Abstract
Owing to increasing concern regarding human well-being and quality of life, the demand for natural products is increased, leading to the development of a market for biological and natural products. This study aimed to investigate the depigmentation and anti-ageing properties of red seaweed (Pyropia yezoensis) extracts for skin care applications. Within the tested range of concentrations (100, 200, 400, and 800 μg mL−1), P. yezoensis extracts did not exert cytotoxic effects on the three skin cell lines tested: mouse melanocytes (Melan-A), human dermal fibroblasts (1064 SK), and human dermal keratinocytes (HaCaT cells). No significant statistical difference (p = 0.05) was detected in the melanin content between cells exposed to 100 μg mL−1 of arbutin and 800 μg mL−1 of P. yezoensis extracts, indicating that P. yezoensis extracts had an potent inhibitory effect on melanogenesis as arbutin. There was a significant decrease in tyrosinase activity by 59.3% and 35.5% after treatment with arbutin and P. yezoensis extracts, respectively. These results indicated that P. yezoensis extracts had strong tyrosinase inhibitory properties but were not as effective as arbutin. P. yezoensis extracts also promoted collagen production by inhibiting collagen-degrading enzymes (matrix metalloproteinase (MMP)-2 and MMP-9) and promoting procollagen synthesis enzymes (tyrosinase-related protein (TRP)-1 and TRP-2). Evaluation of skin brightness and melanin content and dermatological assessment showed that P. yezoensis extracts enhanced skin brightness and reduced melanin content in 23 volunteers. Our results suggest that P. yezoensis extracts can be used as functional cosmetic agents to prevent, or remediate skin ageing and pigmentation.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global cosmetic product market is highly competitive and is projected to reach $69 billion by 2025 (Wise Guy Reports 2018). Cosmetic producers worldwide are increasingly using organic, herbal, and ayurvedic ingredients in their formulations. This trend is primarily a result of increasing interest in health and well-being (Pimentel et al. 2018). As the consumer perceptions of their appearance have increased, the skin whitening products market has grown prominently. According to Zion Market Research (2019) the global market for skin whitening products in 2017 had reached approximately US$ 4075 million and is expected to reach approximately US$ 8895 million by 2024. The global markets for skin whitening products include Europe, North America, Asia-Pacific, Latin America, the Middle East, and Africa. The Asia-Pacific region occupied the largest share of the market for whitening products in 2017, as the usage of these products increased in countries such as China, India, Japan, and Korea.
Melanin is the brown or black pigment derived from the enzymatic oxidation of phenols and is known to play a critical role in protecting the skin from harmful ultraviolet (UV) rays and toxic drugs (Solano et al. 2006). Melanin is produced by melanocytes in the basal layer of the epidermis, and its production can be enhanced by external and internal factors, such as UV-rays and hormonal changes, respectively. Hyperpigmentation is caused by abnormal melanin accumulation in the skin, leading to major skin pigment disorders including melasma, freckles, lentigo, moles, and leucoplakia (Kim et al. 2014; Han et al. 2015). To treat these disorders, the demand for various skin whitening cosmetics containing commercial depigmentation agents is increasing (Azam et al. 2017).
Several synthetic chemical substances have already proven to be effective skin whiteners, but others have recently raised safety concerns that have resulted on the prohibition of their use in some countries. Therefore, given the potential health risks that synthetic ingredients may pose for humans, the search for non-cytotoxic natural depigmentation agents benefits from the increasing interest of consumers on natural ingredients that can be used in cosmetic formulations (Burger et al. 2016).
Seaweeds are widely used as a food product; however they also are gaining attention for applications in the pharmaceutical and chemical industries. They are rich in various biochemical compounds such as minerals, proteins, vitamins, and carbohydrates, which are essential for the human body (Rupérez 2002; Dawczynski et al. 2007; Nova et al. 2020). Seaweeds are also known to be beneficial to the skin, as their ingredients are similar to that of human plasma, and its nutrients can be easily absorbed by the skin (Chapman and Chapman 1980). Owing to its high mineral content, seaweed extract is often used in skin care products, such as moisturizers, facial cleansing products, masks, makeup removers, and bath additives (Quah et al. 2014). A previous study reported that seaweed extract can improve skin elasticity, as well as tone, prevent cellulite, soothe damaged or irritated skin, and increase dermal immunity to cope with external stress (Quah et al. 2014). Considering these benefits, identifying new compounds in seaweeds to reduce pigmentation is important for the development of new cosmetic products to combat skin hyperpigmentation conditions.
The red seaweed Pyropia (formerly, Porphyra), commonly named ‘Kim’ in Korea, ‘Laver’ in the UK and the USA, ‘Nori’ in Japan, and ‘Zicai’ in China, is primarily consumed as food. Pyropia has been cultivated in Korea since the seventeenth century and accounts for one of the most important marine vegetables and aquaculture product in China, Japan, and Korea (Hwang and Park 2020), due to its economic importance and health benefits. Furthermore, owing to its high mineral (e.g., iron), vitamin (e.g., vitamins B and C), and protein content, the demand for Pyropia as a food product is increasing worldwide (Wells et al. 2017; Cho and Rhee 2020).
