Abstract
Cultures of three native macroalgal species (Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei) were developed in open coastal waters of a shallow, potentially eutrophic bay in Southern Spain. Experimental trials to assess the technical feasibility of seaweed cultivation and nitrogen biofiltration potential were conducted between October 2014 and December 2015. Seaweeds were cultivated using submersible rafts in five periods of 9 weeks to identify the most suitable seasons and culture duration (3, 6 or 9 weeks). Using generalized linear models (GLMs), the most relevant environmental factors controlling the growth of the three seaweeds were identified. Maximum net growth rates varied between 1.39% day−1 in C. teedei (winter, 6 weeks) and 4.71% day−1 in G. longissima (autumn, 3 weeks). Overall, the best period to cultivate seaweeds in Cadiz Bay was from mid-winter to early summer. No clear effects of duration of the culture were observed for C. teedei and G. bursa-pastoris. Short culture periods of 3 weeks were more suitable for G. longissima. Tissue N contents generally were lower than the critical quota, and GLMs suggested a critical role of N limiting seaweed growth in this bay. Dissolved inorganic nitrogen was biofiltered from winter to early summer and revealed G. bursa-pastoris as the main biomitigator of nitrogen (up to 80 mg N m−1 month−1 in spring). This species also showed positive growth rates virtually during the entire study period and was the most suitable species for cultivation in this area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macroalgae are used for many purposes since ancient times (Indergaard and Minsaas, 1991), including food, medication, agriculture or animal feed (Buchholz et al. 2012, Mouritsen et al. 2019) and currently are an important resource for the food and pharmaceutical industrial sector (Dhargalkar and Verlecar 2009). Today the global seaweed industry is worth more than US$ 6 billion per annum (FAO 2018). In 2015, the annual production of seaweeds and other aquatic plants harvested from wild stocks was slightly over 1.2 million tonnes fresh weight, a figure similar to that of 2005. In contrast, the aquaculture production of seaweeds was 29.4 million tonnes fresh weight, doubling the figures of 2005 (FAO 2018). In spite of this global context, the situation in Europe is different, and aquatic production (i.e. aquaculture and wild-caught) is stagnant since the end of the twentieth century, with little relevance for seaweed aquaculture.
The European Union considers aquaculture as extremely important for blue growth economy and the 2020 strategy for Europe. Seaweed aquaculture is regarded as a clean and eco-friendly economic activity, providing essential ecological services in coastal waters (Cabral et al. 2016). For instance, cultivated seaweeds can act as habitat forming species, increasing biodiversity and acting as nursery areas for species of commercial interest (Walls et al. 2016; Walls et al. 2019). In addition, as harvesting of seaweed biomass implies a removal of nutrients from coastal waters, seaweed aquaculture has been proposed as a biomitigation action for eutrophic and potentially eutrophic areas (Fletcher 1996; Xu et al. 2011). This becomes especially relevant in a global context of increasing nutrient loadings from non-point sources (Le Moal et al. 2019), where seaweed aquaculture arises as an additional tool to reduce nutrient over-enrichment and associated eutrophication of coastal waters (Kim et al. 2014; Yang et al. 2015; Xiao et al. 2017).
Despite its economic and environmental benefits, the development of macroalgal cultivation in Europe is still in its infancy due to the historically low demand of seaweeds. In contrast with eastern countries (Xia and Abbott 1987; Indergaard and Minsaas 1991), the use of macroalgae for culinary purposes in western countries was anecdotal until the end of the twentieth century (Mouritsen et al. 2019). Although the production of phycocoloids became very important in Spain since the middle of last century (Tasende and Peteiro 2015), most of the biomass used came from the harvesting of natural populations. Nevertheless, in recent decades, the demand of seaweeds as food in Europe is increasing (Pérez-Lloréns et al. 2018; Mouritsen et al. 2019), and some innovative projects in countries such as Ireland or France are being developed (Taelman et al. 2015). Also, on the northwestern coast of Spain, there have been promising results for the culture of Saccharina latissima on the basis of IMTA (integrated multitrophic aquaculture) projects (Freitas et al. 2016), and previous initiatives with other species (Palmaria palmata, Undaria pinnatifida) showed great improvement (Martínez et al. 2006; Peteiro and Freire 2011) but did not succeed due to several reasons such as administrative obstacles or environmental constraints of the species.
The growth of macroalgae is controlled by an array of physical and biological factors such as temperature, photoperiod, water motion, dissolved nutrients or grazing (e.g. Yokoya et al. 1999; Wakibia et al. 2006; Peteiro and Freire 2011). Therefore, knowing of how environmental variables influence field cultures is of paramount importance for the initial steps of the seaweed industry in a particular coastal zone. In this context, the use of exploratory statistical models is very useful in gaining an insight into the importance of the main variables controlling macroalgal growth throughout the year (Lapointe and Bedford 2011).
This study is the first attempt to develop macroalgal cultures in open coastal waters in Southern Spain. So far, only cultures of Gracilariopsis longissima attached to ropes have been carried out in earthen ponds to biomitigate the water nutrient concentration outflowing from a fish farm (Hernández et al. 2006). Also, experimental cultures have been carried out in traditional salinas (Bermejo et al. 2019) as a complement to the traditional salt industry. The demand for seaweeds in the region is still low, although it is increasing every year (Pérez-Lloréns et al. 2018). The aim of the present study included (i) to assess the suitability of the cultivation of three native species of interest for the food industry (Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei) in a shallow, potentially eutrophic bay; (ii) to identify the most relevant environmental factors constraining growth in order to optimize culture conditions; and (iii) to estimate the potential of the three species to biofilter dissolved nitrogen in Cadiz Bay.
