Abstract
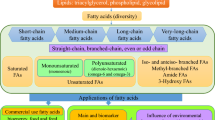
Fatty acids (FAs) and volatile organic compounds (VOCs) are among bioactive substances produced by macroalgae, which have important reported biological activities. The aim of this work was to determine the diversity of FAs and VOCs in brown, green, and red Antarctic macroalgae. Results showed that seaweeds contained 13 to 25 FAs with a predominance of palmitic, oleic, linoleic, and eicosapentaenoic acids. Concerning VOCs, 28 to 55 distinct compounds could be detected, distributed among aldehydes, hydrocarbons, furan derivatives, and ketones, for instance. Generally, hexanal (5.83%–25.51%), heptadecane (0.92%–49.84%) and 2-pentylfuran (3.02%–12.57%) were found in considerable amounts in the analyzed specimens. It is worth noting that, to the best of our knowledge, this was the first time that Antarctic macroalgae had their VOCs elucidated. Therefore, Antarctic seaweeds were composed of several VOCs and FAs which could assist in the elucidation of secondary metabolites from these organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds comprise a diverse group of approximately 10,000 aquatic organisms that can be divided depending on their pigmentation and biochemical and morphological aspects into three main groups that include the Rhodophyta, Ochrophyta, and Chlorophyta (Rodrigues et al. 2015; dos Santos et al. 2019). The biological potential of macroalgae has led to the use of seaweeds as food, fertilizers, bioenergy resources, and additives in cosmetics, for instance, mainly in Asiatic countries (Hamid et al. 2015; Passos et al. 2020). Despite their high potential, macroalgae can still be considered largely unexplored as less than 5% of the known species have been used commercially, making them feasible sources of novel biological applications (Andrade et al. 2013; Rodrigues et al. 2015).
The high adaptive capacity of seaweeds to the most adverse environments enabled their survival in severe habitats that include Polar, sub-Antarctic, and Antarctic regions of the planet (Graeve et al. 2002; Becker et al. 2010). Conditions, such as water temperature, light exposure, nutrient availability, and water salinity, can be extreme in the Antarctic region inducing macroalgae to biosynthesize secondary metabolites as survival mechanisms (Santos et al. 2017; Pacheco et al. 2018; Pacheco et al. 2018). Fatty acids (FAs), sterols, vitamins, carbohydrates, and volatile organic compounds (VOCs) are among the secondary metabolites produced by seaweeds (Santos et al. 2015; Maruti et al. 2018; Schmid et al. 2018).
Although constituting generally less than 5% of the chemical composition of macroalgae, FAs are key secondary metabolites produced by seaweeds in order to help maintain their membrane fluidity, beyond other metabolic functions (Santos et al. 2017). It is known that this biochemical mechanism is one of the possible responses to extreme environmental conditions, which leads to the production of essential FAs that cannot be synthesized by humans (Martins et al. 2016). The consumption of omega-3 (n3) FAs is associated to several biological activities, and the health benefits include antibacterial, antitumor, antioxidant, and antiinflammatory effects as well as prevention of coronary and neurologic diseases (Larsen et al. 2011; Pereira et al. 2012).
VOCs are another vast group of bioactive substances produced by seaweeds that have low molecular weight and are mostly lipophilic compounds, including, for instance, aldehydes, ketones, hydrocarbons, furan derivatives, and alcohols (Hosoglu 2018). Despite early studies dating back to the late 80s regarding VOCs, there are still few research works concerning their determination in macroalgae (Hamid et al. 2015). It is worth noting that VOCs have shown antimicrobial activity, highlighting their biological potential (Paul and Pohnert 2011). However, their mechanism of biosynthesis and action in seaweeds and environment are still largely unknown up to this date.
According to the literature, seaweeds can be a vast source of bioactive compounds, such as VOCs and FAs, that are still largely unexplored to this date. Previous research works indicated that Antarctic seaweeds contain important phytochemicals including FAs (Martins et al. 2016; Santos et al. 2017) and sterols (Pereira et al. 2017; Pacheco et al. 2018). Considering that it is important to continuously develop studies regarding the extraction of compounds from Antarctic macroalgae, the study aimed to highlight novel potential bioactive substances and determine possible chemotaxonomic relationships among the analyzed samples. Therefore, the objectives of this work were to evaluate the volatile composition and the FA profile of seven Antarctic macroalgae (Rhodophyta, Chlorophyta, and Ochrophyta) as well as to differentiate species by their metabolites using statistical approaches.
Materials and methods
Sampling
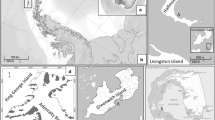
Ochrophyta representatives Desmarestia confervoides and Adenocystis utricularis, Rhodophyta species Myriogramme manginii, Gigartina skottisbergii, Curdia racovitzae, and Georgiella confluens, as well as the Chlorophyta seaweed Ulva intestinalis were collected in several points of the Antarctic Peninsula or in the South Shetland Islands during November and December of 2015 (Table 1). Generally, 6 to 10 individuals of representatives from each macroalgae were manually collected at low tide. Subsequent to collection, the samples were washed with seawater. Morphological identification was performed by expert phycologists by comparing the samples to a database of the Botanic Institute of the University of São Paulo. Finally, individuals were lyophilized, milled, and placed into dark plastic bags, stored at − 20 °C.
