Abstract
The overall objective of this study is to present an assessment of embryonic sporophyte development directly “seeded” on growing substrates using the proprietary binder AtSea and three formulations of calcium alginate. We conducted two independent experiments under controlled conditions in a laboratory setup. First, we compared the effect of salinity (0 ppt, 29 ppt, and 35 ppt) on the performance of the calcium alginate formulations. Second, we assessed the effect of direct seeding with binders on the development of sporophytes of four different size classes (i.e., 83.7, 183.1, 345.2, and 522.0 μm in length). Our results show that salinity affects the performance of calcium alginate with 0 ppt media allowing the formation of a thicker hydrogel than that formulated with 29 and 35 ppt. Results also show that AtSea retained more sporophytes than any formulations of calcium alginate. Although effective when cultures were maintained in complete stillness (i.e., no water motion), we detected 70–80% detachment when aeration was added. We also observed what appeared to be delayed sporophyte development compared with sporophytes growing as free floaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, the production of commercial seaweed accounts for over 32 million tonnes a year worldwide (FAO 2020). Seaweed biomass is the source material for a variety of industrial applications, including the production of food, nutraceuticals, pharmaceuticals, cosmeceuticals, fertilizers, and livestock feed (Barry 2008; Pereira and Yarish 2008; Kim et al. 2015, 2019; FAO 2018). Due to their high productivity, no need for agricultural land, no freshwater or fertilizer usage, and their importance as ecosystem engineers in near-shore coastal areas, seaweed is a promising future source of bioenergy (Aitken et al. 2014; Kerrison et al. 2018; Gegg and Wells 2019; Kim et al. 2019). Among them, the sugar kelp, Saccharina latissima (Linnaeus) C.E.Lane, C. Mayes, Druehl & G.W. Saunders, is a notable one for European and US commercial cultivation (Peteiro 2017; Yarish et al. 2017; Bak et al. 2018; Kim et al. 2019).
Novel seeding methodologies must be adopted in the aquaculture practice to meet the increasing global demand for kelp biomass, as the current methods are not suitable to meet the needs and are highly labor-consuming (Buschmann et al. 2017). Traditionally, kelp seeding for aquaculture relies on wild-collected reproductive sporophytes with meiospores that passively settle onto hatchery string or ropes for outplanting on ocean farms (Pereira and Yarish 2008; Redmond et al. 2014). Although this widespread practice is effective, it is incompatible with significantly expanding the scale of kelp farming in North America and Europe. Also, the dependency on natural populations to provide reproductive material for seeding thousands of hectares may negatively impact wild kelp populations (Smale et al. 2013; Cottier-Cook et al. 2016). Moreover, this seeding technique is labor-intensive and not readily amenable to selective breeding programs for the eventual development of large-scale use of improved cultivars.
Traditional hatcheries rely on meiospores that attach to seed strings which are later deployed at sea onto grow-out ropes. Alternatively, sporophytes can be grown in tumble culture and seeded onto ropes using direct seeding binders (Kerrison et al. 2018), such as the proprietary binder AtSea (AtSeaNovaTechnologies, Ronse, Belgium). This method is compatible with selective breeding techniques as gametophytic stages of kelp can be isolated, sexed, cloned, and cross-fertilized to produce juvenile sporophytes with desirable traits. These juvenile sporophytes can then be seeded directly onto seed-string or grow-out ropes, which will be outplanted on open-water farms (Li et al. 2016; Kerrison et al. 2018). Such direct seeding methodologies could result in permitting flexibility in farming schedules, scaling down nursery operations, and savings in labor and capital costs by reducing the hatchery space, energy, and effort required.
