Abstract
Non-photochemical quenching (NPQ) is one of the most important photo-protection mechanisms in brown macroalgae. Global warming and ocean acidification are predicted to impact physiological characteristics of marine algae. However, little is known about the effects of co-occurrence of the elevation of pCO2 and temperature on photochemical capacity, especially regarding photoprotective mechanisms in brown macroalgae. Here, we studied the separate and combined effects of increases in pCO2 and temperature on the photochemical characteristics and growth performance in sporophytes of the brown macroalga Saccharina japonica. The results showed that the NPQ of S. japonica is mainly dependent on the xanthophyll cycle (XC) which appears to be related only to the activation of the enzyme violaxanthin de-epoxidase (VDE), and the transthylakoid proton gradient (ΔpH) could not induce NPQ alone. The elevation of pCO2 reduced NPQ value of S. japonica under high-temperature stress. After 60-day cultivation under the ambient and elevated pCO2 (400 and 1000 μatm), we further found that the elevation of pCO2 promoted growth and increased the photosynthetic performance of all three cultivar strains of S. japonica that have been traditionally cultured for many years in China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming, as well as the ocean acidification derived from anthropogenic CO2 emissions, is one of the most severe environmental crises in the twenty-first century (Schlüter et al. 2014). The Earth’s surface temperature has risen by 0.7 °C during the last century and is expected to increase by an additional 3 °C by 2100 (Hoegh-Guldberg et al. 2007). As a major global sink for CO2, oceans have absorbed approximately 30% of anthropogenic CO2 emissions, which have mitigated CO2-related global warming (Hall-Spencer et al. 2008). However, CO2 absorption results in a decrease in oceanic pH and changes in seawater chemistry, with increases in the levels of dissolved carbon dioxide and bicarbonate and a lower degree of carbonate saturation, i.e., ocean acidification (Caldeira and Wickett 2005). The results from a growing number of recent studies conducted in the topics indicated that global warming and ocean acidification interact to affect growth and photophysiology of marine primary producers in varying manners and to varying magnitudes, potentially altering community structure and marine ecosystems (Koch et al. 2013). There is evidence that in the brown macroalga Macrocystis pyrifera, the combined increases in temperature and pCO2 have significantly decreased the germination rates and increased the mortality of kelp spores (Gaitán-Espitia et al. 2014). Olischläger and Wiencke (2013) reported that predicted level of ocean acidification may alleviate the effects of low temperature on the growth and photosynthesis of the red alga Neosiphonia harveyi.
As primary producers, photoautotrophs absorb 30% of the anthropogenically emitted carbon and play a significant role in modulating global climate change (García-Gómez et al. 2014). At the surface of the ocean photosynthetic organisms are often exposed to variations in light intensity. Excitation by an excess of light may cause photoinhibition and the production of reactive oxygen intermediates that are detrimental to plant photosynthetic activity, growth, and productivity (Peers et al. 2009). Increase in the amount of reactive oxygen species (ROS) may cause lipid peroxidation and inactivation of enzymes (Babu et al. 2014). To resist the oxidative stress, plants have evolved a variety of photoprotection mechanisms. Non-photochemical quenching (NPQ) is one of the most important and most rapid regulatory strategies to dissipate excess absorbed light as heat and to prevent photodamage to the reaction center in PSII (Mou et al. 2013). Multiple NPQ components based on various molecular mechanisms have been identified in the plant kingdom. In higher plants, the major component is the transthylakoid proton gradient (ΔpH) dependent quenching (qE), which is modulated by the xanthophyll cycle (XC) and the protein PsbS (Johnson and Ruban 2010). During the XC, the prevailing component, violaxanthin (Vx), is converted to antheraxanthin (Ax) and then to zeaxanthin (Zx) by violaxanthin de-epoxidase (VDE), which is regulated by pH, located in the thylakoid lumen and is inhibited by dithiothreitol (DTT) (Masojidek et al. 2004). Zx synthesized via the XC is essential for thermal dissipation. Similar to higher plants, XC plays a key role in NPQ induction of brown algae (Goss and Lepetit 2015). NPQ induction mainly depends on Zx and it is activated by ΔpH in the thylakoid membrane (Ocampo-Alvarez and García-Mendoza 2013). In addition, the PSII antenna subunit PsbS is essential for qE and no PsbS was found in brown algae (García-Mendoza and Colombo-Pallotta 2007). In contrast to higher plants, qE, a fast NPQ induction mechanism is absent in brown algae (García-Mendoza and Colombo-Pallotta 2007). According to previous study, ΔpH alone could not induce NPQ and appears to be related only to the activation of the VDE in M. pyrifera (García-Mendoza and Colombo-Pallotta 2007). Additionally, at the regulatory level, a group of light-harvesting proteins from the LHCSR family is involved in NPQ in green algae, brown algae, and diatoms, but is missing from higher plants (Cao et al. 2013).