Chemicals and materials
A methanolic solution of boron trifluoride (14%, v/v) and a C8–C20 alkane solution were obtained from Sigma-Aldrich (USA), while standards methyl nonadecanoate and a 37-mix fatty acid methyl esters (FAMEs) were acquired from Sigma-Aldrich and Supelco (USA), respectively. Lastly, 20 mL boron-silicate vials and silicone septum were from Shimadzu (Japan) and HPLC-grade n-hexane was obtained from J.T. Baker (USA). All solvent and other chemical reactants were of analytical grade (≤ 98.5%).
Analysis of fatty acids
Extraction
Extraction of FAs from the samples was carried out in triplicate (n = 3) and followed the modified methodology proposed by Bligh and Dyer (1959). Briefly, 1 g of algal biomass, 10 mL of chloroform, 20 mL of methanol, and 10 mL of an aqueous solution of sodium sulfate (1.5%, w/v) were mixed and stirred for 30 min at ambient temperature. Subsequently, 10 mL of chloroform and 10 mL of an aqueous solution of sodium sulfate (1.5%, w/v) were added to the system, and the samples were centrifuged at 3000 rpm for 30 min and, afterwards, had their non-polar organic layer separated and filtered under anhydrous sodium sulfate and evaporated under reduced pressure.
Derivatization
Derivatization of the extracted FAs was performed in triplicate (n = 3), and the procedure described by Moss et al. (1974) was followed. Briefly, the extracted material and 5 mL of a methanolic solution of sodium hydroxide (2%, w/v) were refluxed and stirred for 5 min. Subsequently, 5 mL of a methanolic solution of boron trifluoride (14%, v/v) was added, and the system was allowed to stir under reflux for another 5 min. Then, 20 mL of n-hexane was used to retrieve the non-polar organic layer that was further evaporated to dryness under reduced pressure and nitrogen flow (Moss et al. 1974).
Analysis of volatile organic compounds
Extraction
Headspace extraction of VOCs followed the method described by de Alencar et al. (2017). Briefly, 1 g of algal biomass was introduced into 20 mL boron-silicate vials and wrapped using silicone septum. Samples were incubated at 100 °C for 1 h, and, subsequently, VOCs were injected into a gas chromatograph coupled to a mass spectrometer.
Chromatographic analysis
Chromatographic analysis of FAs was carried out using gas chromatography coupled to a flame ionization detector (GC-FID) model GC-2010 (Shimadzu, Japan). The capillary column used was SP-2560 (100 m × 0.25 mm × 0.2 μm; Supelco, USA), while nitrogen at a gas flow of 1.20 mL min−1 was used as the carrier gas. For the analysis, 1 μL of the samples was injected in split mode (1:100) and subjected to an initial oven temperature of 140 °C increasing 4 °C min−1 to a final oven temperature of 240 °C maintained for 10 min. The injector port was retained at 260 °C. Identification and quantification of the detected FAs were made using a 37-mix standard of FAMEs Mix and the GC Solution software (Shimadzu, Japan).
Chromatographic analysis of VOCs was conducted by Gas Chromatography-Mass Spectrometry (GC-MS) using an equipment model GCMS-QP2010 (Shimadzu). The capillary column used was Rtx-5MS (30 m × 0.25 mm × 0.25 μm; Restek, USA) with helium at a gas flow of 1.28 mL min−1 as carrier gas. For the analysis, 1000 μL of the extracted VOCs was injected in direct mode in the GC–MS system using an initial oven temperature of 30 °C for 2 min growing 4 °C min−1 to 180 °C and then increasing to 20 °C min−1 until reaching the final oven temperature of 280 °C, maintaining this condition for 5 min. The ion source and the injection port were operated at 290 and 250 °C, respectively. Fragments were analyzed from m/z 30 to 450, and identification of the compounds was carried out using NIST-08 library comparing retention indexes to a C8–C20 alkane standard.
Statistical analysis
Two-way analysis of variance (ANOVA) was carried out using GraphPad version 7 (USA), and Tukey’s test (p < 0.05) was used to differentiate FAs from the studied samples. Principal component analysis (PCA) and Ward hierarchical clustering using squared Euclidean distance were conducted by means of Minitab software version 17 (State College, USA) in order to evaluate similarity patterns among FAs and VOCs produced by the studied macroalgae.
Results
Fatty acids
Chromatographic analysis of the FAs extracted from brown, green, and red Antarctic macroalgae (Table 2) revealed that the samples were constituted of 13–25 distinct compounds that varied in aliphatic chain length from 10 to 24 carbons. Qualitatively, D. confervoides had the most diverse FA constitution than the other seaweeds being comprised of 10 saturated FAs (SFAs), 6 monounsaturated FAs (MUFAs), and 9 polyunsaturated FAs (PUFAs). It is worth noting that A. utricularis and G. confluens had more different types of PUFAs and MUFAs, respectively, than G. skottisbergii which was composed of lesser types of FAs than the other species.