Between 2018 and 2019, the Seaweed Biotechnology Lab at the University of Connecticut in collaboration with Woods Hole Oceanographic Institution, Cornell, and Universidad de Los Lagos, Chile, directly seeded onto spools a mixture of embryonic sporophytes and gametophytes from 800 unique crosses using AtSea. This direct seeding was conducted as part of a Saccharina latissima breeding program (MARINER 2017) over two consecutive kelp farming seasons. Researchers observed that sporophyte attachment was inconsistent and that there was a delay in the formation of visible blades (pers. obser, pers. comm. D. Bailey and E. van Sitteren, WHOI). It was hypothesized that (1) the binder had an adverse effect on blade development, (2) the sporophytes were seeded at a suboptimal size, or (3) both.
Although advantageous, there are no low-cost direct seeding binders available in the market, hence limiting the use of such protocols to “seed” commercial farms. Indeed, finding a consistent protocol to attach sporophytes directly onto a growing substrate is of utmost relevance to obtain yields comparable with those by “seeding” kelp farms with meiospores. Such a protocol should allow the reduction in time and effort in the nursery, promote the healthy development of embryonic sporophytes, and facilitate replicable results in a range of environmental conditions. In this study, we evaluated the effects of four binder formulations on the development of embryonic sporophytes. We also determined the optimal sporophyte size to obtain the fastest growth when using AtSea as a direct seeding binder. Outcomes will provide insight that could assist in the formulation of new low-cost direct seeding binders. Overall, these findings will contribute to creating direct seeding protocols to satisfy different application needs in the future.
Materials and methods
Preparation of the embryonic sporophytes
Cloned gametophytes were derived from two populations of sugar kelp isolated from Nubble Light, ME, USA (43.166919 N, 70.591456 W), and Cape Canal, MA, USA (41.773818 N, 70.499448 W). Cloned male (SL-NL-3-MG-X) and female (SL-CC-9-FG-X) gametophytes were combined in a 1:1 ratio finely blended and filtered through 80 μm Nytex mesh. The mixed gametophyte solution was returned to tumble culture (enriched media, 10 °C, 60 μmol photons m−2 s−1) to allow for fertilization. After fertilization was detected (approximately 7 days after processing), microscopic embryonic sporophytes were photographed and measured with an Olympus camera (DS27, Tokyo) mounted onto an Olympus microscope (CKX3_SLP, Tokyo).
Preparation of the slides with twines
Twenty-four microscope glass slides were wrapped around with 2.5 m of Kuralon twine (2 mm). The twine was maintained tightly by securing a rubber band to both ends of the slides. After ensuring proper assemblage, slides were soaked in deionized water for 4 h to remove any potential contaminants. They were then immersed in seawater for 30 min and allowed to air dry for 2 h.
The binder formulation experiments
Calcium alginate (CA) formulations were prepared by blending 100 g of sodium alginate (CAS: 9005-38-3, Acros Organics, Belgium) with either 1 L of synthetic seawater (i.e., 29 and 35 ppt) or distilled water for 60 s. Also, 4 L of 10% calcium chloride (CaCl2; MCB, USA) solution was prepared in seawater (i.e., 29 and 35 ppt) or distilled water to induce the transformation of sodium alginate into calcium alginate (Percival and McDowell 1967). The AtSea binder was made according to the instructions described by the manufacturer, blending 1.25 g of the product in 1 L of seawater (29 ppt as salinity on-site).
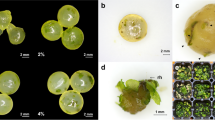
Direct seeding was conducted 15 days after the initial crossing of gametophytes when the sporophytes of 83 ± 5 μm (avg. ± SD) in length were observed. Seeding was conducted by pipetting thirty 5 μL (250 ± 15 embryonic sporophytes per mL) of the crossed culture onto 15 of the slides. Each aliquot (hereafter data point) per slide was seeded individually, ensuring to maintain an even distribution of data points within slides (Fig. 1). As such, each data point per row was separate from the next by 1 cm.