Regarding cosmetic properties, Pyropia extract (4% in sunscreen formulations) has been reported as an excellent active ingredient in sunscreen formulations as it enhances the protective effect of melanin against UV-induced DNA damage (Mercurio et al. 2015). Three sulphated polysaccharide fractions isolated from Pyropia have shown antioxidant activities with IC50 values of 0.06–1.6 mg mL−1, > 1 mg mL−1, and 0.8–1.8 mg mL−1 for superoxide radical activity, hydroxyl radical activity, and lipid peroxidase activity, respectively (Zhang et al. 2003). Enzymatic extracts from Pyropia have also been found to possess high phenolic content and strong antioxidant activities against acetylcholinesterase (Mooberry et al. 2003). Mycosporine-like amino acids (MAAs) are known to be highly active photoprotective candidates for preventing the harmful effects of UV radiation on human skin. Thus, MAAs extracted from Pyropia have been assessed as potential topical sunscreens (Schmid et al. 2006). The content range of MAAs in Pyropia species is between 4.2 and 10.6 mg g−1 (Lawrence et al. 2018). Furthermore, the sunscreen effect of Pyropia extract on HaCaT human keratinocytes has been demonstrated to improve viability with increasing extract concentration (Kim et al. 2014). The Pyropia peptide, PYP1-5, has also been shown to promote collagen synthesis in human dermal fibroblasts, demonstrating its anti-ageing effects (Kim et al. 2017).
Despite of previous reports on the positive effects of Pyropia extracts and its components on the skin, their impact on melanogenesis and skin pigmentation has not been studied yet. In this study we examined the impact of P. yezoensis extracts on skin hypopigmentation and collagen synthesis using both in vitro experiments and clinical studies.
Materials and methods
Seaweed materials
Pyropia yezoensis was collected from Jangbong Island, on the west coast of Korea. The seaweed was washed to remove salt, epiphytes, and sand, and was air-dried in the shade before being coarsely powdered. Pyropia yezoensis was dried and 5 g portions were cut into pieces, suspended in 62.5 mL distilled water, and kept at 4 °C for 16 h. The mixture was centrifuged at 1735×g for 20 min and the supernatant was heated at 95 °C for 20 h. The resultant extract was centrifuged at 1735×g for 10 min and further concentrated using a rotary vacuum evaporator (HS-2005S-N, Hanshin, Korea) at 45 °C.
Cell culture and viability
Cell respiration, an indicator of cell viability, was assessed via the MTT assay, which assess the mitochondrial dependent reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan. Melan-A melanocytes were grown in an RPMI-1640 medium, supplemented with 10% FBS (foetal bovine serum), 1% P/S (penicillin/streptomycin), and 200 nM TPA (12-O-tetradecanoyl phorbol-13-acetate), while human dermal fibroblasts (1064 SK) and human skin keratinocytes (HaCaT cells) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, USA) supplemented with 10% FBS and 1% P/S and incubated at 37 °C and 5% CO2 for 72 h. All cell lines used in this study were purchased from Korea Cell Line Bank (KCLB, Seoul, Korea).
Cells were seeded at the cell density of 2 × 104 cells in 96-well plate and left overnight; subsequently, different concentrations (100–800 μg mL−1) of Pyropia extracts were added, and the cells were incubated for 72 h. MTT dye reagent (2 mg mL−1 PBS) was added to each well and cells were incubated at 37 °C for 3 h. Following medium removal, 0.1% DMSO was added to each well and plates were gently shaken for 10 min. Optical absorbance was determined at 540 nm using an ELISA microplate reader (InfiniteM200PRO, Tecan, Austria). Cell viability was calculated according to Eq. 1:
where Asample is the absorbance from the mixture with Pyropia extracts and Acontrol is the absorbance from the mixture without the addition of the extract. All experiments were conducted in triplicates.
Melanin content assay
Pyropia extracts were diluted with RPMI-1640 medium (500 μL) to a range of concentrations (100, 200, 400, and 800 μg mL−1), added to 24-well plate containing Melan-A melanocytes at a density of 5 × 104 cells per well and incubated at 37 °C and 5% CO2 for 72 h, before being washed twice. Arbutin, a well-known tyrosinase inhibitor, was also added at 100 μg mL−1 (the IC50 value for tyrosinase inhibition was 112 μg mL−1 for arbutin according to Kang et al. (2004)) as a positive control. Cell pellets were dissolved in 1 N NaOH and incubated at 55 °C for 1 h. Melanin content was calculated by comparison of the absorbance at 490 nm using a standard curve of synthetic melanin.