In parallel with the diurnal and seasonal variation of light, global climate change has caused additional stresses to phytoplankton in association with the decline in oceanic pH, the warming of seawater, and the thinning of the upper mixing layer as a result of increased stratification (Doney et al. 2012; Gao et al. 2012). In isolation, the elevation of pCO2 was observed to contribute to a higher capacity for repair of PSII in the diatom Thalassiosira pseudonana (McCarthy et al. 2012), a more rapid recovery of PSII from UV damage in Phaeodactylum tricornutum (Wu et al. 2014), and a decrease in UV B-related photochemical inhibition in Gephyrocapsa oceanica (Jin et al. 2013). In contrast, NPQ mechanisms of the brown macroalgae Padina pavonica and Cystoseira tamariscifolia (Korbee et al. 2014) and in the green macroalga Ulva prolifera were activated under elevated CO2 (Liu et al. 2012). Photosynthesis is also known to be highly sensitive to temperature (Mathur et al. 2014). The greenhouse gas-induced increase in temperature has been associated with a three-fold increase in photoinhibition in intertidal benthic microalgal biofilms (Laviale et al. 2014). Furthermore, the combination of increasing pCO2 with other stresses has been reported to produce antagonistic, synergistic, or neutral effects on physiological performance in various marine organisms (Chen et al. 2014). However, the effects of co-occurrence of the elevation of pCO2 and temperature on photochemical capacity, especially regarding photoprotective mechanisms, remain unclear.
In coastal ecosystems, macroalgal kelp constructs three-dimensional underwater forests and plays significant ecological role in structuring biodiversity in both cold-temperate and polar coastal regions (Wernberg et al. 2010). Unfortunately, predicted increases in seawater temperatures are forecast to decrease the biodiversity of these cold temperate seaweeds and shift the distributional boundaries northward (Raybaud et al. 2013; Redmond 2013). Saccharina japonica is one of the most ecologically and economically important brown macroalgae and is used for food, medicinal, agricultural, and industrial products (Wang et al. 2013). Floating-frame culture using rope rafts has been the most commonly employed cultivation method in the coast of China since the 1950s (Li et al. 2008). During the growing season, from October to June of the following year, this algal frond community forms a dense canopy at the ocean surface, where it is subject to a large diurnal gradient and seasonal variation in light and temperature (Bartsch et al. 2008). Although high capacities for photoacclimation and photoprotection have been reported for another important giant kelp, M. pyrifera (García-Mendoza and Colombo-Pallotta 2007), the exact mechanism of photoprotection is not yet known for S. japonica. Moreover, responses of this productive macroalga to the isolated and combined effects of ocean acidification and the increasing temperature associated with global climate change are of general concern.
Therefore, in the present study, we first characterized NPQ kinetics in S. japonica by using specific chemical inhibitors. Second, we evaluated the effects of temperature and ocean acidification on NPQ in isolation and in combination. To further study the effect of ocean acidification on the growth and photosynthesis in a long-term selection, three strains of S. japonica were cultured under two levels of pCO2 (400 and 1000 μatm) for 60 days. We hypothesized that (1) S. japonica shows a photoprotective mechanism similar to that of M. pyrifera; (2) although increasing temperature induced a strong NPQ response, the elevation of pCO2 may alleviate this response by providing sufficient carbon resources; and (3) increasing pCO2 would produce a general positive effect on photosynthesis and growth.
Materials and methods
Algal material and culture conditions
Samples of S. japonica were collected from Sungo Bay (37°01′–37°09′ N, 122°24′–122°35′ E) in December 2013, when the mean seawater temperature was 10.32 °C. Within 3 h, the algal samples were transported to the laboratory in a tank of natural seawater. The mean size of the collected samples was 15 cm in length and 3 cm in width. In the laboratory, the intact samples were washed several times with sterile seawater until they were free from visible epiphytes and then pre-cultured in Erlenmeyer flasks supplemented with F/2 medium (Guillard 1975) at 10 ± 1 °C with vigorous air bubbling for 2 days. The lighting conditions were set at 100 μmol photons m−2 s−1 supplied by white fluorescent lamps, with a photoperiod of 12 h light/12 h dark.
Chl fluorescence yield and NPQ measurement
In vivo chlorophyll fluorescence measurements were performed at the same temperature (10 ± 2 °C, controlled by an air conditioner) as the natural seawater where the sample was collected by using dual-wavelength pulse amplitude modulated fluorescence monitoring system (Dual-PAM, Heinz Walz, Germany).
Before measurement, the samples were kept in darkness for 20 min and the original fluorescence (F0) was determined under low measuring light. A saturation light pulse (1287 μmol photons m−2 s−1 for 300 ms) was applied to obtain maximum fluorescence (Fm) in the dark-adapted samples. The Fm yield in illuminated samples is denoted as Fm′, and Ft is real-time fluorescence yield. The maximum PSII quantum yield (Fv/Fm) was calculated according to the equation (Hiriart-Baer et al. 2008):
The effective PSII quantum yield (YII) was calculated as follows (Genty et al. 1989):
Relative rate of the photosynthetic electron transport of PSII (rETR) was calculated as (Hiriart-Baer et al. 2008):
where PAR is the irradiance and 0.5 assumes that half of the light absorbed is distributed to PSII.