As can be observed in Table 2, palmitic acid (C16:0) was the prevalent FA in the majority of the studied samples reaching as much as 65.87 ± 0.97% in G. skottsbergii. On the other hand, D. confervoides mostly contained oleic acid (C18:1n9c) in the concentration of 24.50 ± 0.31% while the other samples had amounts that ranged from 4.64 ± 0.62% to 22.11 ± 0.32%. Other FAs can also be highlighted from the results, including linoleic (C18:2n6c), α-linolenic (C18:3n3), arachidonic (C20:4n6), and eicosapentaenoic acid (C20:5n3). Several macroalgae also had unusual FAs in their constitution, such as heptadecanoic (C17:0), heptadecenoic (C17:1), and heneicosanoic acid (C21:0), as well as trans-FAs elaidic (18:1n9t) and linolenaidic acid (18:2n6t).
Generally, the studied macroalgae mainly contained C18-FAs (19.25 ± 0.74%–37.92 ± 0.45%), except for C. racovitzae that had a dominance of C20-FAs (15.26 ± 1.16%). It is worth noting that C20-FAs were also found in higher amounts in brown macroalgae (26.83 ± 0.48%–26.62 ± 1.14%) than the other samples (0.22 ± 0.06%–15.26 ± 1.16%), while C22-FAs were found in minor concentration in the studied seaweeds (0.36 ± 0.05%–2.97 ± 0.59%). Analyzing the classes of FAs (Fig. 1S), it can be observed that SFAs showed predominance in most of the samples varying from 35.81 ± 0.16% to 79.87 ± 1.19% with the exception of D. conferovoides that was dominated by PUFAs (36.13 ± 0.18%). MUFAs were found in middle-ranged concentrations (9.57 ± 0.68%–34.70 ± 1.57%).
Moreover, n3-PUFAs were found in considerable concentrations in D. confervoides (10.53 ± 0.31%), A. utricularis (18.73 ± 0.50%), and M. manginii (9.18 ± 0.51%) while the other samples had amounts that varied from 0.47 ± 0.23% to 7.81 ± 0.69%. Similarly, n6-PUFAs also had their highest concentrations in the brown macroalgae reaching as much as 24.33 ± 0.23% in D. confervoides, while G. skottsbergii only had 0.67 ± 0.10%. The ∑n6/∑n3 ratio is an important parameter to evaluate the nutritional value of a sample, in which the World Health Organization recommends a ratio of less than 10:1. In this sense, results showed that all the studied macroalgae had acceptable ∑n6/∑n3 ratios varying from 0.62 ± 0.06 to 2.30 ± 0.09.
Volatile organic compounds
Chromatographic analysis of the VOCs from brown, green, and red Antarctic macroalgae (Table 1S) indicated the presence of several chemical classes among the samples varying from 28 (A. utricularis) to 55 (G. confluens). Aldehydes (8–12), ketones (5–12), alcohols (2–6), furan derivatives (2–3), hydrocarbons (7–16), and FAs (0–5) were among the chemical classes detected (Fig. 2S). VOCs were mainly found in the form of hydrocarbons (7.28–51.52%) or aldehydes (15.61–45.64%) although ketones (4.86–32.49%) and furan derivatives (4.11–18.51%) were also found in considerable amounts.
Hexanal (5.83%–25.51%), 2-pentylfuran (3.02%–12.57%), and heptadecane (0.92%–49.84%) were the compounds detected in higher concentrations in the studied samples. It is worth noting the presence of heptanal (0.30%–13.07%), 2-heptanone (2.21%–7.41%), 2-pentenylfuran (0.53%–8.65%), and palmitic acid (nd–10.02%) found in the macroalgae. Each seaweed had heptadecane as a major compound although other samples, including A. utricularis, for instance, had hexanal as the predominant VOC.
The VOC profile of the studied samples revealed that seaweeds had particular compounds in their constitution, such as 2-propyl-2-heptenal, β-homocyclocitral, and hexan-2-one in G. confluens, 2,5-dimethyl-5-heptenal, and 6-methyl-5-heptene-2-one in D. confervoides, and dodecanol in A. utricularis. Besides, some VOCs were particular to each phylum as 5-methyl-2-furaldehyde was only found in Ochrophyta samples, 4-oxoisophorone was present in the Chlorophyta representative, and 2-octenal, α-tolualdehyde, and 1-octen-3-one in the Rhodophyta species.
Multivariate analysis
Differences or similarities among the constituents of the Antarctic macroalgae were assessed using PCA (Fig. 1). In this sense, FAs (e.g., C16:0, C18:1n9c, and C20:5n3) that were significantly different in the profile of the samples and VOCs (e.g., heptadecane, hexanal, and 2-pentylfuran) found in noticeable amounts in the seaweeds were chosen as variables. Moreover, parameters, such as ∑C18, ∑PUFAs, and ∑n3, were also used in the statistical analysis due to their significant variations among the samples. The multivariate analysis showed that the chosen variables generated a statistical model that explained 51.19% of differences in the first component (PC1) and 35.48% in the second component (PC2) corresponding to 86.67% of the overall distinction found in the seaweeds.
From Fig. 1b, it can be observed that FAs and their corresponding classes were reasonably distributed in the loading plot as SFAs and PUFAs were found in the negative or positive axis of PC1 while MUFAs were in the positive axis of PC2. As for VOCs, similar patterns could be noticed since compounds of the same class were generally found in the same positions of PC1 and PC2. Regarding the score plot, Ochrophyta representatives were placed in positive regions of PC1 differencing in PC2 as D. confervoides and A. utricularis were in the corresponding positive or negative axis. On the other hand, Rhodophyta species clustered in the negative axis of PC1 with the exception of M. manginii found in the origin of PC1. Similarly to the other phylum, red macroalgae had distinct positions in PC2. U. intestinalis was found near Rhodophyta samples along the negative axis of PC1 and positive PC2.