Intra-cellular tyrosinase activity assay
Melan-A cells were treated with different concentrations of Pyropia extracts for 72 h. The cells were then harvested by trypsinisation and washed with phosphate-buffered saline (PBS). Lysis buffer composed of 0.1 M sodium phosphate buffer (pH 6.8), 0.1% Triton-X, and protease inhibitors was prepared. The cells were then disrupted by sonication and separated by centrifugation at 21,255×g for 20 min. After quantifying the protein content using a protein assay kit (Bio-Rad protein assay kit, USA), the cell lysates were adjusted to the same concentration of protein with the lysis buffer. The reaction mixtures, including cellular extracts, 2 mg mL−1 L-DOPA, and 0.1 M sodium phosphate buffer (pH 6.8), were incubated in a 96-well plate at 37 °C for 2 h. The change in absorbance at 450 nm was measured using an ELISA (enzyme-linked immunosorbent assay) reader (InfiniteM200PRO, Tecan, Austria). The percentage inhibition of tyrosinase activity was calculated using Eq. 2:
where A is absorbance at 450 nm without P. yezoensis extract and B is absorbance at the same wavelength with P. yezoensis extract.
Western blotting
After treatment with different concentrations of Pyropia extracts for 72 h, Melan-A cells were washed twice with cold PBS, and total cellular proteins were extracted using RIPA (radio-immune precipitation assay) lysis buffer containing protease inhibitors.
After electrophoresis, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) at 200 V for 50 min. The membrane was blocked for 2 h in TBST (tris-buffered saline and Tween 20) containing 5% dried skimmed milk powder. After three washes in TBST for over a period of 15 min, the membranes were incubated with primary antibodies: TRP-1 (1:1000, Santa Cruz, USA), TRP-2 (1:1000, Abcam, UK), MITF (1:1000, Abcam, UK), and beta-actin (1:1000, Abcam, UK), overnight at 4 °C. After three washes with TBST, the membranes were incubated with a 1:1000 dilution of HRP-conjugated secondary antibody against the appropriate species for 2 h at room temperature (20–25 °C). Proteins were visualised using a western blotting detection kit (Bio-Rad); β actin was used as an internal control.
Procollagen assay
A 500 μL volume of 1064 SK cells were seeded at a density of 2 × 105 cells per well in 24-well plate, cultured, and treated with different concentrations of Pyropia extracts for 24 h. The media was removed from the wells, and the samples were diluted using media without serum. After 48 h, all media were centrifuged at 434×g for 3 min. The resulting supernatant was measured for the degree of collagen synthesis using a Procollagen type-I c-peptide (PIP) ELISA kit (TaKaRa Biotechnology Inc., Japan) following the manufacturer’s instructions. The absorbance of the final solution was measured at 450 nm (InfiniteM200PRO) to calculate type-I procollagen protein yield; the measured collagen quantity was readjusted after protein quantification.
Gelatin zymography
After 24-h incubation with Pyropia extracts of different concentrations, the HaCaT cell (3 × 103 cells per well) culture media were analysed for gelatine degradation activity through electrophoresis under a non-reducing condition in 10% Tris-Glycin gelatine gel (Invitrogen, USA). Gel was incubated in 1× zymogram renaturing buffer (Invitrogen) containing 2.5% (v/v) Triton X-100 for 30 min at room temperature with gentle agitation, then equilibrated for 30 min at 25 ± 2 °C in 1× zymogram developing buffer (Invitrogen) containing 50 mM Tris base, 40 mM HCl, 200 mM NaCl, 5 mM CaCl2, and 0.02% (w/v) Brij 35, followed by overnight incubation with fresh developing buffer at 37 °C. The zymogram gel was washed three times with deionised water, stained by adding 20 mL SimplyBlue Safestain (Invitrogen) for 1 h at room temperature with gentle shaking, and then washed with 100 mL deionised water for 3 h. The zymogram gel was then taken out of the deionised water and scanned with a GS-710 densitometer (Bio-Rad, USA), digitised using the Quantity One Image Software (Bio-Rad), and the white band intensities were measured. The activities of MMP-2 and MMP-9 were quantified using the Scion Image 4.0.3.2 software (Scion Corporation, USA).
Clinical assessment of Pyropia extract lotion
A human clinical study was conducted in 23 female subjects with an average age of 42.6 ± 8.9 years, including two persons aged 20–29, four aged 30–39, thirteen aged 40–49, three aged 50–59, and one aged 60–69. Pyropia extracts were formulated into a simple lotion to assess the whitening efficacy. After applying the test (with Pyropia extracts) and control products in the designated area (left and right cheek balls) of the same subject twice per day for 4 and 8 weeks, the efficacy of skin whitening was evaluated based on skin brightness, skin melanin content, and a subjective survey.
To ensure accurate evaluation, each subject was allowed to rest for 30 min in a waiting room at a constant temperature (20–25 °C) and humidity (40–60%) to adjust the skin surface temperature and humidity to that of the immediate environment.
The skin whitening effect was measured using a Chroma meter CM700 (Konica Minolta, Japan), at the cheek ball around the temple. The skin brightness value is directly proportional to the whitening of the skin tone.
Skin melanin content was measured using Mexameter MX18 (Courage-Khazaka Electronic GmbH, Germany), a narrow-band reflectance spectrophotometer that emits 568 nm, 660 nm, and 880 nm light from the probe and measures the light reflected from the skin. It records the melanin content within 1 s of skin contact with the sensor. The percentage of whitening was calculated using Eq. 3:
where Xo is the initial melanin content measured at week 0 before application; Xt is the melanin content measured after the tth-week of application.