NPQ was calculated as (Bilger and Björkman 1990):
The rapid light response curve (LRC) was also generated under increasing irradiance from 11, 18, 27, 58, 100, 131, 221, 344, 536 to 830 μmol photons m−2 s−1 and fitted by the function (Platt et al. 1980):
where Ps is defined as the maximum potential ETR, α is the slope of the initial of the RLC, and β is the slope of the RLC beyond the onset of saturation, Ed is the downwelling irradiance (400–700 nm) (Ralph and Gademann 2005). The light utilization efficiency (α), maximum electron transport rate of PSII (ETRmax), and half saturation irradiance (Ek) were obtained from the LRC (Xu et al. 2015).
To investigate NPQ processes under different inhibitor conditions, actinic light of 1287 μmol photons m−2 s−1 was used. Minimal irradiance of > 216 μmol photons m−2 s−1 was necessary to induce NPQ, and irradiances > 1287 μmol photons m−2 s−1 did not induce a higher level of NPQ (Fig. S1). Once steady state fluorescence was achieved, saturating pulses were applied every 30 s to measure the Fm under actinic light (Fm’).
NPQ kinetics of S. japonica
For NPQ kinetics, to minimize the contribution of the saturating light flashes (which are required to determine the NPQ parameter) to NPQ formation, the flashes were separated at 10 s intervals for the first 30 s of induction, 30 s intervals between 30 and 300 s, and 60 s intervals from 300 to 600 s. All measurements were made in triplicate.
The functions of ΔpH and the XC were characterized using the inhibitors ammonium chloride (NH4Cl) and dithiothreitol (DTT). The inhibitor NH4Cl is known as the uncoupler of the ΔpH, while DTT was used as a specific inhibitor of VDE (Mou et al. 2013; Zhang et al. 2014). When appropriate, NH4Cl (8 mmol L−1) or DTT (0.5 mmol L−1) were added at the start of dark incubation to perturb the kinetics of the ΔpH build-up, the XC and NPQ.
The effects of temperature and pCO2 on NPQ kinetics
To identify the isolated and combined effects of temperature and pCO2 on NPQ, a gradient of three temperatures (5, 10, and 20 °C) was established. Under each temperature, four pCO2 levels (400, 700, 1000, and 2000 μatm) were applied the pH of culture medium. The experiment was conducted in triplicate with a flask and tubing system similar to that used by Zou and Gao (2009). Culture medium was bubbled either with air (~ 400 μatm) or air/CO2 mixed gas in a programmed CO2 chamber (HP1000G-D, China) to achieve the desired pCO2 levels and temperature setting. Algal samples were cultured in each condition for 12 h. The pH in each culture medium was measured before and after each transfer using a pH meter (Orion ROSS, Fisher Scientific Instruments) that was calibrated with the National Bureau of Standards (NBS) buffers. Temperature, salinity, and total alkalinity (TA) were also measured periodically (but not at every transfer due to logistical restrictions) throughout the experiment. Other carbonate system parameters were calculated with the CO2SYS using pH and TA (Table S1). Then, the NPQ kinetics were determined using a Dual-PAM-100 fluorometer at an actinic light intensity of 1287 μmol photons m−2 s−1. At the end of the experiment, after NPQ determination, algal samples from each treatment were also frozen in liquid nitrogen and preserved for the analysis of pigments involved in the XC.
Isolated effects of ocean acidification on the physiological performance of S. japonica
To further study the isolated effects of ocean acidification on the physiological performance of S. japonica, mature fronds of three cultivar strains (denoted as BN, Ja, or F) of S. japonica were collected from Sungo Bay (37°01′–37°09′ N, 122°24′–122°35′ E) in August 2014. The three cultivar strains are three of the most important local stains with different phenotypic performance and have been traditionally cultured for many years in China. BN showed the highest biomass with a modest ratio of length/width, Ja showed the highest width and modest biomass, and F showed the highest length and minimum width and matured at the last. Juvenile sporophytes were successfully formed by the summer seedling raising method (Tseng 2001) after cultivation for 60 days. During the cultivation period, the three strains were cultured at 10 ± 1 °C and in a pH-adjusted F/2 medium, which was bubbled at one of two different pCO2 levels (L, 400 μatm and H, 1000 μatm) and exchanged weekly. At the end of experiment, sporophyte size, PSII photosynthetic parameters, and pigment contents were determined.
Xanthophyll determinations
The preserved samples were mechanically disrupted and the pigments were extracted in a tube by adding 3 mL of cold 85% acetone. The extracts were maintained on ice for 1 h, centrifuged at 12,000×g (4 °C) for 5 min, and stored at − 20 °C before analysis by high-pressure liquid chromatography (HPLC). Cellular debris was removed by centrifugation and the supernatants were filtered through 0.22-μm syringe filters into amber HPLC vials. The vials were stored on ice in the dark prior to injection. All extraction procedures were performed in a dimly lit room. Concentrations of XC pigments were determined using reversed phase HPLC as described by Zapata et al. (2000). Pigments were analyzed following Thayer and Bjorkman (1990) with minor modifications using a HP1100 Liquid Chromatograph equipped with a diode array detector (Agilent Technology, USA). An Agilent Technology non-endcapped Zorbax ODS column (4.5 × 250 mm, 5 mm particle sizes) was used in the separation, preceded by a C18 Adsorbosphere guard column (Alltech Associates, Inc., USA). Pigment quantification was performed using calibration curves calculated from HPLC separations using purified pigment standards obtained from DHI Inc. (Denmark). DPS of the XC pigment pool was calculated as (Zx + 0.5Ax)/(Vx + Ax + Zx) (Casper-Lindley and Björkman 1998); here, ΣXC is the sum of the Vx, Ax, and Zx concentrations. DPS represents the photoprotective state of the XC because the carotenoids Ax and Zx are involved in the dissipation of energy as heat, which confers photoprotection to the photosynthetic apparatus.