Hierarchical analysis (Fig. 2) was used to assess possible similarities among the macroalgae based on their FA and VOCs profile following the same variables used in the PCA analysis. The five species were differentiated into two main clusters that were each composed of green and red seaweeds or brown macroalgae. Besides phyla, macroalgae were generally further subdivided up to the same subclass (G. skottsbergii and C. racovitzae of the Rhodymeniophycidae subclass; A. utricularis and D. confervoides of Fucophycidae subclass) or order (G. confluens and M. manginii of the Ceramiales order).
Discussion
Fatty acids
The FA profile of Antarctic macroalgae showed significant differences among their constituents. Genetic and environmental factors, such as species, water temperature, photoperiod, nutrient availability, salinity, and pH, are among the reasons that can explain variations in the concentrations and types of FAs between algae species (Rautenberger and Bischof 2006; Becker et al. 2010). Indeed, analysis of the FA content of Antarctic macroalgae Iridaea cordata, Palmaria decipiens, Plocamium cartilagineum, and Pyropia endiviifolia by Santos et al. (2017) also revealed significant variations among the samples, which highlighted the influence of abiotic parameters in the production of biomolecules in seaweeds.
Given the extreme environmental conditions found in the Antarctic continent, it is thought that macroalgae adjust their biosynthesis metabolism accordingly with a higher production of PUFAs. Although susceptible to lipid peroxidation, PUFAs are essential molecules to maintain membrane’s fluidity of seaweeds in cold temperatures, allowing their survival (Becker et al. 2010; Pacheco et al. 2018). It is worth noting that PUFAs can also participate in the photosynthetic mechanism as they can act as electron carriers assisting in the endogenous production of energy (Sanina et al. 2008). The occurrence of this phenomenon was observed in the Ochrophyta representatives, as well as in M. manginii and C. racovitzae in lesser proportions.
Comparing the results for the red macroalgae G. skottsbergii, G. confluens, and C. racovitzae to the reported in the literature, it can be noted that, qualitatively, the composition of the samples did not vary considerably. Nonetheless, the concentration of PUFAs found in the current work was lower than that indicated by Graeve et al. (2002) and Pacheco et al. (2018), who also reported the analysis of Antarctic seaweeds. Variations of environmental conditions from 2002 to 2015 may be associated to differences in the FA profile, since aspects regarding sample preparation, extraction, and derivatization methods were similar. To the best of our knowledge, this was the first time that the profile of M. manginii has been described with the results being similar to other representatives from the Ceramiales (Schmid et al. 2018).
Regarding brown macroalgae, A. utricularis had similar patterns to that observed in the literature, in which high concentrations of PUFAs were also reported. The presence of other types of FAs is worth noting, for instance, capric (C10:0), lauric (C12:0), and docosadienoic acid (C22:2) that were not observed in a previous study (Pacheco et al. 2018). The profile of D. confervoides was not described in previous works; however, other specimens from Desmarestiales, such as Desmarestia muelleri and Desmarestia antarctica, also had a similar FA composition (Graeve et al. 2002).
Concerning Chlorophyta, the FA composition of U. intestinalis partially agreed with the results reported by Horincar et al. (2014), who analyzed the same alga collected in the Romanian coast. Qualitatively, it can be observed that the Antarctic representative had a higher diversity of detected FAs although the same majoritarian components, such as C16:0 and C18:1n9c, were observed in both seaweeds. The foremost difference among the samples regarded the overall content of PUFAs as one third of the total composition of FAs were PUFAs for the Romanian representative, while the Antarctic representative was only composed of 5.51 ± 0.25%. Fluctuations in the results could be associated to several factors, including environmental conditions, sample treatment, extraction, and derivatization methods. Martins et al. (2016) reported the analysis of U. intestinalis from the sub-Antarctic region resulting in a FA profile similar to the current work.
Generally, the PUFAs found in the studied macroalgae were mainly C18:3n3, C20:5n3, and C20:4n6 with minor contributions of linoleic (C18:2n6c), dihomo-linoleic (20:2), and dihomo-γ-linolenic acid (C20:3n6). The presence of these compounds highlights the importance of seaweeds as natural reservoirs of nutraceutical substances since some PUFAs and their long-chain counterparts cannot be synthesized by humans or terrestrial plants (Pacheco et al. 2018). Moreover, PUFAs also have several pharmaceutical and biotechnological applications for the treatment of coronary, inflammatory, autoimmune, and tumor-related conditions as well as assisting in drug delivery (Pereira et al. 2012; Santos et al. 2016).
α-Linolenic acid (C18:3n3) was among the PUFAs found in greater amounts and is an important compound with reported biological activities reported in the literature, including antioxidant, neuronal protective, anticancer, and antiosteoporotic, for instance (Pereira et al. 2012; Wang et al. 2017). This FA serves as substrate to the synthesis of other relevant PUFAs, such as C20:5n3 and C20:4n6, by mechanisms of elongation and desaturation. Alongside C18:3n3, FAs with higher carbon-chain also have important biological roles acting on brain development, maintenance of cardiovascular health, and inflammatory processes (Larsen et al. 2011; Santos et al. 2016).