Subjects were also evaluated for their skin lightness based on visual assessment by experts. At each visit before and after using the test product, two dermatologists visually evaluated the skin colour of the test site based on the Intensity Score table. The skin colour evaluation criteria table has a skin colour range from 1 (bright and transparent) to 10 (dark and dull). Dermatologists assessed any potential complaints from volunteers and evaluated any unwanted symptoms (such as redness or irritability) and change in its frequency, severity, or specificity every 4 weeks during the test period.
This clinical study was externally monitored by P&K Skin Research Centre (http://www.pnkskin.com/eng) and was conducted in accordance with the ICH guidelines for good clinical practice (GCP). Ethical approval from the institutional ethics committee and informed consent from the participants were obtained (PNK-13613-W1R).
Statistical analysis
Depending upon the outcome of normality testing, paired Student’s t tests (parametric), or Wilcoxon signed ranks (non-parametric) tests, at a significance level of p < 0.05 were used. Additionally, the difference between the control and the test groups was evaluated using the independent t test (parametric) and Mann–Whitney U (non-parametric) tests at a significance level of p < 0.05. The results were expressed as the means ± standard deviation, and the hypothesis mean difference was 5% (p < 0.05) to confirm the significance.
The statistical analysis for the evaluation of whitening improvement of the test product in this research was based on the Korean Functional Cosmetics Codex (Korea Ministry of Drug and Food Safety 2013).
Results and discussion
Pyropia extracts are non-toxic and inhibit melanogenesis
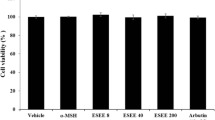
At the tested extract concentrations (100–800 μg mL−1), viability of the three human skin cell lines was not severely diminished, showing over 80% viability compared to the controls (Fig. 1). These results suggest that P. yezoensis extracts do not cause cytotoxic effects when used in the tested concentration range.
Cell viability of a Melan-A cells, b 1064 SK cells, and c HaCaT cells after treatment with Pyropia extracts. Results are expressed as percentages of the control, and values are mean ± SD (n = 3). Different letters indicate statistically significant differences between the groups at p < 0.05, according to Tukey’s test. NS, not significant
We then investigated the hypopigmentation effect of P. yezoensis extracts by evaluating their inhibitory properties on melanin synthesis. Tyrosinase is an enzyme that catalyses the rate-limiting step in melanin synthesis; therefore, arbutin, which acts as a competitive tyrosinase inhibitor, was used as a positive control. When comparing the inhibitory effects of arbutin and P. yezoensis extracts on melanin production, melanin content was reduced by 61.1% in arbutin-treated cells, whereas P. yezoensis extract treatment reduced the melanin content by 44.1 and 53.8% at 400 and 800 μg mL−1, respectively (Fig. 2). No significant difference (p = 0.05) was detected in the melanin content between cells exposed to 100 μg mL−1 of arbutin and 800 μg mL−1 P. yezoensis extracts, indicating that P. yezoensis extracts have a potent inhibitory effect on melanogenesis as arbutin. Although Pyropia extracts did not have the same effect as arbutin at the same concentration, a volume–volume comparison of efficacy of these two compounds was not meaningful as Pyropia extracts were whole crude extracts, and the concentrations of individual active components would likely be extremely low. It should be emphasised here that the tested concentrations of Pyropia extracts were similar to those of arbutin in terms of efficacy. Moreover, the Pyropia extracts are water-based, and obtaining its active compounds would be more cost-effective than obtaining the active compounds of arbutin. Hence, in view of the increasing demand for natural ingredients, it is advantageous to replace arbutin with Pyropia extracts, even if the concentrations of Pyropia extracts needed to exert effects are eight times higher than those of arbutin.
Effects of Pyropia extracts on melanin content by percentage. Arbutin (100 μg mL−1) was used as a positive control. Results are expressed as percentages of control, and values are mean ± SD of three independent experiments. Different letters indicate statistically significant differences between the groups at p < 0.05, according to Tukey’s test
In addition, high arbutin dosage is reportedly toxic to the liver and nephrons (Novak 2010), while products of edible seaweed, such as Pyropia, are known to be mild and biodegradable, exhibiting low toxicity. P. yezoensis extracts can, therefore, be used as an alternative non-toxic whitening agent instead of arbutin.
Pyropia extracts inhibit tyrosinase activity
Tyrosinase-inhibition activity has been found to be supressed in the extracts of various brown seaweeds, including Ecklonia cava, E. stolonifera, Endarachne binghamiae, Ishige okamurae, and Schizymenia dubyi (Kang et al. 2004; Heo et al. 2010; Cha et al. 2011). In red seaweeds, bromaphenol compounds extracted from Symphyocladia latiuscula were reported to be effective tyrosinase inhibitors (Paudel et al. 2019). Recently, considerable attention has been paid to marine algae for the discovery of tyrosinase inhibitors; however, the findings in red seaweed are limited.
