Abstract
Ag-based nanoparticles (NPs) were successfully produced through green synthesis using an aqueous extract from the cultivated seaweed Ulva clathrata as the reducing and stabilizing agent. The biosynthesized NPs had spherical to polymorphic shapes with an average size of 9.5 nm. Microstructural and compositional studies revealed that these particles contained face-centred cubic crystallites of metallic Ag and AgCl. Characteristic peaks in the Fourier-transform infrared spectrum (FTIR) and Raman studies revealed the presence of functional bioactive metabolites from the seaweed extract, such as proteins, polysaccharides, and polyphenols, which are responsible for forming and stabilizing Ag/AgCl NPs. The biosynthesized Ag/AgCl NPs exhibited an important in vitro antibacterial effect against three Vibrio parahaemolyticus strains isolated from farmed shrimp affected with acute hepatopancreatic necrosis disease (AHPND) in northwestern Mexico. Litopenaeus vannamei shrimp were exposed for 7 days to feeds supplemented with Ag/AgCl NPs at 10, 100, 1000, or 10,000 ppm (Ag nominal dietary concentrations). Dietary NP supplement did not affect shrimp survival, growth, or feed conversion ratio, but high concentrations (1000 and 10,000 ppm) decreased the hepatosomatic index significantly. The short-term consumption of Ag/AgCl NPs produced a significant dose-dependent bioaccumulation of Ag in the hepatopancreas and to a lesser extent in the cuticle, while bioaccumulation in the muscle was not significant. The depuration study confirmed a fast Ag assimilation in shrimp’s hepatopancreas and showed a fast depuration rate in the hepatopancreas as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrimp farming is one of the most important aquaculture economic activities in the world, and this activity generates important economic benefits. Mexico is one of the main exporters to important international markets such as USA, Vietnam and France (Conapesca 2017). Newly emerged penaeid shrimp diseases caused by opportunistic pathogens such as Vibrio are producing economic losses in Mexico and worldwide (Aguirre-Guzmán et al. 2004). In 2009, the first cases of acute hepatopancreatic necrosis disease (AHPND), previously referred to as early mortality syndrome (EMS), appeared in China, Vietnam, Thailand and Malaysia. Since 2013, high mortalities (40 to 100% in the first 35 days of shrimp farming) caused by AHPND have been also reported in Mexico and Central American countries. This disease is caused by certain pathogenic strains of Vibrio parahaemolyticus and is characterized by a severe atrophy of the shrimp’s hepatopancreas (HP) (Joshi et al. 2014; Yang et al. 2014; Soto-Rodriguez et al. 2015). To address these issues, several antimicrobial agents were assayed. However, these failed to completely satisfy aquaculture needs as Vibrio species developed resistance to some of these treatments (Sirvakumar et al. 2014; OIE 2014). The use of antibiotics to control Vibrio strains in shrimp aquaculture is not allowed in most of the countries (Defoirdt et al. 2011). Therefore, it is necessary to develop alternative methods for the effective control of Vibrio strains in shrimp production. In the face of this problem, silver nanoparticles (AgNPs) are a promising alternative because of their unique chemical and antimicrobial properties (Rai et al. 2009; Swain et al. 2014; Singh et al. 2015; Sivaramasamy and Zhiwei 2016).
Different varieties of microorganisms and plant extracts have been used in the last years for the production of metallic nanoparticles with low or no toxicity. These procedures have been called ‘green synthesis’ or ‘green chemistry’ (Dehnavi et al. 2013). Green synthesis provides advantages over physical methods and conventional chemical synthesis of nanomaterials because it produces NPs with inexpensive and energy-efficient processes that have little to no impact on the environment and human health. In addition, these processes can be easily scalable and also do not require the use of high pressure, energy, temperature, or toxic chemicals (Abdel-Raouf et al. 2017).