MUFAs detected mostly in the forms of palmitoleic (C16:1) and oleic (18:1n9c) acids were among other lipid classes of interest. Generally, MUFAs were found in intermediate concentrations (9.57 ± 0.68–34.70 ± 1.57) of PUFAs and SFAs in the studied macroalgae, reaching higher amounts in U. intestinalis, M. manginii, and G. confluens. These compounds are building blocks to the further production of PUFAs and have biological activities associated to human health on the regulation of insulin sensitivity and decrease of inflammatory responses (Pacheco et al. 2018).
Finally, SFAs were also important contributors in the profile of the Antarctic macroalgae detected as the main types of FAs in almost all of the studied samples, except for D. confervoides. Higher uptakes of SFAs have been associated with increased risks for the development of coronary diseases, such as myocardial infarction, angina, and arteriosclerosis (Sánchez-Machado et al. 2004). However, since C16:0 was the SFA detected in higher quantity, further risks of consuming these macroalgae would be low as the enzyme ∆9-desaturase found in humans can convert it to C16:1, which itself is not associated with such biological complications (Martins et al. 2016).
Summarizing the obtained results, there is nutraceutical, pharmacological, and biotechnological potential in the studied seaweeds given that they are constituted of important n3 and n6-PUFAs within their FA profile that are linked to health benefits (dos Santos et al. 2019). Pereira et al. (2012) reported that PUFAs could act as drug deliverers as they can penetrate membranes due to their lipophilicity. Moreover, lipophilic extracts of these organisms had biological potential highlighting that FAs could play important roles in antimicrobial and anticancer activities. Even though Antarctic macroalgae have not been explored for commercial purposes, their chemical composition demonstrates the effects of the natural habitat on them, providing a better understanding on the development of these organisms under extremely cold, inhospitable environments (Martins et al. 2018; Pacheco et al. 2018).
Volatile organic compounds
For the first time, macroalgae collected in the Antarctic region had their VOCs profile determined, revealing the presence of several chemical classes that included mostly aldehydes, hydrocarbons, ketones, alcohols, and furan derivatives. The occurrence of these components in seaweeds has been previously reported in organisms analyzed in other regions of the planet (Le Pape et al. 2004; Kamenarska et al. 2006; El Hattab et al. 2007). Concerning the number of compounds detected by the association of headspace extraction with GC-MS, there was a similarity to the ones found in the literature as Osmundaria obtusiloba and Ceramium virgatum were composed of 21 and 36 VOCs, respectively (Horincar et al. 2014; de Alencar et al. 2017). It is worth noting that the application of headspace sorptive extraction could enhance the total number of substances detected as previous research works indicated the presence of 152 VOCs in a mixture of Ulva sp. and Gracilaria sp. (Maruti et al. 2018).
Aldehydes in the forms of hexanal, heptanal, nonanal, and benzaldehyde were found in major proportions among the chemical classes prevalent in the profile of the studied samples. Previous works have also highlighted higher concentrations of these compounds in seaweeds, agreeing with the results found for the Antarctic samples (Nor Qhairul Izzreen and Vijaya Ratnam 2011; Ferraces-Casais et al. 2013). The occurrence of aldehydes in seaweeds can be attributed either to the metabolization of MUFAs and PUFAs (mainly linoleic acid or arachidonic acid) by the enzymes lipoxygenase/fatty acid hydroperoxide lyase or to the biosynthesis process of amino acids (Yamamoto et al. 2014; Balbas et al. 2015). Indeed, U. intestinalis, G. skottsbergii, and G. confluens, for instance, had decreased concentrations of C18:2n6 and C20:4n6 while higher concentrations of aldehydes compared to the other samples possibly indicating the consumption of FAs to produce VOCs.
Aldehydes are known for being part of the many constituents that influence odor and flavor aspects in macroalgae. In this sense, hexanal and benzaldehyde, for instance, have grassy-green and almond odor, respectively, conferring pleasant aromas to them. In this sense, these components could potentially be used in the flavor industry (Horincar et al. 2014). The presence of unsaturated aldehydes, including 2,4-decadienal and hexadecenal, is associated with undesirable flavors due to their fishy aroma (Peinado et al. 2014). On the other hand, aldehydes extracted from seaweeds have been reported for their antimicrobial activity against Erwinia carotovora and Escherichia coli indicating these substances could also have biological applications (Kamenarska et al. 2006).
Hydrocarbons represented mainly as heptadecane were another important group of VOCs detected, although other aliphatic, branched, and cyclic alkanes, alkenes, and aromatic compounds, for instance, were found in minor concentrations. Higher concentrations of heptadecane have also been reported in Ulva prolifera and Ulva linza, while the presence of alkanes has been highlighted in Undaria pinnatifida (Yamamoto et al. 2014; Balbas et al. 2015). Generally, hydrocarbons are produced from degradation processes that occur in FAs and carotenoids. Nonetheless, abiotic factors that include water temperature, photoperiod, and species also play crucial roles in the production of hydrocarbons (López-Pérez et al. 2017). It is thought that these compounds are biosynthesized to act as chemical messengers of male gametes to assist on the reproductive cycle of the organism (de Alencar et al. 2017).