Given that tyrosinase is the key regulatory enzyme for melanin synthesis, measuring its cellular activity is the first crucial step for investigating the hyperpigmentation mechanism of selected agents. Thus, tyrosinase activity was assessed in the three skin cell lines to further confirm melanogenesis inhibition by P. yezoensis extracts. In comparing the effects of arbutin and P. yezoensis extracts on tyrosinase activity, we noted a significant decrease of 59.3% in tyrosinase activity following 100 μg mL−1 arbutin treatment, whereas 800 μg mL−1 P. yezoensis extracts exerted a decrease of 35.5% (Fig. 3). Thus, P. yezoensis extracts were not as effective at inhibiting tyrosinase activity as arbutin, however, showed potent tyrosinase inhibition.
Effects of Pyropia extracts on tyrosinase activity. Arbutin (100 μg mL−1) was used as a positive control. The results are expressed as percentages of control, and values are mean ± SD of three independent experiments. Different letters indicate statistically significant differences between the groups at p < 0.05, according to Tukey’s test
To elucidate the mechanisms underlying the anti-melanogenic activities of P. yezoensis extracts, we examined the effects of P. yezoensis extracts on the expression levels of melanogenic enzymes, such as MITF and tyrosinase-related proteins (TRP-1 and TRP-2). Tyrosinase is modulated by MITF, a master transcription factor in melanogenesis. In addition, TRP-1 and TRP-2 are the major targets of melanogenic enzymes induced by MITF.
As shown in Fig. 4, P. yezoensis extracts (100–800 μg mL−1) were found to decrease the expression of these target proteins. Thus, it can be concluded that P. yezoensis extracts inhibit melanogenic activities by suppressing melanogenic enzymes.
Effects of Pyropia extracts on the expression of melanogenesis-related proteins in Melan-A cells. The cells were treated with the indicated concentrations. Arbutin (100 μg mL−1) was used as a positive control. a The expression levels of TRP-1, TRP-2, and MITF were measured using western blotting with specific antibodies. Equal protein loading was confirmed using β-actin. Western blot quantification was made by densitometry and ImageJ. Relative intensities of b TRP-1, c TRP-2, and d MITF were normalized with β-actin
Naturally occurring skin-whitening agents exert their action via the regulation of melanin production through a number of mechanisms, including the inhibition of the expression and activity of TYR, and the suppression of the uptake and distribution of melanosomes (Qian et al. 2020). MITF plays an essential role in melanogenesis via the control of the transcription of TRPs, such as TYR, TRP-1, and TRP-2 in mammalian cells (Qian et al. 2020). As shown in Figs. 2 and 4, P. yezoensis extracts led to the reduction of the melanin content and to the downregulation of the upstream transcription factor MITF in a dose-dependent manner, while tyrosinase activity was reduced only at higher concentrations of the same extracts.
It has previously been reported that naturally occurring bioactive compounds lead to anti-melanogenesis effects through interference with signalling pathways that downregulate MITF expression (Vance and Goding 2004). Therefore, the hypopigmentation effect of P. yezoensis extracts could be the result of the downregulation of MITF expression, which, in turn, would lead to suppression of TRPs gene and protein expression in a concentration-dependent manner. Overall, these findings suggest that P. yezoensis extracts abrogate melanogenesis first by suppressing the expression of MITF, TRP1, and TRP2, and then by directly inhibiting tyrosinase activity.
Pyropia extracts promote procollagen synthesis and reduce the activity of collagen-degrading enzymes
There are, by definition, two types of ageing—intrinsic ageing and extrinsic ageing. Intrinsic ageing is a natural and gradual process of skin degradation with age. Intrinsic ageing occurs when cells have a lower production of collagen, lower levels of elastin, and slower shedding of dead skin cells and replacement with new skin cells. The cause of intrinsic ageing is attributed to the continuous formation of reactive oxygen species (ROS), a by-product of oxidative cell metabolism (Binic et al. 2013). By contrast, extrinsic ageing is caused by external factors such as gravity, smoking, poor nutrition, and excessive exposure to ultraviolet radiation (photo-ageing). These external factors accelerate skin ageing by causing the skin to develop patchy pigmentation, leathery appearance, sallowness, deep wrinkles, and dryness (Helfrich et al. 2008).
In both types, skin ageing is related to reduced collagen production and the activities of multiple enzymes, including MMPs, which degrade collagen structure in the dermis (Kim et al. 2017). Compounds capable of enhancing collagen expression and inhibiting the activities of hyaluronidase, MMP, and elastase may be potentially used as active ingredients in novel cosmetic products with anti-wrinkle properties.
Therefore, we examined the effect of P. yezoensis extracts on collagen synthesis and observed significant promotion of collagen synthesis in 1064 SK cells following treatment with the extracts (Fig. 5). Type-I collagen constitutes approximately 85% of total collagen and is the main component of the extracellular matrix (ECM) in the skin dermis, conferring tension, elasticity, and flexibility to the skin (Scharffetter et al. 1991). In this study, type-I procollagen synthesis levels were found to be 213, 190, and 199 μg mL−1 after treatment with 200, 400, and 800 μg mL−1 of P. yezoensis extracts, respectively (Fig. 5). This indicates that the extracts have various potential applications in cosmetics and may serve as novel anti-ageing agents.