Materials and methods
Study site
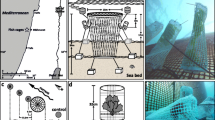
This study was conducted from October 2014 to December 2015 at a selected site in the inner bay of Cadiz; Southern Spain (approx. 36° 28′ 17″ N, 6° 14′ 39″ W), a shallow protected embayment close to a beach named Santibáñez (Fig. 1). The zone is part of the European Nature 2000 Special Area of Conservation ‘Marine bottoms of Cadiz Bay’. The seabed is dominated by the seagrass Cymodocea nodosa (Olivé et al. 2013) and a rich community of marine macroalgae (Hernández et al. 2010). Seawater usually shows low or moderate nutrient levels, with occasional spikes in dissolved inorganic nitrogen or phosphate after heavy rainfall or incoming tidal surge (Morris et al. 2009; Vergara et al. 2012). Despite the good conservation status of the bay, it is also subjected to important anthropogenic pressures as a consequence of high population density and industrial development, and thus it is considered as a potentially eutrophic area (Gómez-Parra and Forja 1992).
Selected species
Three autochthonous macroalgal species were selected for cultivation: Gracilariopsis longissima (S.G. Gmelin) M. Steentoft, L.M. Irvine & W.F. Farnham, Gracilaria bursa-pastoris (S.G. Gmelin) P.C. Silva and Chondracanthus teedei (Mertens ex Roth) Kützing. These species can be found throughout the year in the wild and are of interest for the food industry, although other uses cannot be ruled out. Gracilariopsis longissima is usually found in shallow creeks or in channels near sluice gates of earthen ponds (Pérez-Lloréns et al. 2004). The species is currently consumed in local restaurants and is also a potential resource for biotechnological purposes as a source of lipids or phycocolloids (Stabili et al. 2012). Gracilaria bursa-pastoris thrives within the inner bay and is generally entangled with other macrophytes. It renders a high agar yield of superior quality (Marinho-Soriano 2001), and this is why it has been used in integrated aquaculture projects (Korzen et al. 2016). Finally, C. teedei grows on stones in channels of relatively high tide fluxes. This edible macroalgae is also a valuable source of carrageenan (Chapman and Chapman 1980; Soares et al. 2016).
Physico-chemical data
Once a week during the period of algal cultivation, different environmental variables were monitored. Water temperature, dissolved oxygen and pH were measured at 30 cm depth with a multiparametric probe (sensION MM156 Hach). Salinity was determined using a refractometer (ATAGO). Water samples were collected in triplicate to measure suspended solids (SS) and dissolved inorganic nutrients (nitrate, nitrite, ammonium and phosphate). In the case of SS, 5 L of seawater were collected and immediately transported to the laboratory. The water was shaken to re-suspend solids prior to filtration. Between 1 and 0.5 L of water (depending on the amount of SS) was filtered through a glass fibre filter (Whatman GF/F; effective pore size 0.7 μm). Filters were dried in a desiccation oven at 60 °C for 24 h before the filtration and 48 h after the filtration. Concentration was estimated as the difference in weight of the dried filter after and before filtration divided by the filtered volume. Dissolved inorganic nutrients were estimated from water samples of 10 mL, filtered in situ (GF/F Whatman filter) and determined in a Skalar SAN++ CFA autoanalyzer following standard methods (Grassoff et al. 1983). When available for the study period, additional data of dissolved nutrients were obtained from station 62C2130 of the network of environmental information of Andalusia (REDIAM; http://www.juntadeandalucia.es/medioambiente/site/rediam), which was located in the zone of cultivation.
Raft cultivation
Immediately after collection, harvested seaweeds were transported to the laboratory in a cooler box. Healthy fragments were selected, and visible epiphytes were carefully removed with a wet cloth and a brush. Then, propylene braided ropes (1 cm thickness; ca. 1.3 m long) were seeded with 15–20 g algae (18 tufts) per rope and weighed (fresh weight; fw). Seeded fragments ranged between 12 and 16 cm for G. longissima and 8 and 12 cm for G. bursa-pastoris and C. teedei. Seeded ropes were maintained in 50-L aquaria with aeration and subsequently transported to the experimental site in a cool box, wrapped with humid papers and kept inside plastic bags to avoid desiccation and thermal stress. All the seeded ropes were planted in rafts within 24 h after collection. Rafts consisted of PVC submersible frames of 2.2 m long × 1.3 m wide, which allowed for nine parallel seeded ropes anchored to the frame with plastic shackles (Bermejo et al. 2019). Physical interaction among ropes was minimized by setting a distance of 18 cm between them. Three rafts were placed in the upper subtidal zone and separated 25–50 m following method adopted by Anderson et al. (1999). Rafts were anchored to the bottom with thick ropes and four concrete blocks of 8 kg each and were secured in place by four buoys positioned in each corner. The rafts remained horizontal and submerged at 50 cm depth and occasionally emerging for less than 2 h during extreme spring tides.
Culture monitoring
The study was conducted for five periods of 9 weeks, referred vaguely as autumn 2014 (starting on October 24), winter 2015 (starting on January15), spring 2015 (starting on March 20), summer 2015 (starting on July 30) and autumn 2015 (starting on October 2). To identify the most suitable season and duration of cultivation for each species, algae were harvested after 3, 6 and 9 weeks of growth in any period. Three ropes for each species were collected in each harvesting occasion. Thalli were removed, cleaned carefully and weighed to determine the increase in biomass as well as relative growth rates. The algae were placed in a portable centrifuge to gently drain off excess water. Yield (Y) was calculated following Eq. 1:
where Bt the final algal wet weight for each rope (mg), B0 is the initial biomass, t is the cultivation period (days), dw is the dry weight and L is the length of the rope (m). To estimate dw–fw conversions, samples of macroalgae were dried in the laboratory (5 days in a desiccation oven at 60 °C) after the cultivation period.
To calculate the relative daily growth rate (DGR), an exponential growth was assumed (Eq. 2):
where fwf is the final fresh weight after t days of culture and fw0 is the initial fresh weight.
To evaluate the influence of the internal content of nitrogen (N) on growth, positive net growth values were plotted against tissue N concentrations according to the Droop equation (Droop, 1983):
where DGRmax is the maximal growth rate, NQ is the minimal tissue N content needed to sustain growth (the subsistence N quota; in mg g−1 dw), and N is the actual tissue nitrogen concentration in the seaweed.