Several ketones were also detected in red, brown, and green Antarctic macroalgae, in which the most predominant substances found in the studied species were 2-heptanone and 2,3-octanedione. 2-Heptanone is produced naturally in several foodstuffs, and it is used as additive in food for human consumption (de Alencar et al. 2017). The presence of ketones was also detected in Ceramium virgatum and Monostroma nitidum (Horincar et al. 2014; Yamamoto et al. 2014). Similarly to the other chemical classes, ketones are mainly produced from metabolization processes of FAs, carotenoids, and amino acids within the seaweed (Horincar et al. 2014; Balbas et al. 2015). In aquatic organisms, the role of ketones has still been unknown, although they can act as repellent or attractive compounds to insects in terrestrial plants (Kamenarska et al. 2006). This can be associated to the low odor thresholds of most ketones that, alongside aldehydes, are important contributors to the aroma of macroalgae, in which α-ionone and 6,10-dimethyl-5,9-undecadien-2-one can be cited as examples linked to violet-like and green odors, respectively (Yamamoto et al. 2014; Balbas et al. 2015).
In diminished concentrations when compared to the other classes, alcohols were detected in the studied seaweeds mainly as 1-octen-3-ol and p-ethylphenol. These compounds were also present in the macroalgae Dictyopteris membranacea and Palmaria palmata from Algeria and France, respectively (Le Pape et al. 2004; El Hattab et al. 2007). Alcohols can be biosynthesized by several mechanisms that include secondary decomposition of PUFAs, glycolysis of carbohydrates, reduction of aldehydes, and from amino acids (Zhou et al. 2017; Jerković et al. 2018). The main role of alcohols is still little understood; however, it is thought that these compounds can assist in defense mechanisms of macroalgae (Kamenarska et al. 2006; Sun et al. 2012). Moreover, alcohols, such as 1-octen-3-ol, can also contribute to the aroma of the aquatic organism as they have low odor thresholds (Peinado et al. 2014).
Previous works regarding halogenated VOCs from Antarctic macroalgae highlighted the presence of diiodomethane, bromoform, dibromomethane, dibromochloromethane, chloroiodomethane, and bromodichloromethane in seaweeds (Laturnus et al. 1996, 1998). Although G. skottsbergii contained decyl chloride and α-chlorotoluene, generally halogenated hydrocarbons were not observed for the studied macroalgae. Among the reasons that could explain differences between the results are the distinct harvest approaches, algae treatment, and chromatographic settings. In general lines, halogenated VOCs are produced by the enzyme haloperoxidase that can fix halide ions into organic molecules. This leads to the production of halogenated metabolites that assess defensive mechanisms in macroalgae against microorganisms and competitive seaweeds (Kamenarska et al. 2006; Sun et al. 2012).
Other types of VOCs were also detected in Antarctic macroalgae, including sulfur metabolites, furan derivatives, FAs (low carbon-chain), and phthalates. Compounds containing sulfur have often been reported in seaweeds, mainly as dimethyl trisulfide, dimethyl sulfide, and dimethyl sulfoxide (Hosoglu 2018; Maruti et al. 2018). Furan derivatives compose several chemicals that influence the aroma of macroalgae, being produced from the degradation of linoleic acid or carbohydrates (Sun et al. 2012; de Alencar et al. 2017). Phthalates mainly as diisobutyl phthalate, diethyl phthalate, and dimethyl phthalate were also observed in the studied organisms. These substances are not associated to endogenous processes in macroalgae, but they could be found in seaweeds as a result of environmental pollution, such as those reported for Capsosiphon fulvescens collected in South Korea (Sun et al. 2012).
According to reports from the literature, VOCs produced by macroalgae are considerably affected by environmental factors and species, which could also be observed in the present work. Nonetheless, few seaweeds have had their profile of VOCs elucidated, indicating further research studies need to be conducted for the identification of aquatic components (Maruti et al. 2018). In this manner, it would be possible to detect chemicals with commercial or biological applications in the food, biotechnological, and pharmaceutical industries, for instance, as it has been demonstrated that seaweeds comprehend a vast and biorenewable source of interesting substances (Horincar et al. 2014; de Alencar et al. 2017).
Multivariate analysis
According to the PCA results, FA and VOCs profiles could be used to differentiate macroalgae in their respective phylum. In this sense, higher concentrations of variables that included C18-FAs, C20-FAs, PUFAs (C20:4n6 and C20:5n3), and 1-octen-3-ol assisted in the distinction of Ochrophyta representatives from the other phyla. Moreover, distinct amounts of 2-pentylfuran and C18:1n9c differentiated the brown algae D. confervoides and A. utricularis. Similar patterns were observed for Rhodophyta as samples of this phylum could be distinguished by the presence of SFAs (mainly C16:0), MUFAs (mainly C16:1), 2-heptanone, 2-pentenylfuran, benzaldehyde, 2,3-octanedione, and hexanal. Further differences in the concentrations of these variables also allowed the distinction of species on the score plot.
Using PCA, Van Durme et al. (2013) also observed that distinct concentrations of VOCs could differentiate several species of microalgae. Multivariate analysis was also employed by Bravo-Linares and Mudge (2009) who showed that physico-chemical and environmental conditions, including wind speed, water temperature, and seasoning, influenced the biosynthesis of VOCs in marine organisms. Moreover, the use of FAs as means to establish distinctions among seaweeds and their phyla has also been reported in the literature (Kumari et al. 2009).