MMPs are responsible for changes in the structure and function of skin tissue that occur during skin ageing (Rittié and Fisher 2002). Of the several human MMPs, MMP-2 and -9 (gelatinase A and B) are secreted as inactive soluble zymogens and degrade several ECM substrates, such as gelatine, collagen, and fibronectin (Zamilpa et al. 2010). Thus, we evaluated the effect of P. yezoensis extracts on MMP expression and observed reduced activity of MMP-2 by 12.2% at 800 μg mL−1, and MMP-9 by 39.5% and 62.6% at 400 and 800 μg mL−1 of P. yezoensis extract treatment, respectively, than the vehicle-treated cells (Fig. 6).
Effects of Pyropia extracts on a MMP-2 and b MMP-9 activity. Values are expressed as percentages of control, and the data are mean ± SD (n = 3). Statistical significance was determined using Tukey’s test. Different letters indicate statistically significant differences between the groups at p < 0.05
These results further suggest that P. yezoensis extracts attenuate ageing effects in dermal fibroblasts and keratinocytes by promoting type-I collagen synthesis and reducing MMP expression. It is interesting to note that keratinocytes participate in the maintenance of skin homeostasis via active cross-talk with fibroblasts in healthy skin (Russo et al. 2020). Moreover, the production of type-I collagen was found to be enhanced in fibroblasts exposed to keratinocytes (Dufour et al. 2020). Meanwhile, the production of MMPs, including MMP-2 and MMP-9, consistently increase in fibroblasts under the influence of keratinocytes (Chinnathambi and Bickenbach 2005; Sawicki et al. 2005). Hence, keratinocytes promote ECM turnover by favouring MMP production over simultaneously reduced, or alternatively increased, collagen production by fibroblasts. Fibroblasts were also reported to enhance the deposition of basal membrane components by keratinocytes (El Ghalbzouri and Ponec 2004; Sorrell et al. 2004) while keratinocytes exhibit increased proliferation, decreased apoptosis, physiological differentiation, and increased basement membrane deposition in the presence of fibroblasts (Russo et al. 2020). Thus, fibroblasts modulate the viability, proliferation, and differentiation of keratinocytes. The reciprocal roles of keratinocytes and fibroblasts, as well as their interactions, may underline the need to simultaneously study keratinocytes and fibroblasts, as exemplified in this study, for a better understanding of skin mechanisms. Further investigations using in vitro ageing models, such as oxidative stress, or UV-induced ageing models, will be useful, and certainly another avenue to determine the suitability of Pyropia extracts as anti-ageing agents.
Basic cosmetics formulated with Pyropia extracts have significant whitening effects
The search for novel hypopigmentation compounds should be complemented with studies on the safety for application in human cosmetics. Certain commercial skin whitening compounds, such as hydroquinone, were eventually banned due to adverse side effects (Kooyers and Westerhof 2006; Burger et al. 2016). Therefore, long-term preclinical and clinical trials are needed to determine the side effects of experimental compounds in humans. Thus, 23 subjects were recruited in a clinical study conducted for 4 and 8 weeks. To confirm whether a change in skin brightness resulted from using P. yezoensis extracts, the skin brightness value was measured at visiting intervals. Daily application of lotion containing 0.1% w/w P. yezoensis extract to the cheeks of subjects (n = 23) elicited a more significant whitening effect than the control product after 8 weeks (Fig. 7a, p < 0.05). The skin brightness of the cheek treated with P. yezoensis extract increased by 1.32% after 8 weeks of use, while those treated with the control product showed an increase of 0.46% (Fig. 7a). The Wilcoxon signed rank (non-parametric) test revealed a significant statistical difference at 5% (p < 0.05) for skin brightness between subjects treated with the test product (lotion containing Pyropia extracts) and those treated with the control product (without the extracts).
The skin melanin content was found to decrease by 2.4% and 3.0% from the initial content after 4 and 8 weeks of using the test product containing P. yezoensis extracts, respectively, whereas there was no change in the melanin content in cheek balls treated with the control product. There was a statistically significant difference between the effect of the test and control products on skin melanin content in both the 0–4 and 0–8 week treatments (Fig. 7b). Clinical efficacy evaluation via visual assessment by a dermatologist after using the formulation containing P. yezoensis extracts for 8 weeks revealed a significant skin-lightening effect than the control (Fig. 7c). Notably, during the test period, dermatologists did not document or observe a single case of complaints or undesirable symptoms (such as redness or irritability) among the 23 volunteers.
Conclusions
To our knowledge, this is the first study demonstrating the efficacy and safety of using P. yezoensis extracts to prevent melanogenesis. P. yezoensis extracts showed potential to enhance collagen synthesis, while preventing its degradation, in human skin fibroblasts. Therefore, these extracts could be used as functional cosmetic agents for preventing or alleviating skin wrinkle formation.