The critical N quota (NC) is the necessary concentration of tissue N to maintain growth at the maximum possible rate and has been estimated as the cut-off point between the minimum slope and the maximum growth, with the minimum slope being the line joining the points between DGR = 0 and DGR = 0.5 DGRmax (Pedersen and Borum 1996).
The tissue N content was estimated from macroalgal samples collected before and after cultivation. The samples were dried (5 days at 60 °C) and ground. The N content was estimated after combustion at 1050 °C in a LECO CHNS-932 Elementary Chemical Analyser. Nitrogen was measured as NOx.
Biomitigation assessment
The biomitigation capacity of all species was computed according to the following expression:
where Nt and N0 (mg N g−1 dw) are the tissue N contents at the end and at the beginning of the cultivation period, respectively.
Statistical analysis
The cultivation trial of the three species was aimed to assess the effects of ‘season’ (four levels: autumn, winter, spring and summer) and ‘duration of culture period’ (three levels: 3, 6, 9 weeks) on the growth rate, following a randomized complete block design. In the case of G. longissima and G. bursa-pastoris, where there were data for autumn 2014 and autumn 2015, a third factor (year) was considered and was nested with ‘season’. Three-way ANOVAs for G. longissima and G. bursa-pastoris and two-way ANOVAs in the case of C. teedei were tested to assess the effect of the different factors on growth rate. Previously, data were assessed for normality and homoscedasticity (Shapiro-Wilks and Levene tests, respectively). The level of significance was set at 5% probability, excluding G. longissima, which was confined to 1% to reduce type I error, as the normality hypothesis did not fulfil for some interactions, in spite of the fact that the data were homoscedastic (Underwood 1997).
To identify the main variables influencing DGR, a generalized linear model (GLM) was used, assuming Gaussian distribution due to the existence of negative data (Vaz-Pinto et al. 2013). The model has been shown to be a suitable approach to assess the significance of the main variables controlling macroalgal growth (Lapointe and Bedford 2011). To avoid collinearity issues and to make the interpretation of results easier, the variable Dissolved Inorganic Nitrogen (DIN) concentration was used instead of [NO3−], [NO2−] and [NH4+]. Suspended solids and phosphates also showed a strong correlation (r > 0.7), and only the former was retained for GLM. Similarly, a strong correlation was observed between solar irradiance and temperature, and only temperature was retained for the analysis. Thus, the following explicative variables were included in the model: mean water temperature, tidal coefficient, DIN, tissue N content, SS, salinity and days of cultivation. Meteorological data were obtained from the historical data of the REDIAM. The selection criterion and selection procedure used were ‘best subset’ and ‘Akaike information criteria’, respectively. The percentage of changes in DGR explained by the model was calculated according to (Zuur et al. 2009) the following:
where Nd is the null deviance and Rd is the residual deviance obtained from the linear model.
Statistical analyses were carried out using the software R-program (R Development Core Team 2011).
Results
Environmental factors
Table 1 shows the physical and chemical variables monitored throughout the study period. Seawater temperature ranged between 9.5 and 28.4 °C, with maximum mean values in August and minimum in February. The pH ranged between 7.83 and 8.80. The greater mean values were measured in spring. These two variables followed a marked seasonal pattern. Salinity ranged between 31 and 41 ‰ but did not show a clear pattern as it was influenced by rainy periods or high evaporation within the bay and surrounding saltmarshes and the managing of water fluxes in traditional salinas. The greatest mean salinities were recorded in winter and spring. Dissolved oxygen concentrations were highest during the spring, with values ranging from 5.3 and 12.8 mg L−1. Finally, SS showed marked differences as they were influenced by the wind. Values reached up to 253 mg L−1 during strong eastern winds, which occur specially during the summer.
Ammonium was the main form of DIN throughout the study period. Overall, concentrations were highest during the winter, after the season of heavy rainfall (data not shown). Highest concentrations were normally associated with the mixing of the water column due to strong windy conditions and river discharges in the basin after heavy rainfall. Phosphate was generally lower than 0.5 μM (Table 1).
Relative growth rates and yield
Gracilariopsis longissima
The statistical data showed that the relative growth rate of G. longissima was affected significantly by the duration of culture period, season and year (Table 2). Generally, the longer the cultivation period, the lower the growth rates (Fig. 2a). The DGR was even negative during part of the year, particularly after 9-week period of cultivation, when many thalli were partially broken. Overall, winter and early spring were the most recommended period for the cultivation of this species, although the highest rate was obtained in autumn 2014 after 3 weeks of cultivation (4.71% day−1). Yield was highest in early spring; i.e., 195.04 mg dw m−1 day−1 (1.905 g fw m−1 day−1) produced during the first 3 weeks.
The GLM (Table 3) suggested that the most important environmental variables explaining growth were tissue N content (p < 0.001), period of cultivation (p < 0.001), seawater temperature (p = 0.027) and the interaction between DIN and period of cultivation (p < 0.001). According to the model, the linear combination of these variables explained 61.15% of the changes in DGR.
Gracilaria bursa-pastoris
The statistical data showed that DGR of G. bursa-pastoris was influenced significantly by season and year. The interaction between the duration of culture period and season was also significant (Table 4). Relative growth rates were positive throughout the study period, with maximum values reached in autumn 2014 (3.47% day−1) and spring 2015 (3.24% day−1) after 3 and 6 weeks of cultivation, respectively. Growth rates were usually higher when the period of cultivation was longer (Fig. 2b). Due to the reliability of the observed DGRs (mean coefficient of variation we 16.27%) for the considered culture periods (i.e., 3, 6 and 9 weeks), it was suitable to estimate DGR every 3 weeks (Fig. 3). The pattern suggested that G. bursa-pastoris could be cultivated in the bay from mid-winter to the end of the summer. On the contrary, the low or even negative growth rates in autumn (both in 2014 and 2015) indicated the less favourable period for cultivation. The highest yield was obtained in spring (325.24 mg dw m−1 day−1; 1.91 g fw m−1 day−1), after 9 weeks of cultivation.