The application of hierarchical analysis reinforced the results obtained in the PCA analysis as similar macroalgae tended to cluster in the dendrogram. In this sense, it was observed that the distinct FA and VOC profiles present mostly in brown and red seaweeds could differentiate them into two main groups. Similarities among the variables also caused the clustering of red and green macroalgae, which could be associated to their genetic closeness compared to brown seaweeds. Analyzing the FA profile of 27 macroalgae, Kumari et al. (2009) successfully differentiated or clustered samples based on their chemical composition agreeing with the results of the current research work.
Conclusions
In the present study, the FA and VOC profiles of red, brown, and green Antarctic macroalgae were successfully analyzed. Results showed that palmitic, oleic, linoleic, and eicosapentaenoic acids were the predominant FAs while hexanal, heptadecane, and 2-pentylfuran were the major types of VOCs in the samples. Furthermore, statistical analysis of the algal chemical components indicated distinct degrees of similarities among them. Therefore, this study elucidated that Antarctic macroalgae had a wide range of constituents which are associated to defense mechanisms and could lead to the development of novel bioactive compounds for applications in food, pharmaceutical, and biotechnological industries.
References
Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P (2013) Valuable compounds in macroalgae extracts. Food Chem 138:1819–1828
Balbas J, Hamid N, Liu T, Kantono K, Robertson J, White WL, Ma Q, Lu J (2015) Comparison of physicochemical characteristics, sensory properties and volatile composition between commercial and New Zealand made wakame from Undaria pinnatifida. Food Chem 186:168–175
Becker S, Graeve M, Bischof K (2010) Photosynthesis and lipid composition of the Antarctic endemic rhodophyte Palmaria decipiens: effects of changing light and temperature levels. Polar Biol 33:945–955
Bravo-Linares CM, Mudge SM (2009) Temporal trends and identification of the sources of volatile organic compounds in coastal seawater. J Environ Monit 11:628–641
de Alencar DB, Diniz JC, Rocha SAS, Pires-Cavalcante KMS, Freitas OJ, Nagano CS, Sampaio AH, Saker-Sampaio S (2017) Chemical composition of volatile compounds in two red seaweeds, Pterocladiella capillacea and Osmundaria obtusiloba, using static headspace gas chromatography mass spectrometry. J Appl Phycol 29:1571–1576
dos Santos MAZ, de Freitas SC, Berneira LM, Mansilla A, Astorga-España MS, Colepicolo P, de Pereira CMP (2019) Pigment concentration, photosynthetic performance, and fatty acid profile of sub-Antarctic brown macroalgae in different phases of development from the Magellan Region, Chile. J Appl Phycol 31:2629–2642
Dyer WJ, Bligh EG (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
El Hattab M, Culioli G, Piovetti L, Chitour SE, Valls R (2007) Comparison of various extraction methods for identification and determination of volatile metabolites from the brown alga Dictyopteris membranacea. J Chromatogr A 1143:1–7
Ferraces-Casais P, Lage-Yusty MA, Rodríguez-Bernaldo de Quirós A, López-Hernández J (2013) Rapid identification of volatile compounds in fresh seaweed. Talanta 115:798–800
Graeve M, Kattner G, Wiencke C, Karsten U (2002) Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. Mar Ecol Prog Ser 231:67–74
Hamid N, Ma Q, Boulom S, Liu T, Zheng Z, Balbas J, Robertson J (2015) Seaweed minor constituents. In: Tiwari BK, Troy DJ (eds) Seaweed sustainability. Elsevier, NY, pp 193–242
Horincar VB, Parfene G, Tyagi AK, Gottardi D, Dinică R, Guerzoni ME, Bahrim G (2014) Extraction and characterization of volatile compounds and fatty acids from red and green macroalgae from the Romanian Black Sea in order to obtain valuable bioadditives and biopreservatives. J Appl Phycol 26:551–559
Hosoglu IM (2018) Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem 240:1210–1218
Jerković I, Marijanović Z, Roje M, Kuś PM, Jokić S, Čož-Rakovac R (2018) Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS One 13:0196462
Kamenarska Z, Ivanova A, Stancheva R, Stoyneva M, Stefanov K, Dimitrova-Konaklieva S, Popov S (2006) Volatile compounds from some Black Sea red algae and their chemotaxonomic application. Bot Mar 49:47–56
Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B (2009) Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem 120:749–757
Larsen R, Eilertsen KE, Elvevoll EO (2011) Health benefits of marine foods and ingredients. Biotechnol Adv 29:508–518
Laturnus F, Wiencke C, Klöser H (1996) Antarctic macroalgae—sources of volatile halogenated organic compounds. Mar Environ Res 41:169–181
Laturnus F, Wiencke C, Adams FC (1998) Influence of light conditions on the release of volatile halocarbons by Antarctic macroalgae. Mar Environ Res 45:285–294
Le Pape M-A, Grua-Priol J, Prost C, Demaimay M (2004) Optimization of dynamic headspace extraction of the edible red algae Palmaria palmata and identification of the volatile components. J Agric Food Chem 52:550–556
López-Pérez O, Picon A, Nuñez M (2017) Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res Int 99:1002–1010