Immediately after inoculation, slides were coated with 1 mL of one of the four binder solutions (AtSea, CA 0 ppt, CA 29 ppt, CA 35 ppt). Each treatment consisted of three replicates. Seeded and coated slides remained exposed to air (17 °C, 65% humidity) for 15 s before transferring them to individual 250 mL square containers. No aeration was provided. Media changes were done every 7 days.
The embryonic sporophyte size experiments
Four sporophyte size classes were tested in this experiment to determine the optimum size of sporophytes for direct seeding. The first seeding was conducted 15 days after crossing, when sporophytes of 83 ± 5 μm (size 1) in length were observed, followed by a second seeding 20 days after crossing, with sporophytes of 183 ± 5 μm in length (size 2). A third seeding and a fourth seeding were conducted 26 and 33 days after crossing when sporophytes were 345 ± 15 μm (size 3) and 522 ± 50 μm (size 4), respectively (Fig. 2). Data points were seeded as described above, with the coating done using AtSea only. No aeration was provided for the first 28 days. Cultures were only subject to gentle aeration for 10 min every 7 days starting 35 days post direct seeding and until no detachment was observed. The remainder of the cross was maintained in tumble culture to allow sporophytes to develop as free floaters.
Data collection and statistical analysis
For both experiments, microscope slides were photographed using the rear camera of a smartphone to allow tracking for blade development. Data points were quantified every 7 days to quantify data point retention over time. In the second experiment, the optimum size of sporophytes for direct seeding was determined by measuring the total length of five sporophytes selected randomly per size 28 days after seeding. The data points and total blade length at the end of the experimental periods were analyzed separately after confirmation of normality (Shapiro–Wilk test), independence of variables (Durvin–Watson test), and homogeneity of variances (Cochran’s test). One-way ANOVA was applied to find the differences for each group in both experiments. LSD post hoc comparisons were conducted where differences were found (α = 0.05). All analyses were done using R (R Core Team 2016).
Results
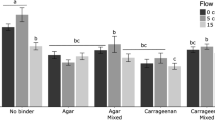
Data points attached with AtSea and CA 29 ppt remained coated by an apparent more fluid matrix than data points coated with CA 0 ppt. CA 35 ppt coating, which showed the highest apparent fluidity of all binders, was the first to wash off after placing the slides into the growing media. Visible blades were first observed 16 days after direct seeding with AtSea and CA 29 ppt. CA 0 ppt and CA 35 ppt treatments showed incipient blades 21 days following direct seeding; however, only a limited number of sporophytes developed into bigger blades (Fig. 3). Overall, AtSea showed a significantly highest retention of sporophytes (p < 0.05; Fig. 3).
Regardless of the binder used (i.e., CA or AtSea), sporophytes that developed into visible blades showed a wrinkled appearance, particularly at the edges. This morphology contrasted with blades developed as free floaters, which showed smooth edges. The wrinkled appearance smoothed out as blades grew larger (Fig. 4).
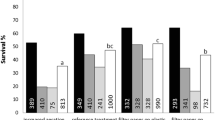
The size of initial sporophytes seeded showed significant effects for the development of blades. Twenty-eight days after seeding, sporophytes with an average initial size of 183 μm (size 2) showed significantly larger blades than any other size class (p < 0.05; Fig. 5; Table 1). Size 2 sporophytes also showed better attachment following 28 days post direct seeding, with an average retention of 27.7 ± 0.89 data points per slide (Table 1). Our results revealed that the development of sporophyte blades directly seeded can be optimized if sporophytes of ~ 183 μm are used. Slides holding size 1 and size 4 sporophytes had the least number of data points (p < 0.05). Attachment of sporophytes decreased abruptly after aeration was added to the cultures. Regardless of the sporophyte initial size class, a 70% loss was observed after the first and second aeration treatments (day 35 and 42 post direct seeding). Detachment persisted with subsequent aeration treatments until day 56 post direct seeding.