According to the GLM (Table 3), only the interaction between the duration of the culture period and the tissue N content showed a significant effect in the growth rate of this species (p < 0.001). The combination on these variables explained 44.31% of the changes in DGR.
Chondracanthus teedei
The statistical data showed that DGR of C. teedei was not significantly affected by any of the tested factors (data not shown). Mean values did not show a clear pattern and were relatively low throughout the period of study, with negative rates found during autumn 2015 when thalli were cultivated for more than 3 weeks (Fig. 2c). The highest DGR was found in winter after 6 weeks of cultivation (1.39% day−1). Yield was greatest in summer, after 3 weeks of cultivation (93.55 mg dw m−1 day−1; 495 mg fw m−1 day−1).
For this species, the GLM suggested that the net growth rate was significantly influenced by DIN, tissue N, salinity, the length of the cultivation period and the interaction between DIN and tissue N (Table 3). However, the linear combination of all variables explained only 23.36% of the net growth rate of this species.
Tissue N and biofiltration
Gracilariopsis longissima
Figure 4 a shows the tissue N content of G. longissima during the study period. The maximum N content was estimated in winter after 3 weeks of cultivation (3.29%). During this time, values were even higher than NC (the critical quota, considered at ca. 2%). Values were always higher than NQ but generally lower than Nc, which indicated that algae were growing below the maximum theoretical DGR for most of the study period.
Seasonal variation of the tissue N content in thalli of Gracilariopsis longissima (a), Gracilaria bursa-pastoris (b) and Chondracanthus teedei (c). Error bars represent the standard deviation (n = 3). Horizontal lines within the figures represent reference values for the critical (NC) and subsistence (NQ) quota for N
In this species, the positive DGR was related to the tissue N, as shown by the significant fit to the Droop model (R2 = 0.5; p < 0.01; Fig. 5). According to the model, the maximum theoretical growth rate in Cadiz Bay was 4.29 ± 0.51% day−1, the value of NQ was 0.54 ± 0.08% and NC was 1.85 ± 1.00%. In addition, N content in G. longissima was directly related to the ammonium concentrations in the sampling station (Table 1), as suggested by the high significant relationship between both variables (Tissue N = 0.2022 [NH4+] + 0.7074; F1,14 = 34.51, p < 0.001, R2 = 0.73).
From the values of tissue N and the total net biomass after and before any period of cultivation, the computed biofiltration rate showed that G. longissima biofiltered effectively DIN only during the winter (Fig. 6a), when the macroalgae showed positive net growth rate throughout the season. Maximum mean biofiltration rates were estimated in 34.51 ± 10.88 mg N m−1 month−1.
Gracilaria bursa-pastoris
The tissue N content in G. bursa-pastoris revealed a clear seasonal pattern (Fig. 4b), with values higher than NC at the end of autumn 2014 and winter 2015, which did not correspond with periods of maximum growth rates. By contrast, tissue N content decreased dramatically in summer, when values nearly reached the theoretical NQ. No relationship was found between DGR and tissue N, but, as it was also found for G. longissima, the tissue N content in G. bursa-pastoris was significantly related to the ammonium concentration in the cultivation site (Tissue N = 0.3301 [NH4+] + 0.5796; F1,14 = 17.64, p < 0.01, R2 = 0.57).
Gracilaria bursa-pastoris biofiltered DIN from the bay during most of the year (Fig. 6b) with the exception of autumn 2015, when no positive rates were found. The estimated mean biofiltration rates reached 80.42 ± 31.36 mg N m−1 month−1 during the spring, when the maximum DGRs were measured.
Chondracanthus teedei
The tissue N content of C. teedei also showed a clear seasonal pattern (Fig. 4c), with the highest values found in winter and spring and then a decrease in N content during summer and autumn. All concentrations, however, were always lower than Nc, which suggested that the macroalgae were never growing at their maximal potential. Similarly to G. bursa-pastoris, the Droop model did not explain a possible relationship between growth rate and tissue N. There was no significant relationship found either between tissue N content and ammonium or nitrate in the seawater from the cultivation site, although significance for nitrate was marginal (F1,23 = 3,43, p = 0.067, R2 = 0.33). Regarding DIN biofiltration, C. teedei showed positive rates during winter and spring, when tissue N was greater. Maximum mean rates of biomitigation were estimated after 6 weeks of cultivation in spring (25.22 ± 17.71 mg N m−1 month−1).
Discussion
The present investigation addressed the possibility of cultivation of three common and valuable red seaweeds in the bay of Cadiz. This is the first study to investigate field cultivation of seaweeds in open waters in southern Spain, where the demand of marine macroalgae is increasing (Pérez-Lloréns et al. 2018; Mouritsen et al. 2019). At the same time, one of the main ecological services provided by macroalgal cultures (biomitigation of nutrients; Fletcher, 1996) has been explored as an additional tool to tackle potential coastal eutrophication. Cultures of G. longissima and C. teedei in earthen ponds were previously carried out (Hernández et al. 2006; Bermejo et al. 2019), but this is the first time that cultures of G. bursa-pastoris have been tested in Spain.