Statistical analysis
One-way and two-way ANOVAs were employed to analyze the isolated and combined effects, respectively, of pCO2 and temperature on various physiological parameters using the software SPSS 17.0 (SPSS, USA). Post hoc tests, Tukey’s test for two-way ANOVA, and Dunnett’s test for multivariate ANOVA were also performed. The assumptions of homogeneity of variance and normality were assessed by examining the scatter plots of the residuals and the normal curves of the residuals, respectively. A significance level of 0.05 was set for all tests.
Results
The general NPQ feature of S. japonica
Multiple intensities of actinic light were applied to measure NPQ kinetics (Fig. S1). Minimal irradiance of > 216 μmol photons m−2 s−1 was necessary to induce NPQ, and irradiances > 1287 μmol photons m−2 s−1 did not induce a higher level of NPQ. Therefore, an actinic light of 1287 μmol photons m−2 s−1 was established as the final applied irradiance to saturate photosynthesis and induce maximal NPQ. At the actinic light intensity of 1287 μmol photons m−2 s−1, the basic characteristics of NPQ of S. japonica were further studied by Chl a fluorescence quenching (Fig. 1). In S. japonica, NPQ was rapidly induced when the light switched on and exhibited reversibility in darkness (Fig. 1a). To further study the role of ΔpH and XC in NPQ, the various concentrations of uncoupling agents NH4Cl or DTT were added at the start of the dark incubation (Fig. S2). NPQ decreased significantly with an increase in the concentration of NH4Cl or DTT. The final concentrations of NH4Cl and DTT determined to be the minimum concentrations required to achieve complete inhibition were 8 mmol L−1 and 0.5 mmol L−1, respectively.
a Chlorophyll a fluorescence quenching in S. japonica. b Effects of NH4Cl or DTT on NPQ kinetics. Before measuring NPQ values, samples were pre-incubated in the dark for 20 min with NH4Cl (8 mmol L−1) or DTT (0.5 mmol L−1). c Effect of NH4Cl on fluorescence quenching in S. japonica over 15 min of exposure to actinic light (up arrow), with NH4Cl added after 10 min. d Effect of NH4Cl on fluorescence quenching in S. japonica over 15 min of exposure to actinic light (up arrow), with NH4Cl added before light exposure. e Chlorophyll a fluorescence quenching with DTT and NH4Cl added after light exposure for 10 min. f Chlorophyll a fluorescence quenching with DTT and seawater added after light exposure for 10 min. The values are represented as means ± SD, n = 3
The NPQ values of DTT-treated and control samples were determined within 10 min of illumination at 1287 μmol photons m−2 s−1 (Fig. 1b). The mean NPQ value in the control samples was approximately 1.87. In contrast to control samples, there was an obvious decrease in NPQ (0.7) after the addition of 0.5 mmol L−1 DTT, indicating that the de-epoxidation of XC pigments is required for NPQ induction in S. japonica. NPQ also decreased significantly with the addition of 8 mmol L−1 NH4Cl at the start of the dark incubation (Fig. 1b). Chl a fluorescence quenching was also monitored under 15 min of high light exposure, by adding NH4Cl during the process (Fig. 1c) or before light exposure (Fig. 1d). Both assays induced a relaxation of fluorescence quenching, which appears to be correlated with the ΔpH induced by NPQ. However, the samples incubated with DTT before light exposure did not exhibit the slow relaxation of NPQ after the addition of NH4Cl (Fig. 1e), although a slight increase may be induced by seawater (Fig. 1f). These results indicated that the slow reversibility of NPQ only occurred in the presence of de-epoxidated XC pigments and that the role of this gradient appears to be related only to the activity of the XC.
The levels of the pigments involved in the XC under the treatment conditions evaluated were determined by HPLC (Table 1). The high light condition induced higher levels of Zx/Chls and DPS, indicating that the high light was effective in inducing Zx synthesis and increasing the de-epoxidation index of the XC. When samples were treated with inhibitors, the Zx/Chls and DPS of the HL-NH4Cl samples decreased from 0.509 ± 0.042 to 0.295 ± 0.004 and from 0.080 ± 0.043 to 0.024 ± 0.001, respectively (p < 0.05), which indicates that the ΔpH may affect the activation of the XC. DTT-treated samples induced substantially lower levels of Zx/Chls and DPS, demonstrating that 0.5 mmol L−1 DTT can almost entirely block the conversion of Vx into Ax and Zx under high light conditions. Similarly, the correlation analysis further indicates that NPQ showed a strong positive correlation with Zx/Chls (R2 = 0.802) (Fig. 2a), and NPQ also showed a strong positive correlation with DPS (R2 = 0.788) (Fig. 2b).