Seaweeds are commercially-important, renewable, marine living resources (Kumar et al. 2012) with biologically-active compounds that have activities against pathogens such as viruses, bacteria and fungi (Ibañez et al. 2012). Scientists have researched the green synthesis of gold and silver nanoparticles using several seaweed species and obtained nanoparticles with sizes averaging from 5 to 200 nm, a high synthesis rate, and an excellent colloidal stability. The phytochemicals present in seaweed extracts such as carbohydrates, peptides, alkaloids, steroids, phenolics, saponins and flavonoids are responsible for the reduction and stabilization of nanoparticles (Rai et al. 2009; Prasad et al. 2013; Swain et al. 2014; Singh et al. 2015; Sivaramasamy and Zhiwei 2016; Vieira et al. 2016).
Ulva clathrata (Roth) C. Agardh is a green alga from the Ulvaceae family with a worldwide distribution, and it can be also produced by aquaculture. This seaweed has several applications in shrimp farming as a functional feed additive or as fresh food in co-culture with white shrimp Litopenaeus vannamei, allowing healthier shrimp and improved water quality (Cruz-Suarez et al. 2010, 2013; Laramore et al. 2018; Peña-Rodríguez et al. 2010). Cultivated U. could be a promising source of reduction agents for the synthesis of nanoparticles, which production would be then easily scalable.
Although two recent reports evidenced the efficacy of AgNPs in vivo (Morales-Covarrubias et al. 2016) and in vitro against AHPND-causing V. parahaemolyticus (Sivaramasamy et al. 2017), none had been synthetized with seaweed extracts. Considering these antecedents, the aim of this study was to purify and characterize Ag-based NPs produced through green chemistry using an aqueous extract of cultivated U. clathrata (AEU), and to evaluate their in vitro antibacterial activity against three V. parahaemolyticus strains obtained from farmed shrimp affected with AHPND in northwestern Mexico. In addition, this project sought to examine, through short-term dietary exposure experiments, the effects of Ag-based NPs on shrimp survival and growth, as well as the Ag bioaccumulation-distribution in different tissues along with its depuration kinetics.
Materials and methods
Chemical reagents
Water sterile-filtered (W3500), silver nitrate (209139), resazurine sodium salt (R7017), and sodium chloride (V000106) were from Sigma-Aldrich (USA). The bacteriological mediums Tryptic Soy Agar (TSA), Tryptic Soy Broth (TSB), Mueller Hinton Agar (MHA), Mueller Hinton Broth (MHB) and Agar Agar (AA) were obtained from Difco (USA).
Seaweed
Ulva clathrata (Roth) C. Agardh was cultivated on a large-scale system in a shrimp earthen pond in Sinaloa, Mexico. Details of production and processing conditions are reported by Peña-Rodríguez et al. (2011).
Bacterial strains
Vibrio parahaemolyticus strains were obtained from laboratory culture collections at CIAD Mazatlan, Sinaloa, Mexico. Samples were isolated from the stomach and hepatopancreas of farmed shrimp affected with AHPND in northwestern Mexico. The molecular identification, characterization and pathogenicity of the bacterial isolates used in this work were reported by Soto-Rodriguez et al. (2015); V. parahaemolyticus strains M9-04 and M6-05 are classified as pathogenic, and M5-28 as non-pathogenic. Strains were maintained in cryopreservation (− 80 °C) until activated in TSB supplemented with 2% NaCl.
Preparation of aqueous extract of Ulva (AEU)
Ten gram of seaweed powder was extracted with 200 mL of distilled hot water (90 °C) stirred constantly for 15 min and left to stand in darkness for 12 h before centrifugation (2500 rpm) for 15 min (IEC Centra MP4R, International equipment company, USA). The pellet was washed twice with 100 mL hot water at 90 °C. The soluble extracts were pooled and frozen at − 80 °C (ULT 1386-3-A14, Revco Scientific Inc., USA) for subsequent freeze-drying (Freezone Benchtop 6L, Labconco, USA).