Relative growth rates and yield
Studies of G. longissima showed higher net growth rates up to 6.0% day−1 in Cadiz Bay, during the winter in the outflow reservoir of a fish farm (Hernández et al. 2006), or up to 8% day−1 measured in natural populations of this species in a nearby tidal creek (Pérez-Lloréns et al. 2004). The maximum DGRs measured during the present study were similar to those reported by Bermejo et al. (2019) in a nearby traditional salina (3.2% day−1), Álvarez-Gómez et al. (2019) in laboratory cultures (ca. 4% day−1) or He et al. (2014) in an IMTA mesocosm system of G. longissima and the fish Sciaenops ocellatus (3.0% day−1). However, G. longissima in this study showed long periods of net biomass losses, and generally, net growth rates were not sustained for more than 3 weeks. Considering that the N content in G. longissima was sufficient to sustain growth (i.e. tissue N content higher than NQ; Fig. 6a) during the entire study period, the observed negative growth rates might be a consequence of the biomass loss by grazing or thalli breaking due to the hydrodynamic conditions. The only time of the year when the culture of G. longissima seems to be feasible was winter and early spring. This growth pattern partially agreed with previous studies developed in this area, which identify winter and mid spring as suitable periods (from November to April) for the growth of this species, and late spring and early summer (May and June) as unsuitable (Pérez-Lloréns et al. 2004; Bermejo et al. 2019).
The relative net growth rates of G. bursa-pastoris obtained in this study (3.5% day−1) were lower than those found for this species in the Thau lagoon (up to 6.2% day−1; De Casablanca et al. 1997) or in an IMTA study in Israel (up to 10% day−1; Korzen et al. 2016). These differences could be attributed to two main reasons: (i) De Casablanca et al. (1997) and Korzen et al. (2016) cultivated the macroalgae in cages, which reduces the loss of biomass by thalli breakage and grazing by macrofauna; and (ii) nutrient availability in both coastal ecosystems were higher than that in Cadiz Bay. The tissue N content observed for G. bursa-pastoris in this study was lower than the critical quota for most of the year, which supported this explanation. The observed seasonal growth pattern agreed with that described by Marinho-Soriano et al. (1998) in the Thau Lagoon, where this seaweed exhibited a marked seasonal peak in late spring.
The maximum net growth rates in C. teedei (1.6% day−1) were lower than those found in thalli cultured in a traditional salina (2.0% day−1; Bermejo et al. 2019) and also in those estimated in the closely related species Chondracanthus chamissoi in Chile (ca. 4% day−1; Bulboa et al. 2005), despite that rates in the latter were less than 2% day−1 for a large part of the year. The growth rates observed in this study could be partially explained by nitrogen limitation, as tissue N was always lower than NC. However, considering the results obtained by the ANOVA and GLM, other factors or combination of factors not considered in this model might explain the growth pattern of this species. Overall, DGRs obtained in suspended rope cultivation in the field were far from the ones observed by Zinoun (1993) under controlled conditions in laboratory, which were close to 8% day−1. This species showed the higher relative growth rates from winter to late spring (Pereira and Mesquita 2004; Bermejo et al. 2019). However, no clear seasonal pattern in growth was observed during this study, which indicated that culture conditions were suboptimal.
Maximum yield varied between ca. 94 mg dw m−1 day−1 (C. teedei) and 325 mg dw m−1 day−1 (G. bursa-pastoris) (495–1911 mg fw m−1 day−1). Regarding the Gracilariales, the maximum mean yield in G. longissima (1905 mg fw m−1 day−1) almost trebled mean values found in earthen ponds under high hydrodynamic conditions (745 mg fw m−1 day−1; Bermejo et al. 2019). However, high production was only sustained for less than a month. Even when yield was highest for G. bursa-pastoris, net production was far from maximum yields obtained for Gracilaria lemaneiformis in integrated open cultures with fish species Sebastodes fuscescens in north China (maximum 120 ± 19.5 g fw m−1 day−1; Zhou et al. 2006) or Gracilaria chilensis cultivated with salmon in Chile (8.67 g fw m−1 day−1; Halling et al. 2005). These preliminary results show the possibility of further improvements in the cultivation of these species in Cadiz Bay. For C. teedei, maximum yield values were half of the net production reported by Bermejo et al. (2019) in winter under high seedling density and high hydrodynamics (965 ± 324 mg fw m−1 day−1), which significantly improved the culture of this species.
The GLMs and tissue N contents (Fig. 4) indicated a crucial role of nitrogen limitation controlling the growth of the three species cultivated in Cadiz Bay. Tissue N contents were below the critical quota for most of the year (Pedersen and Borum 1997; Pedersen and Johnsen 2017), which might limit the potential growth as well as the fitness and ability to cope with environmental stress (Kumar et al. 2010; Parages et al. 2014; Van Alstyne 2018). The GLMs also indicated a relevant role of culture duration, which was especially significant in the case of G. longissima. This species, as well as C. teedei, usually showed lower DGR when cultivated during longer periods (Fig. 2), which might be related to the more likely occurrence of stressful events over their tolerance limits as the duration of culture increased. These two species were naturally present in the surrounding area of Cadiz Bay, in sites of low irradiance with enhanced water flowing conditions, such as the lock gates of salinas, narrowing channels or bridge spans (Bermejo et al. 2019). Although in the inner bay of Cadiz key environmental factors for the development of macrophytes, such as salinity, nutrient availability or temperature (Hurd et al. 2014) showed a broad range of variability, this range was similar or even lower to those found in adjacent tidal creeks and saltmarshes, where these species thrive. Thus, in order to optimize the cultures of the two species, light and water motion conditions should be adjusted together with the increase in nitrogen availability. Particularly, the enhancement of DIN availability would greatly improve yield and growth of G. bursa-pastoris, the most suitable seaweed species for culture in Cadiz Bay.
Tissue N and biofiltration
Nitrogen availability was essential to regulate the growth of the three species. In fact, in spite that tissue N content was determined from macroalgae cultivated in the field, the value of the NC estimated for G. longissima was close to that reported in the literature (2.14%) for Gracilaria vermiculophyla (Pedersen and Johnsen 2017) or the reference value of 2% suggested for macroalgae (Hanisak 1983; Wheeler and Björnsäter 1992) and so was NQ, which was close to values reported for similar macroalgal species by Pedersen and Borum (1997) or slightly lower than NQ suggested by Pedersen and Johnsen (2017) in G. vermiculophyla (0.706 ± 0.095%). In addition, tissue N content was strongly related with external ammonium in the two Gracilariales. The importance of ammonium for the growth of Gracilaria was pointed out by Naldi and Wheeler (1999), who found that tissue N content increased more under ammonium enrichment that under nitrate enrichment in Gracilaria pacifica. Abreu et al. (2011a) and Pedersen and Johnsen (2017) also highlighted the importance of ammonium in the tissue N accumulation in G. vermiculophyla due to its higher uptake efficiency vs. nitrate under a large range of ambient concentrations.