The isolated and combined effects of pCO2 and temperature on NPQ in young sporophyte of S. japonica
Statistical analysis showed that there were significant effects of temperature and pCO2 on NPQ kinetics across 1200 s of S. japonica (Fig. 3, Table S2). Although NPQ increased with the increasing temperature from 5 to 10 °C and 20 °C (Table S3), the elevation of pCO2 reduced value of S. japonica (Table S4). Analysis of pigments involved in Zx indicated that Zx/Chls and DPS showed trends similar to those of the various treatments; high temperature increased these parameters, and the elevation of pCO2 reduced them, except for the variation in Zx/Chls at the lowest temperature of 5 °C (Table 2). Consistently, correlation analysis demonstrates that the correlation coefficient R2 (0.280) between Zx/Chls and NPQ was substantially lower than that between DPS and NPQ (0.735) (Fig. 4).
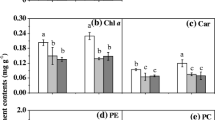
The physiological performances of various strains of S. japonica under ambient and elevated pCO2 are shown in Fig. 5. Although there were significant differences in blades size among the three tested strains, the elevation of pCO2 dramatically promoted growth in all three strains (p < 0.001) (Fig. 5a). In terms of the PSII photosynthetic parameters, the Fv/Fm, YII (Fig. 5b) and ETRII (Fig. 5c) values were increased significantly under the elevated pCO2 treatment for all three strains. From the LRCs, other parameters including α, ETRmax, and Ek were calculated (Table 3). Statistical analysis revealed significant differences in ETRmax (p < 0.001), Ek (p < 0.001), and α (p = 0.036) among the three strains. The elevation of pCO2 treatment resulted in a significant decrease in α (p < 0.001), but marked increases in ETRmax (p < 0.001) and Ek (p < 0.001). Additionally, although one-way ANOVA indicated that the effects of pCO2 on the levels of the pigments Chl a, Chl c, and carotenoids were not significant, all of these levels were, on average, greater under the pCO2 of 1000 μatm (Fig. 5d).
The isolated effect of increasing pCO2 on growth (a), the PSII photosynthetic parameters of Fv/Fm, YII (b), and ETRII (c), and the pigment contents (d) of S. japonica cultured under either a low pCO2 of 400 μatm (L) or a high pCO2 of 1000 μatm (H) for 60 days. The values are represented as means ± SD, n = 3
Discussion
According to the results, we speculated that the NPQ mechanism in brown macroalga S. japonica is mainly associated with the XC and that a change in the ΔpH alone could not induce NPQ (Fig. 1). The elevation of pCO2 reduced NPQ values of S. japonica, which could save energy spent in thermal dissipation and invest more energy to promote growth and photosynthesis (Figs. 3 and 5). Although the basic principle of NPQ, the dissipation excessive light energy as heat is identical across photoautotrophs, the regulation mechanisms vary significantly in organisms (Zhang et al. 2014). In green algae, NPQ is mainly controlled by the ΔpH and further depends on the activation of the XC to varying extents depending on taxon or lineage (Lunch et al. 2013; Mou et al. 2013). For diatoms and brown algae, the ΔpH is not sufficient to induce NPQ, which has been mainly associated with the formation of diatoxanthin (Dtx) and Zx (Cao et al. 2013). In this study, the NPQ kinetics in S. japonica was examined by the addition of the chemical uncouplers NH4Cl and DTT (Fig. 1).
The significant decrease in NPQ with the addition of NH4Cl indicates that the disruption of ΔpH may induce a reduction in NPQ (Fig. 1 b, c and d). An even more highly significant decline in NPQ was also induced by the addition of DTT (Fig. 1b). Chlorophyll a fluorescence quenching analysis further demonstrates that the addition of NH4Cl to DTT-pretreated samples did not relax NPQ (Fig. 1e). The slight decline in fluorescence quenching may be attributable to the addition of seawater (Fig. 1f). Therefore, ΔpH was not the factor inducing NPQ. Further pigment analysis indicated that a high light intensity induces significant increases ΣXC/Chls and DPS; in the presence of DTT, the ΣXC/Chls and DPS decreased significantly from 0.293 ± 0.054 and 0.509 ± 0.042 to 0.121 ± 0.043 and 0.112 ± 0.084, respectively (Table 1). In particular, the Zx/Chls ratio decreased to a barely detectable level. This indicates that the NPQ induction in S. japonica closely followed the synthesis of Zx in XC (Fig. 2a) and that ∆pH alone could not induce NPQ, which appears to be solely related to the activation of the VDE, similar to the NPQ regulation mechanism of the giant kelp M. pyrifera (García-Mendoza and Colombo-Pallotta 2007).
