Abstract
Objective
To compare fertilization, implantation and pregnancy rates in donor oocyte cycles triggered for final oocyte maturation with either human chorionic gonadotropin (hCG) or gonadotropin releasing hormone (GnRH) agonist in the same donor population in two sequential stimulation cycles.
Design
Prospective randomized cross-over trial.
Setting
Private infertility clinic.
Patient(s)
Eighty-eight stimulation cycles in 44 egg donors.
Interventions
Controlled ovarian hyperstimulation (COH) with GnRH antagonist protocol triggered with hCG or GnRH agonist (leuprolide acetate 0.15 mg) in the same egg donors in two consecutive cycles.
Main outcome measure(s)
The primary outcome measure was the proportion of mature and fertilized oocytes per donor cycle. Secondary outcome measures were implantation and pregnancy rates in the recipients and incidence of ovarian hyperstimulation syndrome (OHSS) in oocyte donors.
Result(s)
The proportion of mature oocytes, fertilized oocytes and mean embryo scores were comparable between the two triggering agents. While implantation (36.53% vs, 32.93%), pregnancy (69.08% vs. 68.81%) and clinical pregnancy (41.3% vs. 40.2%) rates were comparable for the groups, the incidence of OHSS was significantly lower in GnRH than in hCG triggered cycles.
Conclusion(s)
Fertilization, implantation and pregnancy rates from donor oocytes stimulated with GnRH antagonist protocol were identical for donor cycles triggered with hCG and GnRH agonist. GnRH antagonist triggering in egg donors was associated with lower rates of OHSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to solving a variety of reproductive problems, the introduction of human egg donation in 1983 has enhanced our understanding of the physiology and pathophysiology of human reproduction. One important medical problem was the need to develop a stimulation protocol suitable for egg donors. Egg donors are chosen among young, normally fertile women, a group prone to ovarian hyperstimulation syndrome (OHSS). There is a need, however, for OHSS-free stimulation in oocyte donation. Different stimulation protocols have been proposed to overcome this harmful and sometimes fatal complication. Human chorionic gonadotropin (hCG) has been used successfully in the final maturation of dominant follicles in assisted reproduction protocols. Due to the prolonged luteotrophic effects of hCG, ovulatory serum concentrations of this hormone may increase the incidence of OHSS [1]. A new treatment option for egg donors undergoing ovarian stimulation is the gonadotropin-releasing hormone (GnRH) antagonist protocol, in which a single bolus of GnRH agonist can trigger a mid-cycle LH surge. This protocol is efficient in triggering ovulation and may prevent OHSS in high responders [2]. High responders showed acceptable rates of fertilization, implantation, clinical pregnancy, ongoing pregnancy and early pregnancy loss after triggering final maturation with GnRH agonist and hCG [3]. This dual trigger protocol seems to be safe and effective for oocyte maturation in patients with significant risk factors for OHSS [3]. Agonist vs. hCG triggering has been tested in fresh autologous IVF cycles. A meta-analysis showed that the use of GnRH or hCG for final oocyte maturation, and in which GnRH antagonist was used to inhibit premature LH surge, yielded a comparable number of oocytes capable of undergoing fertilization and subsequent embryonic cleavage [4]. Moreover, a retrospective analysis of the use of GnRH agonist to trigger final oocyte maturation on the outcome of IVF cyclesfound that the use of a flexible multidose GnRH-antagonist protocol with GnRH-agonist for final oocyte maturation in fresh autologous cycles in high-responder patients eliminated the risk of OHSS [5]. However, the likelihood of an ongoing clinical pregnancy and implantation was significantly lower after GnRH agonist triggering compared with the standard hCG treatment [4, 5]. Agonist triggering has also been tested in frozen-embryo replacement IVF cycles; these studies found that the likelihood of a live birth after GnRH triggering of final oocyte maturation was not impaired, suggesting that the impaired clinical pregnancy and implantation rates in IVF cycles may be due to endometrial factors in antagonist cycles triggered for oocyte maturation with GnRH agonists [6]. Since ovarian stimulation in donor egg cycles may increase the risk of OHSS, triggering with agonist vs. hCG was also compared in oocyte donation cycles. A retrospective study of 74 oocyte donor cycles showed that the agonist trigger is a viable alternative for oocyte donors at significant risk of OHSS [7]. Moreover, a large study, involving 2,077 donor cycles, showed that oocyte developmental potential and embryo quality were not affected when GnRH agonist was used for final oocyte maturation [8]. In that study the agent for final oocyte maturation was chosen after taking into account the total number of follicles present on the day of final triggering, with GnRH agonist preferred for donors with more follicles and higher E2 levels. This bias could be eliminated by assessing stimulation in the same donors using two different triggering agents.