The results also showed that the three species were able to biomitigate DIN, at least during part of the year, which suggested the usefulness of nutrient biofiltration through native seaweeds culture as a mitigation measure to manage coastal eutrophic waters. The bioremediation potential was especially relevant during the winter, when the three species showed positive net rates, and during the spring, when G. bursa-pastoris and C. teedei reached the maximum biofiltration rates. Although biomitigation was generally lower than that obtained under high dissolved nitrogen concentration from a fish farm (ca. 510 mg N m−1 month−1; Hernández et al. 2002 for Gracilariopsis longissima), the nutrient biofiltration estimated in this study contributes to the natural biofiltration process carried out by wild macroalgae and seagrass populations of the bay (Van Engeland et al. 2011). Considering a mean biofiltering value of our species of 25 mg N m−1 month−1 during the winter, each raft biomitigated ca. 2.5 g N in this season, which would be retained as algal biomass. A conservative value of 750 rafts per hectare might biomitigate ca. 0.660 kg N ha−1. However, these hypothetical estimations are far from those calculated by Kim et al. (2015) in a eutrophic estuary, using longline cultures of the kelp Sacharina latissima as a biomitigator (10–139 kg N ha−1 year−1). Scaling the cultures would also help to mitigate the effect of spikes of nutrients from a nearby river after heavy rainfall or from discharges of several fish farms in the area. That is especially important in Cadiz Bay as a marine protected area.
Conclusions and further recommendations
The cultures could benefit from several improvements in the raft cultivation method. The importance of enhanced water motion on the growth rate has been demonstrated (Ryder et al., 2004; Bermejo et al. 2019) as well as the strong effect of dissolved nutrients on the increase of yield and growth rate as different studies have shown when dealing with IMTA projects (Abreu et al. 2011b; He et al. 2014). It is possible to find suitable conditions in the bay, close to the main channel and fish farms; however, these deployments would require permission from the administrative authorities. The depth where rafts are placed can also be adjusted so that irradiance can be partially controlled. As mentioned above, G. longissima and C. teedei prefer conditions of low irradiance (Bermejo et al. 2019), and, possibly, this is one of the reasons why these two species grew better in winter.
In conclusion, the experiments carried out with submersible rafts to cultivate three native macroalgae in Cadiz Bay (G. longissima, G. bursa-pastoris and C. teedei) suggested that G. bursa-pastoris is the most suitable one for cultivation. The scaling-up and technical improvements of the cultivation method can lead to a profitable and eco-friendly activity that increases seaweed production, especially of those with the highest demand for consumption in the region. In addition, the seaweed aquaculture of the macroalgae could contribute to maintain the water quality of the bay, an area where several fish farms are currently in operation.
References
Abreu MH, Pereira R, Buschman AH, Sousa-Pinto Yarish C (2011a) Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. J Exp Mar Biol Ecol 407:190–199
Abreu MH, Pereira R, Yarish C, Buschman AH, Sousa-Pinto (2011b) IMTA with Gracilaria vermiculophylla: productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312:77–87
Álvarez-Gómez F, Korbee N, Figueroa FL (2019) Effects of UV radiation on photosynthesis, antioxidant capacity and the accumulation of bioactive compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J Phycol 55:1258–1273
Anderson RJ, Smit AJ, Levitt GJ (1999) Upwelling and fish-factory waste as nitrogen sources for suspended cultivation of Gracilaria gracilis in Saldanha Bay, South Africa. Hydrobiologia 398-399:455–462
Bermejo R, Macías M, Cara CL, Sánchez-García J, Hernández I (2019) Culture of Chondracanthus teedei and Gracilariopsis longissima in a traditional salina from southern Spain. J Appl Phycol 31:561–573
Buchholz CM, Krause G, Buck BH (2012) Seaweed and man. In: Wiencke C, Bischof K (eds) Seaweed biology. Springer, Dordrecht, pp 471–493
Bulboa CR, Macchiavello JE, Oliveira EC, Fronck E (2005) First attempt to cultivate the carrageenan-producing seaweed Chondracanthus chamissoi (C. Agardh) Kützing (Rhodophyta; Gigartinales) in Northern Chile. Aquac Res 36:1069–1074
Cabral P, Levrel H, Viard F, Frangoudes K, Girard S, Scemama P (2016) Ecosystem services assessment and compensation costs for installing seaweed farms. Mar Policy 71:157–165
Chapman VJ, Chapman DJ (1980) Seaweeds and their uses, 3rd edn. Chapman and Hall, London, p 334
De Casablanca ML, Marinho-Soriano E, Laugler T (1997) Growth of Gracilaria bursa-pastoris in a Mediterranean lagoon: Thau, France. Bot Mar 40:29–37
Dhargalkar VK, Verlecar XN (2009) Southern Ocean seaweeds: a resource for exploration in food and drugs. Aquaculture 287:229–242
Droop MR (1983) 25 years of algal growth kinetics: a personal view. Bot Mar 26:99–112
FAO (Food and Aquaculture Organisation of the United Nations) (2018) The global status of seaweed production, trade and utilization. Globefish Programme 124, FAO, Rome
Fletcher RL (1996) The occurrence of ‘green tide’. In: Schramm W, Nienhuis PH (eds) Marine benthic vegetation: recent changes and the effects of eutrophication. Springer Verlag, Berlin, pp 7–43