Discussion
Results in this study show that formulations of calcium alginate were not as effective as AtSea in binding sporophytes onto Kuralon twine. Calcium chloride is one of the most frequently used compounds to cross-link sodium alginate to create hydrogels (Lee and Mooney 2012; Ropp 2013). However, calcium chloride is highly soluble in aqueous solutions (Lee and Mooney 2012), which probably interfered with its effectiveness to gelate sodium alginate and thus create an effective binder. Additionally, aqueous solutions prepared with seawater contained sodium chloride possibly interfered with the cross-linking of Ca++ and alginate. This interference could explain the weak bonding and gelation observed in the binders when prepared with saline media. Results in this study also show that sporophytes will have different growth rates depending on the original size when seeded. These findings coincide to some extent with results by Forbord et al. (2020). In their assessments, these authors compared the performance of blades seeded onto ropes at different days from fertility induction. Authors found that sporophytes directly seeded 35 days after fertilization showed the longest length at harvest.
On the other hand, the lag in development and wrinkled appearance of directly seeded sporophytes compared with those growing as free floaters could have resulted from the resistance exerted by the viscosity of the binders on the embryonic sporophytes. Despite what appeared to be a delayed development of blades, AtSea is a better binding option than any of the formulations of CA tested here. Nevertheless, its binding potential was affected by water motion caused by aeration. Kerrison et al. (2018) indicate that AtSea binding allows for an immediate outplanting at sea. In our study, we did not conduct any outplanting at sea. Still, we detected an increase in detachment with water motion, even though aeration was first added starting 35 days after direct seeding of the embryonic kelp onto the twine. Even though our experiments were conducted in the nursery only, results coincide with those of Kerrison et al. (2018), where the final density of sporophytes that remained attached to the grow-out substrate was a tenth of the initial seeding density.
It is important to highlight that we did not test the performance of the binders on different textiles. Neither do we assess different ways to apply the binders. Instead, we focused on assessing the development and attachment of sporophytes seeded onto Kuralon twine and coated with four binder formulations. Our results are consistent with observations made during the first two seasons of our selective S. latissima breeding program (MARINER 2017; Umanzor et al. in prep.), in which a high percentage of detachment was noticed, particularly after aeration was added to the directly seeded spools. Similar to what has been described in this contribution, we also detected a delay in sporophyte development in our first season of our kelp breeding program, with visible blades showing after 5 to 6 weeks post direct seeding. For now, based on our results, AtSea appears as a better direct seeding binder than the hydrogel formed by sodium alginate and calcium chloride. However, additional research is needed to understand the relationship between the consistencies of the binders with the development and attachment of sporophytes. It will also be imperative to calculate the variability in the final sporophyte density caused by detachment over time, and if this variability is consistent across different environmental scenarios.
Data availability
Data obtained in the experiments herein can be available upon request.
Change history
04 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s10811-020-02345-4
References
Aitken D, Bulboa C, Godoy-Faundez A, Turrion-Gomez JL, Antizar-Ladisla B (2014) Life cycle assessment of macroalgae cultivation and processing for biofuel production. J Clean Prod 75:45–56
Bak UG, Mols-Mortensen A, Gregersen O (2018) Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res 33:36–47
Barry L (2008) Seaweed, potential as a marine vegetable and other opportunities. Australian Government, Rural Industries Research and Development Corporation, Canberra. RIRDC Publication No 08/009, RIRDC Project No CON-9A. 46 pp