The aim of the present prospective study was to compare IVF outcomes (oocyte number and quality, and fertilization, pregnancy and implantation rates) for antagonist protocols with hCG and GnRH agonist for final oocyte triggering in egg donation cycles using the same donor population.
Materials and methods
Donors
All oocyte donation cycles were performed in a private fertility center in Cyprus between January and December 2008. Donors were assigned to two different ovulation triggering protocols using a computer based table of random numbers by the registered nurse. Once a donor was assigned to one protocol, she underwent the second protocol during the subsequent cycle. The follow-up physician performing the ovum pick-up procedure was blinded to patient assignment. A total of 50 egg donors were included in the study and 44 were eligible for the analysis. The time interval between the two stimulations was 3–10 months. Donations were anonymous, and donors were required to be between 20 years and 33 years of age. Each donor underwent a conventional clinical and psychological workup and was assayed for serological markers for hepatitis B, hepatitis C and HIV. The study protocol was approved by our Institutional Review Board, and all donors provided written informed consent.
Ovarian stimulation
Donor stimulation was performed using recombinant FSH (Puregon, Organon, Istanbul, Turkey) or hMG (Merional 75 IU, IBSA, Lugano, Ticino, Switzerland), starting on day 3 of each donor’s menstrual cycle. The starting dose of gonadotropin was between 150 IU and 450 IU, with the daily dose adjusted according to ovarian response. The GnRH antagonist (Orgalutran, Organon, Istanbul, Turkey) was introduced according to a multiple-dose protocol (0.25 mg/day) when a leading follicle of 14 mm was present. Triggering was performed when at least three follicles ≥20 mm were present, either with 3,000–10,000 IU hCG IM (Pregnyl ampul 5,000 IU, Organon, Istanbul, Turkey) or with 0.15 mg leuprolide acetate SC (Lucrin Flakon 5 mg, Abbot, Istanbul, Turkey). The hCG dose was adjusted according to E2 level and OHSS risk factors. Patients with ≤25 follicles on the day of triggering or E2 ≤ 5,000 pg/mL received 10,000 IU hCG whereas those with 25–35 follicles or E2 in the range 5,000–10,000 pg/mL received 5,000 IU HCG. Donors at highest risk of OHSS, determined by the presence of ≥35 follicles on the day of triggering and E2 level >10,000 pg/mL received 3,000 IU of hCG for the final stimulation of the follicles. Oocyte retrieval was organized 35 h after triggering. The last dose of gonadotropin and the GnRH antagonist was injected ≤24 h before triggering. After oocyte retrieval, the donors were informed about the possible risks of OHSS. Each donor was followed-up by telephone for at least 2 weeks after the procedure. Ultrasound scans and physical examinations were performed if patients experienced any symptoms. None of our patients experienced severe OHSS.
Recipients
Before treatment, oocyte recipient candidates were carefully assessed, including general physical and gynecological examinations, blood tests, cervical smears, and pelvic ultrasound. The uterine cavity of each was assessed by hysterosalpingography, with or without hysteroscopy. Oral estradiol valerate (Estrofem, Novo Nordisk, Istanbul, Turkey) was used in a incremental regimen, starting at 2 mg daily, for endometrial preparation. Patients on standby received up to 10 mg a day, with the duration of treatment varying in accordance with the availability of oocytes. The endometrium was assessed by serial ultrasound, and recipients with inadequate endometrium or endometrium >12 mm by ultrasound were excluded. Individuals with severe oligoasthenoteratozoospermia or azoospermia were also excluded. Beginning on the day of oocyte retrieval, each recipient was given 600 mg micronized progesterone (Progestan capsule, Koçak Pharma, Istanbul, Turkey). For standardization of the study and to increase fertilization rate, donor eggs were routinely fertilized by ICSI (intracytoplasmic sperm injection). Embryos were transferred, by the same physician, into the uterine cavity on day 3 after ICSI under ultrasound guidance. If clinical pregnancy was successful, as assessed by the presence of at least one intrauterine sac and a fetal heart beat, as shown by ultrasound 5 weeks after transfer, hormone therapy was continued until the 12th weeks of gestation. Ongoing pregnancy was defined as a viable pregnancy confirmed by ultrasound at 12 weeks of gestation. The implantation rate was defined as the ratio of gestational sacs to the number of embryos transferred. Pregnancy was defined by serum hCG ≥5 IU/L 12 days after embryo transfer.