Freitas JR, Salinas JM, Cremades J (2016) Sacharina latissima (Laminariales, Ochrophyta) farming in an industrial IMTA system in Galicia (Spain). J Appl Phycol 28:377–385
Gómez-Parra A, Forja JM (1992) Significance of benthic regeneration in nutrient balance in the Bay of Cadiz, south-west Spain (a shallow semi-closed coastal ecosystem). In: Vollenweider RA, Marchetti R, Viviani R (eds) Marine coastal eutrophication: proceedings of an international conference, Bologna, Italy, 21–24 march 1990. Elsevier, Amsterdam, pp 1079–1086
Grassoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analysis. Verlag Chemie, Weinheim, p 419
Halling C, Aroca G, Cifuentes M, Buschmann AH, Troell M (2005) Comparison of spore inoculated and vegetative propagated cultivation methods of Gracilaria chilensis in an integrated seaweed and fish cage culture. Aquacult Int 13:409–422
Hanisak MD (1983) The nitrogen relationship of marine macroalgae. In: Carpenter EJ, Capone DG (eds) Nitrogen in the marine environment. Academic Press, New York, pp 669–730
He QY, Zhang J, Chai Z, Wu H, Wen S, He P (2014) Gracilariopsis longissima as biofilter for an integrated multi-trophic aquaculture (IMTA) system with Sciaenops ocellatus: bioremediation efficiency and production in a recirculating system. Indian J Mar Sci 43:528–537
Hernández I, Martínez-Aragón JF, Tovar A, Pérez-Lloréns J, Vergara JJ (2002) Biofiltering efficiencies for dissolved nutrients in three species of estuarine macroalgae cultivated with sea bass (Dicentrarchus labrax) waste waters 2. Ammonium J Appl Phycol 14:375–384
Hernández I, Pérez-Pastor A, Vergara JJ, Martínez-Aragón J, Fernández-Engo MA, Pérez-Lloréns JL (2006) Studies on the biofiltration capacity of Gracilariopsis longissima: from microscale to macroscale. Aquaculture 252:43–53
Hernández I, Bermejo R, Pérez-Lloréns JL, Vergara JJ (2010) Contribución al conocimiento de los macrofitos marinos del saco interno y caños adyacentes de la bahía de Cádiz. Algas, Bulletin of the Spanish Phycological Society 43:11–16
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Indergaard M, Minsaas J (1991) Animal and human nutrition. In: Guiry MD, Blunden G (eds) Seaweed resources in Europe: uses and potential. John Wiley & Sons, New York, pp 21–65
Kim JK, Kraemer GP, Yarish C (2014) Field scale evaluation of seaweed aquaculture as a nutrient bioextraction strategy in Long Island Sound and the Bronx River Estuary. Aquaculture 433:148–156
Kim JK, Kraemer GP, Yarish C (2015) Use of sugar kelp aquaculture in Long Island Sound and the Bronx River Estuary for nutrient extraction. Mar Ecol Prog Ser 531:155–166
Korzen L, Abelson A, Israel A (2016) Growth, protein content and carbohydrate contents in Ulva rigida and Gracilaria bursa-pastoris integrated with an offshore fish farm. J Appl Phycol 28:1835–1845
Kumar M, Kumari P, Gupta V, Reddy CRK, Jha B (2010) Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J Exp Mar Biol Ecol 391:27–34
Lapointe BE, Bedford BJ (2011) Stormwater nutrient imputs favour growth of non-native macroalgae (Rhodophyta) on O'ahu, Hawaiian Islands. Harmful Algae 10:310–318
Le Moal M, Gascuel-Odoux C, Ménesguen A, Souchon Y, Étrillard C, Levain A, Moatar F, Pannard A, Souchu P, Lefebvre A, Pinay G (2019) Eutrophication: a new wine in an old bottle? Sci Total Environ 651:1–11
Marinho-Soriano E (2001) Agar polysaccharides from Gracilaria species (Rhodophyta, Gracilariaceae). J Biotech 89:81–84
Marinho-Soriano E, Laugier T, Casabianca D (1998) Reproductive strategy of two Gracilaria species, G. bursa-pastoris and G. gracilis, in a Mediterranean Lagoon (Thau, France). Bot Mar 41:559–564
Martínez B, Viejo RM, Rico JM, Rodde R, Faes VA, Oliveros J, Álvarez D (2006) Open sea cultivation of Palmaria palmata (Rhodophyta) on the northern Spanish coast. Aquaculture 254:1–4
Morris EP, Peralta G, Benavente J, Freitas R, Rodrigues AM, Quintino V, Álvarez O, Varcárcel-Pérez N, Vergara JJ, Hernández I, Pérez-Lloréns JL (2009) Caulerpa prolifera stable isotope ratios reveal anthropogenic nutrients within a tidal lagoon. Mar Ecol Prog Ser 390:117–128
Mouritsen OG, Rhatigan P, Pérez-Lloréns JL (2019) The rise of seaweed gastronomy: phycogastronomy. Bot Mar 62:210–226
Naldi M, Wheeler PA (1999) Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. J Phycol 35:70–77
Olivé I, Vergara JJ, Pérez-Lloréns JL (2013) Photosynthetic and morphological photoacclimation of the seagrass Cymodocea nodosa to season, depth and leaf position. Mar Biol 160:285–297
Parages ML, Figueroa FL, Conde-Álvarez RM, Jiménez C (2014) Phosphorylation of MAPK-like proteins in three intertidal macroalgae under stress conditions. Aquat Biol 22:213–226
Pedersen MF, Borum J (1996) Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar Ecol Prog Ser 142:261–272
Pedersen MF, Borum J (1997) Nutrient control of estuarine macroalgae: growth strategy and the balance between nitrogen requirements and uptake. Mar Ecol Prog Ser 161:155–163
Pedersen MF, Johnsen KL (2017) Nutrient (N and P) dynamics of the invasive macroalgae Gracilaria vermiculophyla: nutrient uptake kinetics and nutrient release through decomposition. Mar Biol 164:172
Pereira L, Mesquita JF (2004) Population studies and carrageenan properties of Chondracanthus teedei var. lusitanicus (Gigartinaceae, Rhodophyta). J Appl Phycol 16:369–383