Marine algae experience diurnal and annual changes in irradiance and temperature, which may vary from limitation to levels potentially inhibiting photosynthesis. Moreover, de-synchronized variations in light and temperature may lead to photoinhibition occurring at a relatively low light saturation (Vonshak et al. 2001). Although photosynthesis is known to be highly sensitive to temperature, information on the effects of changing temperature on associated photoprotective mechanisms is rather scarce. A few studies have reported that high temperature may increase the de-epoxidation rate in the XC, the repair rate of PSII and RUBISCO activity, leading to a reduction of photoinhibition in the diatom T. pseudonana (Cabrerizo et al. 2014; Halac et al. 2014). Low temperature has also been identified to play a synergistic role with excess illumination, limiting electron transport and carbon fixation rates, thereby inducing photoinhibition even under relatively weak light conditions (Gerotto et al. 2011; Halac et al. 2014). Thus, a suboptimal physiological temperature, either high or low, may result in photodamage to photosynthetic organisms (Fig. 3). Similar to our results, previous studies also showed that NPQ increased with temperature in the kelp Alaria esculenta (Roleda. 2009). In parallel, there were, in general, increases in the Zx/Chls, ΣXC/Chls, and DPS involved in the ZX under high temperature (Table 2).
The ongoing CO2 emission-induced ocean acidification has been reported to result in various physiological effects on phytoplankton, indicating complex responses among various species and numerous interactions with other environmental factors (Chen et al. 2014). Even within the brown macroalgae, there is inconsistency among the findings on the effects of ocean acidification on species. Swanson and Fox (2007) reported that CO2 enrichment promoted growth in the emergent kelp species Nereocystis luetkeana but inhibited growth in the submergent Saccharina latissima. Species-specific responses of photosynthesis to changes in seawater carbonate chemistry have also been identified within brown algae (Xu and Gao 2012). In the present study, the predicted pCO2-induced ocean acidification promoted the growth and photosynthesis of the sporophytes of S. japonica, one of the most typical giant kelps in China, during long-term cultivation (Fig. 5). The positive effect of ocean acidification may be attributable to the increased concentration of inorganic carbon resources. As the main form of inorganic carbon for photosynthesis, HCO3− can be acquired by algal cells either via active transport or via a carbon-concentrating mechanism (CCM). For the kelp Laminaria digitata, an increase in carbon resources increased the DIC uptake, but decreased the activity of external carbonic anhydrase (exCA), which is involved in CCM (Klenell et al. 2004). The energy savings from the down-regulation of CCM may benefit growth and photosynthesis. However, except for the positive effect of excess carbon availability, the elevation of pCO2 also induces a lower value of α in S. japonica sporophytes (Table 3), suggesting that the projected level of ocean acidification may reduce its light use efficiency, which contradicts the findings of Li et al. (2014) for the diatom P. tricornutum.
Considering the effect of ocean acidification on NPQ, the elevation of pCO2 has been demonstrated to increase the susceptibility to photoinhibition of several temperate phytoplankton species (Trimborn et al. 2014). In contrast, the projected increase in ocean acidification was found to alleviate UV inhibition in the diatom P. tricornutum and the coccolithophore Gephyrocapsa oceanica (Jin et al. 2013, Wu et al. 2014). Unfortunately, no data were available regarding the effect of ocean acidification on NPQ within the scope of brown macroalgae. Our present study thus presents the first evidence that CO2-induced seawater acidification reduces the NPQ response of S. japonica to high light and alleviates the effect of high temperature on NPQ. Consistent with the NPQ results, the DPS of the XC decreased significantly in response to the increase in pCO2 from 400 to 2000 μatm (Table 2). Although the underlying mechanism remains unknown, the decrease in NPQ may have been attributed to the increased level of PAR at which light becomes excessive for electron transport rate, as evidenced by the higher ETRmax and Ek under the elevation of pCO2 (Table 3). In addition, the correlation analysis indicates that there are poor relationships between Zx/Chls and NPQ (R2 = 0.280), and there are strong positive between DPS and NPQ (R2 = 0.735) (Fig. 4). According to the results of Ocampo-Alvarez and García-Mendoza (2013), DPS instead of absolute concentration of Zx play a key role during NPQ induction in M. pyrifera (Fig. 6). Moreover, we speculate that elevate pCO2 reduced the activity of Zx epoxidase enzyme and inhibited the conversion from Zx to Vx at lower (5 °C) and higher (20 °C) temperatures and the underlying mechanisms should be further investigated.
In conclusion, with progressive climate change, the enhanced stratification in association with global warming and the increased acidification resulting from ongoing CO2 emissions will expose marine primary producers to a more complex marine environment and will enhance light stress for photoautotrophs living in the upper mixing layer. We speculated that the photoprotective mechanism of NPQ to high light is mainly associated with the XC in the giant kelp S. japonica. CO2-induced ocean acidification and high temperature exert antagonistic effects on NPQ. In isolation, the projected level of ocean acidification promoted growth and photosynthesis by S. japonica sporophytes, but decreased the NPQ activity under high light conditions. Thus, the predicted ocean acidification may alleviate the effect of high temperature on giant kelp, conserving energy for growth and photosynthesis.