Outcome measures and statistical analysis
Since the primary goal of our study was to show the effectiveness of GnRH agonist as a triggering agent in donor oocyte cycles, the primary outcomes included proportion of mature (MII) oocytes, fertilized oocytes per donor cycle and mean embryo score. Secondary outcomes included implantation and pregnancy rates. Although we used a relatively small patient population, other secondary outcomes included the rate of mild to moderate OHSS in oocyte donors. All statistical analyses were performed using GraphPad Instat 3, Software for Windows 2008. Values are expressed as mean ± standard deviation. Metric variables were analyzed by Student’s t-test and nominal variables by the McNemar test. A p value < 0.05 was considered statistically significant.
Results
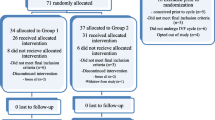
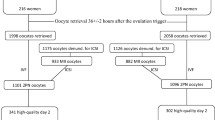
The present study was a crossover clinical trial. Fifty donors were randomized to two different stimulation protocols using a computer based table of random numbers. Two of the donors in hCG arm and three of the donors in GnRH agonist arm left the study without giving a reason. One of the donors in GnRH agonist arm was stopped because of the poor response. At the end, 44 donors were stimulated with both regimens. (Fig. 1). The results obtained when the 44 donors were triggered with the two different stimulation agents are listed in Table 1. Since the second stimulation of each donor was performed using the same protocol and the same gonadotropin as in the previous cycle, the same percentages of women in the two groups were treated with rFSH or HMG. The total dose of gonadotropins and the total days of stimulation were comparable for the two protocols, as were the numbers of oocytes retrieved (cumulus-oocyte complex and mature), and proportions of mature (MII) and fertilized (2pn) oocytes. The mean number of embryos transferred per patient in the antagonist protocols triggered with hCG and GnRH agonist were almost identical (3.40 ± 0.70 vs 3.39 ± 0.07). Oocytes per donor, triggered with these two protocols, were transferred to 2.32 ± 0.83 and 2.13 ± 0.80 recipients, respectively. None of the donors triggered with GnRH agonist developed OHSS, while four donors triggered with hCG had symptoms of mild to moderate OHSS (p = NS). None of these donors required hospitalization, and conservative management was sufficient for treatment.
Pregnancy results are summarized in Table 2. Embryos were transferred to 105 and 93 patients in the hCG and GnRH agonist groups, respectively. Recipient age and number of embryos transferred per patient were similar in the two groups. Although the implantation rate in the hCG arm (36.53%) was higher than in the GnRH agonist arm (32.93%), this difference was not statistically significant. Rates of pregnancy, clinical pregnancy rate, and multiple pregnancy, defined as >1 gestational sac on ultrasound during the fifth gestational week, were comparable for the two groups.
Dıscussıon
New protocols in egg donation cycles remain to be assessed, including investigating the potency of GnRH agonist for the final maturation of oocytes in donation procedures. Donation programs, including ours, increasingly utilize GnRH antagonist protocols, including the use of GnRH agonists for final triggering in oocyte maturation, making it important to compare old and new protocols.
Although hCG is widely used for final oocyte maturation, its prolonged luteotrophic effect leads to supraphysiological levels of estradiol and progesterone, leading, in many cases, to the development of hyperstimulation syndrome [2]. The most common and severe complication of ovarian hyperstimulation is OHSS. GnRH agonist triggering has gained wider popularity after the finding that GnRH agonist triggering does not result in similar prolonged luteotrophic effects, thus preventing OHSS. It is unclear, however, if GnRH agonist use results in comparable pregnancy rates. For example, one study found that GnRH agonist use resulted in significantly lower implantation and clinical pregnancy rates, in addition to a significantly higher rate of early pregnancy loss, than observed with hCG, with these differences likely due to luteal phase deficiency [9]. The likelihood of live births was improved after GnRH use in frozen-thawed embryo replacement cycles, further suggesting that GnRH agonist triggering is accompanied by luteal phase deficiency [10, 11]. Results of a recent meta-analysis suggested that the numbers of oocytes capable of being fertilized and undergoing subsequent embryonic cleavage were comparable with GnRH agonist and hCG [12]. However, the likelihood of an ongoing clinical pregnancy was significantly lower after GnRH agonist triggering than after standard HCG treatment [4]. Other studies have also indicated that the use of a protocol consisting of GnRH agonist triggering after GnRH antagonist cotreatment, combined with adequate luteal phase and early pregnancy E2 and progesterone supplementation, reduces the risk of OHSS in high-risk patients undergoing IVF without affecting the implantation rate [12, 13].
