Ku SY, Suh CS, Kim KC, Lee WD, Kim SH. Serum anti-Müllerian hormone and inhibin B levels at ovulation triggering day can predict the number of immature oocytes retrieved in in vitro fertilization cycles. J Korean Med Sci. 2008;23:657–61.
Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BCJM. Antimüllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–23.
La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, et al. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19:2738–41.
Feyereisen E, Mendez DH, Taieb J, et al. Anti-Müllerian hormone: clinical insights into a promising biomarker of the ovarian follicular status. Reprod Biomed Online. 2006;12:695–703.
Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, et al. Anti-Müllerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online. 2008;16:664–70.
Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201.
Arbo E, Vetori DV, Jimenez MF, Freitas FM, Lemos N, Cunha-Filho JS. Serum anti-Mullerian hormone levels and follicular cohort characteristics after pituitary suppression in the late luteal phase with oral contraceptive pills. Hum Reprod. 2007;22:3192–6.
Mohamed KA, DaviesWA LH. Antimullerian hormone and pituitary gland activity after prolonged down-regulation with goserelin acetate. Fertil Steril. 2006;86:1515–7.
La Marca A, Giulini S, Orvieto R, De Leo V, Volpe A. Anti-Mullerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569–72.
Hehenkamp WJ, Looman CW, Themmen APN, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63.
La Marca A, Stabile G, Carducci Artenisio A, Volpe A. Serum antimullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–7.
Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–40.
Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90:395–400.
Lahlou N, Chabbert-Buffet N, Gainer E, Roger M, Bouchard P, VA2914 Study Group. Diphasic pattern of anti-mullerian hormone (AMH) in ovulatory cycles as evidenced by means of an ultra-sensitive assay: new insights into ovarian function. Fertil Steril. 2006;86(Suppl):S11.
Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. 2008;89:927–33.
Freour T, Mirallié S, Bach-Ngohou K, Denis M, Barrière P, Masson D. Measurement of serum anti-Müllerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART). Clin Chim Acta. 2007;375:162–4.
Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, de Ziegler D. Clinical uses of anti-Müllerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91:226–30.
Knauff EA, Eijkemans MJ, Lambalk CB, Broekmans FJ, Themmen AP, Fauser BC. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J Clin Endocrinol Metab. 2009;94:786–92.
Lekamge DN, Lane M, Gilchrist RB, Tremellen KP. Increased gonadotrophin stimulation does not improve IVF outcomes in patients with predicted poor ovarian reserve. J Assist Reprod Genet. 2008;25:515–21.
McIlveen M, Skull JD, Ledger WL. Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod. 2007;22:778–8.
Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23:1359–65.
Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–14.
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2008; Nov 29. [Epub ahead of print].
Hendricks DJ, Kwee J, et al. Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative metaanalysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87:764–75.
Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89:1670–6.
Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–6.
Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414–21.
Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, et al. Anti-Mullerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–75.
La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS; on behalf of the ESHRE Special Interest Group for Reproductive Endocrinology – AMH Round Table. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009 Jun 11. [Epub ahead of print].
Financial Disclosure
The authors have no potential conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Serum basal AMH correlates better than age, FSH, and inhibin B with the number of retrieved oocytes and may offer a better prognostic value for clinical pregnancy in IVF cycles.
Rights and permissions
About this article
Cite this article
Wu, CH., Chen, YC., Wu, HH. et al. Serum anti-Müllerian hormone predicts ovarian response and cycle outcome in IVF patients. J Assist Reprod Genet 26, 383–389 (2009). https://doi.org/10.1007/s10815-009-9332-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-009-9332-8