References
Babu MY, Palanikumar L, Nagarani N, Devi VJ, Kumar SR, Ramakritinan CM, Kumaraguru AK (2014) Cadmium and copper toxicity in three marine macroalgae: evaluation of the biochemical responses and DNA damage. Environ Sci Pollut Res 21:9604–9616
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda M, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Cabrerizo MJ, Carrillo P, Villafañe VE, Helbling EW (2014) Current and predicted global change impacts of UVR, temperature and nutrient inputs on photosynthesis and respiration of key marine phytoplankton groups. J Exp Mar Biol Ecol 461:371–380
Caldeira K, Wickett ME (2005) Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res Oceans 110:1–12
Cao S, Zhang X, Xu D, Fan X, Mou S, Wang Y, Ye N (2013) A transthylakoid proton gradient and inhibitors induce a non-photochemical fluorescence quenching in unicellular algae Nannochloropsis sp. FEBS Lett 587:1310–1315
Casper-Lindley C, Björkman O (1998) Fluorescence quenching in four unicellular algae with different light-harvesting and xanthophyll-cycle pigments. Photosynth Res 56:277–289
Chen SW, Beardall J, Gao KS (2014) A red tide alga grown under ocean acidification up-regulates its tolerance to lower pH by increasing its photophysiological functions. Biogeosciences 11:6303–6328
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4:11–37
Gaitán-Espitia JD, Hancock JR, Padilla-Gamiño JL, Rivest EB, Blanchette CA, Reed DC, Hofmann GE (2014) Interactive effects of elevated temperature and pCO2 on early-life-history stages of the giant kelp Macrocystis pyrifera. J Exp Mar Biol Ecol 457:51–58
Gao KS, Xu JT, Gao G, Li Y, Hutchins DA, Huang BQ, Wang L, Zheng Y, Jin P, Cai XN, Häder D, Li W, Xu K, Liu N, Riebesell U (2012) Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat Clim Chang 2:519–523
García-Gómez C, Gordillo FJ, Palma A, Lorenzo MR, Segovia M (2014) Elevated CO2 alleviates high PAR and UV stress in the unicellular chlorophyte Dunaliella tertiolecta. Photochem Photobiol Sci 13:1347–1358
García-Mendoza E, Colombo-Pallotta MF (2007) The giant kelp Macrocystis pyrifera presents a different nonphotochemical quenching control than higher plants. New Phytol 173:526–536
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen Subjects 990:87–92
Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T (2011) Role of PSBS and LHCSR in Physcomitrella patens acclimation to high light and low temperature. Plant Cell Environ 34:922–932
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Halac SR, Villafañe VE, Gonçalves RJ, Helbling EW (2014) Photochemical responses of three marine phytoplankton species exposed to ultraviolet radiation and increased temperature: Role of photoprotective mechanism. J Photochem Photobiol B 141:217–227
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Hiriart-Baer VP, Arciszewski TJ, Malkin SY, Guildford SJ, Hecky RE (2008) Use of pulse-amplitude-modulated fluorescence to assess the physiological status of Cladophora sp. along a water quality gradient. J Phycol 44:1604–1613
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Jin P, Gao K, Villafañe VE, Campbell DA, Helbling EW (2013) Ocean acidification alters the photosynthetic responses of a coccolithophorid to fluctuating ultraviolet and visible radiation. Plant Physiol 162:2084–2094
Johnson MP, Ruban AV (2010) Arabidopsis plants lacking PsbS protein possess photoprotective energy dissipation. Plant J 61:283–289
Klenell M, Snoeijs P, Pedersén M (2004) Active carbon uptake in Laminaria digitata and L. saccharina (Phaeophyta) is driven by a proton pump in the plasma membrane. Hydrobiologia 514:41–53
Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol 19:103–132
Korbee N, Navarro NP, García-Sánchez M, Celis-Plá PSM, Quintano E, Copertino PA, Mariath R, Mangaiyarkarasi N, Pérez-Ruzafa Á, Figueroa FL, Martínez B (2014) A novel in situ system to evaluate the effect of high CO2 on photosynthesis and biochemistry of seaweeds. Aquat Biol 22:245–259
Laviale M, Barnett A, Ezequiel J, Lepetit B, Frankenbach S, Méléder V, Serôdio J, Lavaud J (2014) Response of intertidal benthic microalgal biofilms to a coupled light-temperature stress: evidence for latitudinal adaptation along the Atlantic coast of southern Europe. Environ Microbiol 17:3662–3677
Li XJ, Liu J, Cong YZ, Qu SC, Zhang ZZ, Dai HL, Luo SJ, Han XB, Huang SS, Wang Q, Liang GJ, Sun J, Jin Y, Wang DQ, Yang GP (2008) Breeding and trial cultivation of Dongfang No. 3, a hybrid of Laminaria gametophyte clones with a more than intraspecific but less than interspecific relationship. Aquaculture 280:76–80
Li Y, Xu J, Gao K (2014) Light-modulated responses of growth and photosynthetic performance to ocean acidification in the model diatom Phaeodactylum tricornutum. PLoS One 9:e96173
Liu Y, Xu J, Gao K (2012) CO2-driven seawater acidification increases photochemical stress in a green alga. Phycologia 51:562–566