Pérez-Lloréns JL, Brun FG, Andría J, Vergara JJ (2004) Seasonal and tidal variability of environmental carbon related physico-chemical variables and inorganic C acquisition in Gracilariopsis longissima and Enteromorpha intestinalis from los Toruños salt marsh (Cadiz Bay, Spain). J Exp Mar Biol Ecol 304:183–201
Pérez-Lloréns JL, Hernández I, Vergara JJ, Brun FG, León A (2018) Those curious and delicious seaweeds: a fascinating voyage from biology to gastronomy. UCA Press, Cadiz
Peteiro O, Freire O (2011) Effect of water motion on the cultivation of the commercial seaweed Undaria pinnatifida in a coastal bay of Galicia, Northwest Spain. Aquaculture 314:269–276
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ryder E, Nelson SG, McKeon C, Glenn EP, Fitzsimmons K, Napolean S (2004) Effect of water motion on the cultivation of the economic seaweed Gracilaria parvispora (Rhodophyta) on Molokai, Hawaii. Aquaculture 238:207–219
Soares F, Fernandez C, Silva P, Pereira L, Gonçalves T (2016) Antifungal activity of carrageenan extracts from the red alga Chondracanthus teedei var. lusitanicus. J Appl Phycol 28:2991–2998
Stabili L, Acquaviva MI, Biandolino F, Cavallo RA, De PSA, Fanizzi FP, Narracci M, Petrocelli A, Cecere E (2012) The lipid extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): a potential resource for biotechnological purposes? New Biotech 29:443–450
Taelman SE, Champenois J, Edward MD, De Meester S, Dewulf J (2015) Comparative environmental life cycle assessment of two seaweed cultivation systems in North West Europe with a focus on quantifying sea surface occupation. Algal Res 11:173–183
Tasende MG, Peteiro C (2015) Explotación de las macroalgas marinas: Galicia como caso de estudio hacia una gestión sostenible de los recursos. Ambienta 111:116–132
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, p 504
Van Alstyne KL (2018) Seawater nitrogen concentration and light independently alter performance, growth, and resource allocation in the bloom-forming seaweeds Ulva lactuca and Ulvaria obscura (Chlorophyta). Harmful Algae 78:27–35
Van Engeland T, Bouma TJ, Morris EP, Brun FG, Peralta G, Lara M, Hendrisks IE, Soetaert K, Middelburg JJ (2011) Potential uptake of dissolved organic matter by seagrasses and macroalgae. Mar Ecol Prog Ser 477:71–81
Vaz-Pinto F, Olabarria C, Arenas F (2013) Role of top-down and bottom-up forces on the invasibility of intertidal macroalgal assemblages. J Sea Res 76:178–186
Vergara JJ, García-Sánchez MP, García-Marín P, Brun FG, Pérez-Lloréns JL, Hernández I (2012) Seasonal functioning and dynamics of Caulerpa prolifera meadows in shallow areas: an integrated approach in Cadiz Bay Natural Park. Estuar Coast Shelf Sci 112:255–264
Wakibia JG, Bolton JJ, Keats DW, Raitt LM (2006) Factors influencing the growth rates of three commercial euchemoids at coastal sites in southern Kenya. J Appl Phycol 18:565–573
Walls AM, Kennedy R, Fitzgerald RD, Blight AJ, Johnson MP, Edwards MD (2016) Potential novel habitat created by holdfasts from cultivated Laminaria digitata: assessing the macroinvertebrate assemblages. Aquacult Env Interact 8:157–169
Walls AM, Edwards MD, Firth LB, Johnson MP (2019) Ecological priming of artificial aquaculture structures: kelp farms as an example. J Mar Biol Assoc U K 99:729–740
Wheeler PA, Björnsäter BR (1992) Seasonal fluctuations in tissue nitrogen, phosphorus, and N:P for five macroalgal species common to the Pacific northwest coast. J Phycol 28:1–6
Xia B, Abbott IA (1987) Edible seaweeds of China and their place in the Chinese diet. Econ Bot 41:341–353
Xiao X, Agusti S, Lin F, Li K, Pan Y, Yu Y, Zheng Y, Wu J, Duarte CM (2017) Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci Rep 7:46613
Xu D, Gao Z, Zhang X, Qi Z, Meng C, Zhuang Z, Ye N (2011) Evaluation of the potential role of the macroalga Laminaria japonica for alleviating coastal eutrophication. Bioresour Technol 102:9912–9918
Yang Y, Chai Z, Wang Q, Chen W, He Z, Jiang S (2015) Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res 9:236–244
Yokoya NS, Kakita H, Obika H, Kitamura T (1999) Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophyla from Shikoku Island, Japan. Hydrobiologia 398:339–347
Zhou Y, Yang H, Hu H, Liu Y, Mao Y, Zhou H, Xu X, Zhang F (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture 252:264–276
Zinoun M, Cosson J, Deslandes E (1993) Influence of culture conditions on growth and physicochemical properties of carrageenans in Gigartina teedii (Rhodophyceae — Gigartinales). Bot Mar 36:131–136
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effect models and extensions in ecology with R. Springer, New York
Acknowledgements
The authors are thankful to R. Love and F. Barrena for field assistance. We appreciate the comments and helpful English revision of the manuscript by M. O’Donnell and Dr. N. Golden.
Funding
This study was financially supported by the excellence project 1235 (EAlga) of the Consejería de Economía y Conocimiento de la Junta de Andalucía and the Spanish Ministry of Education, Culture and Sport within the framework of the State Programme Salvador de Maradiaga to Promote Talent and Employability in R&D&i, Subprogramme of Mobility in R&D&i.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bermejo, R., Cara, C.L., Macías, M. et al. Growth rates of Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei (Rhodophyta) cultured in ropes: implication for N biomitigation in Cadiz Bay (Southern Spain). J Appl Phycol 32, 1879–1891 (2020). https://doi.org/10.1007/s10811-020-02090-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02090-8