Pyropia contain several amino acids, such as alanine, glutamic acid, and taurine, as well as carbohydrates, proteins, flavonoids, and various minerals and vitamins. Previous studies have paid special attention to the unique components of red seaweed, including proteins and derived peptides (e.g., phycobiliproteins, glucoproteins containing “cellulose binding domains”, phycolectins, and the related MAAs), along with polysaccharides (e.g., floridean starch and sulphated galactans such as carrageenans and agarans) and minerals (Cian et al. 2015). Nevertheless, it is also important to obtain information on the biological activities of these compounds and to determine whether any particular compound can act alone or complement others to exert optimal beneficial effects. In the current study, however, instead of further attempting to characterize single active compounds, we have only tested the efficacy of the whole crude extracts of P. yezoensis due to three main reasons: efficacy, cost-effectiveness, and applicability. In traditional medicine, whole plants or mixtures of plants are used rather than isolated compounds, as there is evidence that crude plant extracts often have a higher in vitro or/and in vivo activity than isolated constituents at an equivalent dose (Rasoanaivo et al. 2011). Pure drugs that are industrially produced or isolated from plants may be selected for their high activity, but, they rarely reach the same level of activity as in unrefined extract, even assessed at equivalent concentrations or dose of the active component (Wagner and Ulrich-Merzenich 2009). This phenomenon is attributed to the absence of interacting substances that are in the whole extract. In addition, many plants contain substances that inhibit multi-drug resistance (Rasoanaivo et al. 2011). The poor quality of processed plant materials, preclinical laboratory protocols, inadequate fractionation process, degradation of active ingredients during fractionation, and poor biological models may also contribute for the lower activities of purified compounds.
Another drawback of isolating and characterizing pure compounds is that they are often more expensive to produce and distribute and, thus, represent a high associated cost to the manufacturer or are often unavailable and/or unaffordable to customers. In contrast, crude extracts can be produced at lower costs.
Extract of P. yezoensis are already recognized ingredients within the International Nomenclature of Cosmetic Ingredients (INCI) list, although information on their functions might not be available. The INCI system was introduced in the early 1970s by the Personal Care Products Council (former Cosmetic, Toiletry, and Fragrance Association [CTFA]) and the list is maintained by the Personal Care Products Council. INCI names are used in the USA, the European Union, China, Japan, and several other countries for listing ingredients on the labels of cosmetic products. There are currently more than 16,000 ingredients on the INCI list, and extracts of P. yezoensis itself are, therefore, available for immediate application in cosmetic formulations.
Nevertheless, we propose that the chemical separation and characterization of potentially bioactive compounds of P. yezoensis extracts should be performed based on their chemical structure, conformation, bioavailability, and the type and position of functional groups to enhance their commercial use. These assessments should be conducted using techniques such as supercritical CO2 extraction, membrane separation, and ultrasonic-aided extraction to ensure a better outcome.
Altogether, our results suggest that Pyropia extracts could be used as safe, effective, and inexpensive cosmetic agents for skin whitening and prevention, or alleviation, of skin wrinkle formation. These extracts could have the potential for mass production at an industrial scale, given the Pyropia annual harvest of 2,563,048 t in 2017 (FAO 2019). Further clinical studies aimed at clarifying the other health-related effects and potential benefits of P. yezoensis extracts may reveal additional value for the cosmetology industry.
References
Azam MS, Choi J, Lee M-S, Kim H-R (2017) Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar Drugs 15:297
Binic I, Lazarevic V, Ljubenovic M, Mojsa J, Sokolovic D (2013) Skin ageing: Natural weapons and strategies. Evid-Based Complement Alternat Med 2013:827248
Burger P, Landreau A, Azoulay S, Michel T, Fernandez X (2016) Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 3:36
Cha SH, Ko SC, Kim D, Jeon YJ (2011) Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J Dermatol 38:354–363
Chapman V, Chapman D (1980) Seaweed as animal fodder, manure and for energy. In: Seaweeds and their uses, 3rd edn. Chapman & Hall, London, pp 30–61
Chinnathambi S, Bickenbach JR (2005) Human skin and gingival keratinocytes show differential regulation of matrix metalloproteinases when combined with fibroblasts in 3-dimensional cultures. J Periodontol 76:1072–1083
Cho TJ, Rhee MS (2020) Health functionality and quality control of laver (Porphyra, Pyropia): Current issues and future perspectives as an edible seaweed. Mar Drugs 18:14
Cian RE, Drago SR, De Medina FS, Martínez-Augustin O (2015) Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar Drugs 13:5358–5383
Dawczynski C, Schubert R, Jahreis G (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103:891–899
Dufour AM, Borowczyk-Michalowska J, Alvarez M, Truchetet M-E, Modarressi A, Brembilla NC, Chizzolini C (2020) IL-17A dissociates inflammation from fibrogenesis in systemic sclerosis. J Invest Dermatol 140:103–112 e108
El Ghalbzouri A, Ponec M (2004) Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen 12:359–367