Martins RM, Dos Santos MAZ, Pacheco BS, Mansilla A, Astorga-España MS, Seixas F, De Pereira CMP (2016) Fatty acid profile of the Chlorophyta species from Chile’s sub-Antarctic region. Acad J Sci Res 4:93–98
Martins RM, Nedel F, Guimarães VBS, Silva AF, Colepicolo P, Pereira CMP, Lund RG (2018) Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Frontiers in Microbiology 9:412
Maruti A, Durán-Guerrero E, Barroso CG, Castro R (2018) Optimization of a multiple headspace sorptive extraction method coupled to gas chromatography-mass spectrometry for the determination of volatile compounds in macroalgae. J Chromatogr A 1551:41–51
Moss CW, Lambert MA, Merwin WH (1974) Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol 28:80–85
Nor Qhairul Izzreen M, Vijaya Ratnam R (2011) Volatile compound extraction using solid phase micro extraction coupled with gas chromatography mass spectrometry (SPME-GCMS) in local seaweeds of Kappaphycus alvarezii, Caulerpa lentillifera and Sargassum polycystum. Int Food Res J 18:1449–1456
Pacheco BS, dos Santos MAZ, Schultze E, Martins RM, Lund RG, Seixas FK, Colepicolo P, Collares T, Paula FR, de Pereira CMP (2018) Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front Bioeng Biotechnol 6:185
Passos LF, Berneira LM, Poletti T, Mariotti KDC, Carreño NL, Hartwig CA, Pereira CMP (2020) Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust J Forensic Sci:1–10. https://doi.org/10.1080/00450618.2020.1715478
Paul C, Pohnert G (2011) Production and role of volatile halogenated compounds from marine algae. Nat Prod Rep 28:186–195
Peinado I, Girón J, Koutsidis G, Ames JM (2014) Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res Int 66:36–44
Pereira H, Polo C, Rešek E, Engelen A (2012) Polyunsaturated fatty acids of marine macroalgae: potential for nutritional and pharmaceutical applications. Mar Drugs 10:1920–1935
Pereira CMP, Nunes CFP, Zambotti-Villela L, Streit NM, Dias D, Pinto E, Gomes CB, Colepicolo P (2017) Extraction of sterols in brown macroalgae from Antarctica and their identification by liquid chromatography coupled with tandem mass spectrometry. J Appl Phycol 29:751–757
Rautenberger R, Bischof K (2006) Impact of temperature on UV-susceptibility of two Ulva (Chlorophyta) species from Antarctic and Subantarctic regions. Polar Biol 29:988–996
Rodrigues D, Freitas AC, Pereira L, Rocha-Santos TAP, Vasconcelos MW, Roriz M, Rodríguez-Alcalá LM, Gomes AMP, Duarte AC (2015) Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem 183:197–207
Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P (2004) Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem 85:439–444
Sanina NM, Goncharova SN, Kostetsky EY (2008) Seasonal changes of fatty acid composition and thermotropic behavior of polar lipids from marine macrophytes. Phytochemistry 69:1517–1527
Santos SAO, Vilela C, Freire CSR, Abreu MH, Rocha SM, Silvestre AJ (2015) Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem 183:122–128
Santos SAO, Oliveira CSD, Trindade SS, Abreu MH, Rocha SS, Silvestre AJ (2016) Bioprospecting for lipophilic-like components of five Phaeophyta macroalgae from the Portuguese coast. J Appl Phycol 28:3151–3158
Santos MAZ, Colepicolo P, Pupo D, Fujii MT, de Pereira CMP, Mesko MF (2017) Antarctic red macroalgae: a source of polyunsaturated fatty acids. J Appl Phycol 29:759–767
Schmid M, Kraft LGK, van der Loos LM, Kraft GT, Virtue P, Nichols PD, Hurd CL (2018) Southern Australian seaweeds: a promising resource for omega-3 fatty acids. Food Chem 265:70–77
Sun SM, Chung GH, Shin TS (2012) Volatile compounds of the green alga, Capsosiphon fulvescens. J Appl Phycol 24:1003–1013
Van Durme J, Goiris K, De Winne A, De Cooman L, Muylaert K (2013) Evaluation of the volatile composition and sensory properties of five species of microalgae. J Agric Food Chem 61:10881–10890
Wang HMD, Li XC, Lee DJ, Chang JS (2017) Potential biomedical applications of marine algae. Bioresour Technol 244:1407–1415
Yamamoto M, Baldermann S, Yoshikawa K, Fujita A, Mase N, Watanabe N (2014) Determination of volatile compounds in four commercial samples of Japanese green algae using solid phase microextraction gas chromatography mass spectrometry. Sci World J 2014:1–8
Zhou L, Chen J, Xu J, Li Y, Zhou C, Yan X (2017) Change of volatile components in six microalgae with different growth phases. J Sci Food Agric 97:761–769
Acknowledgments
The authors are grateful to the people from the logistic support from the Brazilian Antarctic Program. The authors are also thankful for the financial and fellowship support from the Brazilian research funding agencies National Council for Scientific and Technological Development (CNPq), Coordination for Improvement of Higher Level Personnel (CAPES, Finance Code 001), Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS), and Foundation for Research Support of the State of São Paulo (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 263 kb)
Rights and permissions
About this article
Cite this article
Berneira, L., da Silva, C., Poletti, T. et al. Evaluation of the volatile composition and fatty acid profile of seven Antarctic macroalgae. J Appl Phycol 32, 3319–3329 (2020). https://doi.org/10.1007/s10811-020-02170-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02170-9