Buschmann, AH, Camus, C, Infante, J, Neori, A, Israel, A, & Hernández-González, MC Pereda, SV (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Cottier-Cook EJ, Nagabhatla N, Badis Y, Campbell M, Chopin T, Dai W, Fang J, He P, Hewitt C, Kim GH, Huo Y, Jiang Z, Kema G, Li X Liu F, Liu H, Liu Y, Lu Q, Luo Q, Mao Y, Msuya FE, Rebours C, Shen H, Stentiford GD, Yarish C, Wu H, Yang X, Zhang J, Zhou Y, Gachon, CMM (2016) Safeguarding the future of the global seaweed aquaculture industry. United Nations University (INWEH) and Scottish Association for Marine Science Policy Brief. 12pp
FAO (2018) The state of world fisheries and aquaculture. Available from: http://www.fao.org/fishery/en. Accessed 23 May 2020
FAO (2020) The state of world fisheries and aquaculture. Available from: http://www.fao.org/fishery/en. Accessed 8 Sept 2020
Forbord S, Steinhovden KB, Solvang T, Handå A, Skjermo J (2020) Effect of seeding methods and hatchery periods on sea cultivation of Saccharina latissima (Phaeophyceae): a Norwegian case study. J Appl Phycol 32:2201–2212
Gegg P, Wells V (2019) The development of seaweed-derived fuels in the UK: an analysis of stakeholder issues and public perceptions. Energy Policy 133:110924
Kerrison PD, Stanley MS, Hughes AD (2018) Textile substrate seeding of Saccharina latissima sporophytes using a binder: an effective method for the aquaculture of kelp. Algal Res 33:352–357
Kim JK, Kraemer GP, Yarish C (2015) Use of sugar kelp aquaculture in Long Island Sound and the Bronx River Estuary for nutrient extraction. Mar Ecol Prog Ser 531:155–166
Kim JK, Stekoll M, Yarish C (2019) Opportunities, challenges and future directions of open water seaweed aquaculture in the United States. Phycologia 58:446–461
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Li X, Li X, Zhang Z, Qu S, Liang G, Sun J, Zhao N, Cui C, Cao Z, Li Y, Pan J, Yu S, Wang Q, Luo S, Song S, Guo L, Yang G (2016) Improving seedless kelp (Saccharina japonica) during its domestication by hybridizing gametophytes and seedling-raising from sporophytes. Sci Rep 6:21255
Mariner (2017) Seaweed hatchery and selective breeding technologies. Available at https://arpa-e.energy.gov/?q=slick-sheet-project/seaweed-hatchery-and-selective-breeding-technologies. Accessed 13 May 2020
Percival E, McDowell RH (1967) Chemistry and enzymology of marine algal polysaccharides. Academic Press, London, p 219
Pereira R, Yarish C (2008) Mass production of marine macroalgae. Encycl Ecol:2236–2247
Peteiro C (2017) Alginate production from marine macroalgar, with emphasis on kelp farming. In: Rehm BHA, Moradali MF (eds) Alginates and their biomedical applications. Springer, Cham, pp 27–66
Ropp RC (2013) Group 17 (H, F, Cl, Br, I) alkaline earth compounds. In: Ropp RC (ed) Encyclopedia of the alkaline earth compounds. Elsevier, Amsterdam, pp 25–104
Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3:4016–4038
Yarish C, Kim JK, Lindell S, Kite-Powell H (2017) Developing an environmentally and economically sustainable sugar kelp aquaculture industry in southern New England: from seed to market. Stamford, CT
Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England seaweed culture handbook-nursery systems. Connecticut Sea Grant, 93 pp. Available at http://www.digitalcommons.uconn.edu/seagrant_weedcult/1/ or http://www.seagrant.uconn.edu/publications/ aquaculture/handbook.pdf. Accessed 2 May 2020
R Core Team, 2016. R: A Language and Environment for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/ Accesed 10 June 2020
Acknowledgments
We thank S. Lindell for providing the AtSea binder. We also thank D. Bailey and E. van Sitteren for their insight.
Funding
This study was supported by the Macroalgae Research Inspiring Novel Energy Resources (MARINER, DE-FOA-0001726) to CY as a sub-award to Contract DE-AR0000911.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Umanzor, S., Li, Y. & Yarish, C. Effect of direct “seeding” binders and embryonic sporophyte sizes on the development of the sugar kelp, Saccharina latissima. J Appl Phycol 32, 4137–4143 (2020). https://doi.org/10.1007/s10811-020-02277-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02277-z