The probable endometrial effect of the GnRH agonist used in antagonist cycles was eliminated by comparative studies in egg donor models. Beside that the egg donors we used were young, their overstimulation puts them at high risk for OHSS. A recent study found that the risk of ovarian hyperstimulation in egg donors was 0.82%, and was observed only in donors given hCG for final oocyte maturation [14]. Another large study suggested that recipient outcome did not differ significantly using oocytes from GnRH antagonist-treated donor cycles triggered with hCG or GnRH agonist, although GnRH agonist triggering was associated with a lower incidence of moderate/severe OHSS in egg donors [7]. This study had several limitations, including its retrospective design and the physician choice of triggering agent. The final E2 level, as well as the numbers of follicles and retrieved oocytes, were significantly higher in the GnRH agonist arm. In that study, GnRH agonist was used mainly in high responders and donors at risk for OHSS. To overcome these biases, we designed a prospective study comparing the effectiveness of GnRH agonist and hCG in the same donors in two sequential cycles. We found no differences in pregnancy, implantation and fertilization rates between the two triggering agents. Despite our finding that final E2 concentration and number of retrieved oocytes were higher in the GnRH agonist arm, the differences were not significant, and OHSS was not observed following GnRH agonist use. Thus, even in the same patient population administered the same number of gonadotropin ampules, GnRH agonist resulted in lower rates of OHSS, without compromising embryo number and qualities. The homogeneity of our donor population contributed to a large extent to the increasing tendency to use GnRH agonist as a final triggering agent in egg donation cycles. The best way to avoid OHSS is to reduce ovarian stimulation and to use GnRH agonist as an alternative to hCG for triggering oocyte maturation. To our knowledge, our study is the first prospective randomized cross-over study supporting the hypothesis that GnRH agonist is an effective alternative to hCG for the final oocyte maturation in oocyte donor cycles and should be the method of choice, especially for donors with evident risk factors for OHSS.
References
Shapiro BS, Daneshmand ST, Garner FC, Aquirre M, Ross R, Morris S. Effects of the ovulatory serum concentration of human chorionic gonadotropin on the incidence of ovarian hyperstimulation syndrome and success rates for in vitro fertilization. Fertil Steril. 2005;84:93–8.
Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication. Hum Reprod. 2000;15:1965–8.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90:231–3.
Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–68.
Orvieto R, Rabinson J, Meltzer S, Zohav E, Anteby E, Homburg R. Substituting HCG with GnRH agonist to trigger final follicular maturation—a retrospective comparison of three different ovarian stimulation protocols. Reprod Biomed Online. 2006;13:198–201.
Griesinger G, Kolibianakis EM, Papanikolaou EG, Diedrich K, Van Steirteghem A, Devroey P, et al. Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril. 2007;88:616–21.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Comparison of human chorionic gonadotropin and gonadotropin-releasing hormone agonist for final oocyte maturation in oocyte donor cycles. Fertil Steril. 2007;88:237–9.
Bodri D, Guillén JJ, Galindo A, Mataró D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: findings of a large retrospective cohort study. Fertil Steril. 2008;91:365–71.
Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Reprod. 1992;7:117–9.
Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grøndahl ML, Westergaard L, et al. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20:1213–20.
Eldar-Geva T, Zylber-Haran E, Babayof R, Halevy-Shalem T, Ben-Chetrit A, Tsafrir A, et al. Similar outcome for cryopreserved embryo transfer following GnRH-antagonist/GnRH-agonist, GnRH-antagonist/HCG or long protocol ovarian stimulation. Reprod Biomed Online. 2007;14:148–54.
Acevedo B, Gomez-Palomares JL, Ricciarelli E, Hernández ER. Triggering ovulation with gonadotropin-releasing hormone agonists does not compromise embryo implantation rates. Fertil Steril. 2006;86:1682–7.
Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89:84–91.
Bodri D, Guillén JJ, Polo A, Trullenque M, Esteve C, Coll O. Complications related to ovarian stimulation and oocyte retrieval in 4052 oocyte donor cycles. Reprod Biomed Online. 2008;17:237–43.
Acknowledgements
The authors thank Nevin Demir, Handan Canca and Cyprus Yasam Hospital IVF team for the data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is the first prospective randomized cross-over study supporting the hypothesis that GnRH agonist is an effective alternative to hCG for the final oocyte maturation in oocyte donor cycles and should be the method of choice, especially for donors with evident risk factors for OHSS.
Rights and permissions
About this article
Cite this article
Sismanoglu, A., Tekin, H.I., Erden, H.F. et al. Ovulation triggering with GnRH agonist vs. hCG in the same egg donor population undergoing donor oocyte cycles with GnRH antagonist: a prospective randomized cross-over trial. J Assist Reprod Genet 26, 251–256 (2009). https://doi.org/10.1007/s10815-009-9326-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-009-9326-6