Lunch CK, LaFountain AM, Thomas S, Frank HA, Lewis LA, Cardon ZG (2013) The xanthophyll cycle and NPQ in diverse desert and aquatic green algae. Photosynth Res 115:139–151
Masojidek J, Kopecká J, Koblížek M, Torzillo G (2004) The xanthophyll cycle in green algae (Chlorophyta): its role in the photosynthetic apparatus. Plant Biol 6:342–349
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol B 137:116–126
McCarthy A, Rogers SP, Duffy SJ, Campbell DA (2012) Elevated carbon dioxide differentially alters the photophysiology of Thalassiosira pseunonana (Bacillariophyceae) and Emiliania huxleyi (Haptophyta). J Phycol 48:635–646
Mou S, Zhang X, Ye N, Miao J, Cao S, Xu D, Fan X, An M (2013) Analysis of ΔpH and the xanthophyll cycle in NPQ of the Antarctic sea ice alga Chlamydomonas sp. ICE-L. Extremophiles 17:477–484
Ocampo-Alvarez H, García-Mendoza E (2013) Antagonist effect between violaxanthin and de-epoxidated pigments in nonphotochemical quenching induction in the qE deficient brown alga Macrocystis pyrifera. Biochim Biophys Acta Bioenerg 1827:427–437
Olischläger M, Wiencke C (2013) Ocean acidification alleviates low-temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (Rhodophyta). J Exp Bot 64:5587–5597
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–521
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Raybaud V, Beaugrand G, Goberville E, Delebecq G, Destombe C, Valero M, Davoult D, Morin P, Gevaert F (2013) Decline in kelp in west Europe and climate. PLoS One 8:e66044
Redmond S (2013) Effects of increasing temperature and ocean acidification on the microstages of two populations of Saccharina latissima in the Northwest Atlantic. Master’s thesis. University of Connecticut
Roleda MY (2009) Photosynthetic response of Arctic kelp zoospores exposed to radiation and thermal stress. Photochem Photobiol Sci 8:1302–1312
Schlüter L, Lohbeck KT, Gutowska MA, Gröger JP, Riebesell U, Reusch TB (2014) Adaptation of a globally important coccolithophore to ocean warming and acidification. Nat Clim Chang 4:1024–1030
Swanson AK, Fox CH (2007) Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs. Glob Chang Biol 13:1696–1709
Thayer SS, Bjorkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331e343
Trimborn S, Thoms S, Petrou K, Kranz SA, Rost B (2014) Photophysiological responses of Southern Ocean phytoplankton to changes in CO2 concentrations: short-term versus acclimation effects. J Exp Mar Biol Ecol 451:44–54
Tseng CK (2001) Algal biotechnology industries and research activities in China. J Appl Phycol 13:375–380
Vonshak A, Torzillo G, Masojidek J, Boussiba S (2001) Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ 24:1113–1118
Wang Y, Xu D, Fan X, Zhang X, Ye N, Wang W, Mao Y, Mou S, Cao S (2013) Variation of photosynthetic performance, nutrient uptake, and elemental composition of different generations and different thallus parts of Saccharina japonica. J Appl Phycol 25:631–637
Wernberg T, Thomsen MS, Tuya F, Kendrick GA, Staehr PA, Toohey BD (2010) Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol Lett 13:685–694
Wu Y, Campbell DA, Gao K (2014) Faster recovery of a diatom from UV damage under ocean acidification. J Photochem Photobiol B 140:249–254
Xu D, Wang D, Li B, Fan X, Zhang XW, Ye NH, Zhuang Z (2015) Effects of CO2 and seawater acidification on the early stages of Saccharina japonica development. Environ Sci Technol 49:3548–3556
Xu J, Gao K (2012) Future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol 160:1762–1769
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine containing mobile phases. Mar Ecol Prog Ser 195:29–45
Zhang X, Mou S, Cao S, Fan X, Xu D, Ye N (2014) Roles of the transthylakoid proton gradient and xanthophyll cycle in the non-photochemical quenching of the green alga Ulva linza. Estuar Coast Shelf Sci 163:69–74
Zou D, Gao K (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia 48:510–517
Funding
This work was supported by National key research and development program of China (2018YFD0901503, 2018YFD0900703, 2016YFC1402102); Major Scientific and Technological Innovation Project of Shandong Provincial Key Research and Development Program (2019JZZY020706); National Natural Science Foundation of China (41976110; 41676145); Shandong key Research and Development Plan (2018GHY115010); Special Scientific Research Funds for Central Non-Profit Institutes, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (20603022019006, 20603022016001); Youth Talent Program Supported by Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018-MFS-01); Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (NO. 2018SDKJ0406-3); China Agriculture Research System (CARS-50).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 424 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Xu, D., Guan, Z. et al. Elevated CO2 concentrations promote growth and photosynthesis of the brown alga Saccharina japonica. J Appl Phycol 32, 1949–1959 (2020). https://doi.org/10.1007/s10811-020-02108-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02108-1