FAO (2019) Fisheries and aquaculture statistics. FAO, Rome
Global Market Research Reports (2018) Global cosmetics market - by product type, ingredient, region - market size, demand forecasts, industry trends and updates (2018—2025)
Han D, Wang S, Hu Y, Zhang Y, Dong X, Yang Z, Wang J, Li J, Deng X (2015) Hyperpigmentation results in aberrant immune development in silky fowl (Gallus gallus domesticus Brisson). PLoS One 10:e0125686
Helfrich YR, Sachs DL, Voorhees JJ (2008) Overview of skin aging and photoaging. Dermatol Nursing 20:177
Heo S-J, Ko S-C, Kang S-M, Cha S-H, Lee S-H, Kang D-H, Jung W-K, Affan A, Oh C, Jeon Y-J (2010) Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem Toxicol 48:1355–1361
Hwang EK, Park CS (2020) Seaweed cultivation and utilization of Korea. Algae 35:107–121
Kang HS, Kim HR, Byun DS, Son BW, Nam TJ, Choi JS (2004) Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch Pharm Res 27:1226–1232
Kim C-R, Kim Y-M, Lee M-K, Kim I-H, Choi Y-H, Nam T-J (2017) Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-β/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int J Mol Med 39:31–38
Kim S, You DH, Han T, Choi E-M (2014) Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). Photochem Photobiol B 141:301–307
Kooyers T, Westerhof W (2006) Toxicology and health risks of hydroquinone in skin lightening formulations. J Eur Acad Dermatol Venereol 20:777–780
Korea Ministry of Drug and Food Safety (2013) Korean functional cosmetics codex. Cosmetic Act
Lawrence KP, Long PF, Young AR (2018) Mycosporine-like amino acids for skin photoprotection. Curr Med Chem 25:5512–5527
Mercurio D, Wagemaker T, Alves V, Benevenuto C, Gaspar L, Campos PM (2015) In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. Photochem Photobiol B 153:121–126
Mooberry SL, Leal RM, Tinley TL, Luesch H, Moore RE, Corbett TH (2003) The molecular pharmacology of symplostatin 1: A new antimitotic dolastatin 10 analog. Int J Cancer 104:512–521
Nova P, Martins AP, Teixeira C, Abreu H, Silva JG, Silva AM, Freitas AC, Gomes AM (2020) Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J Appl Phycol 32:1789–1802
Novak J (2010) Arbutin-a risk substance in herbs? Zeitschr Arznei Gewürzpflanz 15:170–173
Paudel P, Wagle A, Seong SH, Park HJ, Jung HA, Choi JS (2019) A new tyrosinase inhibitor from the red alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Mar Drugs 17:295
Pimentel FB, Alves RC, Rodrigues F, Oliveira P, Beatriz M (2018) Macroalgae-derived ingredients for cosmetic industry—an update. Cosmetics 5:2
Qian W, Liu W, Zhu D, Cao Y, Tang A, Gong G, Su H (2020) Natural skin-whitening compounds for the treatment of melanogenesis. Expe Therapeut Med 20:173–185
Quah CC, Kim KH, Lau MS, Kim WR, Cheah SH, Gundamaraju R (2014) Pigmentation and dermal conservative effects of the astonishing algae Sargassum polycystum and Padina tenuis on guinea pigs, human epidermal melanocytes (HEM) and Chang cells. Afr J Tradit Compl Alternat Med 11:77–83
Rasoanaivo P, Wright CW, Willcox ML, Gilbert B (2011) Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar J 10:1–12
Rittié L, Fisher GJ (2002) UV-light-induced signal cascades and skin aging. Ageing Res Rev 1:705–720
Rupérez P (2002) Mineral content of edible marine seaweeds. Food Chem 79:23–26
Russo B, Brembilla NC, Chizzolini C (2020) Interplay between keratinocytes and fibroblasts: A systematic review providing a new angle for understanding skin fibrotic disorders. Front Immunol 11:618
Sawicki G, Marcoux Y, Sarkhosh K, Tredget EE, Ghahary A (2005) Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and-9 and their inhibitors. Mol Cell Biochem 269:209–216
Scharffetter K, Wlaschek M, Hogg A, Bolsen K, Schothorst A, Goerz G, Krieg T, Plewig G (1991) UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res 283:506–511
Schmid D, Schürch C, Zülli F (2006) Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmetics 9:1–4
Solano F, Briganti S, Picardo M, Ghanem G (2006) Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res 19:550–571
Sorrell JM, Baber MA, Caplan AI (2004) Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol 200:134–145
Vance KW, Goding CR (2004) The transcription network regulating melanocyte development and melanoma. Pigment Cell Res 17:318–325
Wagner H, Ulrich-Merzenich G (2009) Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 16:97–110
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: Revisiting our understanding. J Appl Phycol 29:949–982
Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML (2010) Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics 10:2214–2223
Zhang Q, Li N, Zhou G, Lu X, Xu Z, Li Z (2003) In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodophyta) in aging mice. Pharmacol Res 48:151–155
Zion Market Research (2019) Skin lightening products market by type (lotion and cream, foam, gel, serum & toner, scrub, and others), by end-user (men and women), by nature (natural/herbal, synthetic, and organic), and by distribution channel (pharmacy, specialty outlet, supermarket/hypermarket, convenience store, beauty salon, e-retailer, and others): global industry perspective, Comprehensive Analysis, and Forecast, 2017—2024
Acknowledgments
The authors thank the anonymous reviewers for their helpful comments. This study was supported by the Regional Innovation System (RIS) funded by the Korea Institute for Advancement of Technology (KIAT) of the Republic of Korea (Grant No. R0002107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, J., Lee, H., Choi, S. et al. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J Appl Phycol 33, 653–662 (2021). https://doi.org/10.1007/s10811-020-02305-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02305-y