Synthesis of NPs
NPs were obtained through green synthesis with our patented method (Luna et al. 2015a, 2015b) by adding 100 mL AgNO3 (1 × 10−3 M) aqueous solution to a flask containing 170 mg AEU previously dissolved in 100 mL Sigma sterile-filtered water. The mixture was kept in constant magnetic stirring at room temperature for 48 h. The formation of NPs was confirmed by solution colour change. NPs were washed three times using absolute ethanol (ratio 1:3) as an antisolvent for precipitation assistance and centrifuged (10,000 rpm). Finally, the purified precipitate was re-dispersed using ultrasound (Ultrasonic cleaner 50HT, VWR International, USA) in Sigma sterile-filtered water for further characterization studies. Ag concentration was measured by atomic absorption spectroscopy (AAS) (ICE AAS 3500, ThermoScientific, UK) according to the AOAC method 990.08 (AOAC 1997) and was expressed in μg Ag mL−1.
Characterization of NPs
NPs presence in the brown purified colloidal sample were confirmed through UV-vis spectroscopy (Nicolet 60S, ThermoFisher Scientific, USA) analysis in the wavelength range of 300–1100 nm. Surface plasmon resonance (SPR) was observed for AgNPs. AEU without the addition of AgNO3 solution was also analysed.
Crystal phases present in the sample were identified by powder X-ray diffraction (XRD) studies (X′Pert PRO diffractometer, Panalytical, USA) with Cu Kα (λ = 1.5418 Å) radiation. In this study, purified colloidal samples were dried at 50 °C overnight, obtaining dried samples in powder form. Values of the mean coherence length perpendicular to the crystallographic planes (200) of AgCl and (111) of metallic Ag, L200,AgCl and L111,Ag, were estimated using the Scherrer equation (Luna et al. 2015a, 2015b)
where λ is the X-ray wavelength, β is the broadening of the diffraction peak (after subtracting the instrumental broadening), and θ is the Bragg angle.
Particle morphology and size distributions of the biosynthesized samples were examined with transmission electron microscopy (TEM) using a microscope (TITAN 80–300 kV, FEI, USA) operated at 300 kV. To obtain more detailed information about the microstructural properties of the samples, high-resolution transmission electron microscopy (HRTEM) and selected-area electron diffraction (SAED) analyses were also carried out. Moreover, energy-dispersive spectrometry (EDX) measurements were performed with an EDS analyser attached to the TEM. For these studies, the colloidal sample was properly diluted with water and a drop was deposited onto a lacey-carbon copper grid and allowed to dry in ambient conditions in a free-powder area. No evidence of structural or chemical transformations of the samples was present during the TEM examination. Processing of TEM micrographs and analysis of HRTEM images by fast Fourier transforms (FFT) was carried out using the software Digital Micrograph 3.7.0 (Gatan Inc., USA).
FTIR and Raman studies were performed to identify the functional groups of the AEU responsible for the reduction of Ag+ ions to Ag0, and their stabilizing action on Ag-based nanoparticles. FTIR spectra (Nicolet 510 Fourier transform spectrometer, ThermoFisher Scientific, USA) were collected after diluting the specimen in KBr pellets in the wavenumber range of 500–4000 cm−1. Raman spectra (HR800 UV Confocal Raman Microscope, Horiba Scientific, USA) were recorded across the range of 500–4000 cm−1 using a green laser (532.14 nm), working at 600 line mm−1, × 100 objective, 20 mW, and 0.1-mm pinhole.
Antibacterial activity in vitro tests
Disc diffusion assay was performed on Mueller Hinton agar (MHA) plates plus 2% NaCl spread with 100 μL of each V. parahaemolyticus bacterial suspension (1 × 106 UFC mL−1). Under aseptic conditions, filter paper discs (6 mm) were saturated with 20 μL of each stock solution test from AEU (50 μg dry extract mL−1), Ag/AgCl NPs, AgNO3 solution (50 μg Ag mL−1) or distilled water (negative control), and were placed on MHA plates. The final concentration was 1 μg disc−1. After 24 h of incubation at 29 °C, the inhibition zone (IZ) was measured with a digital Vernier (CD-6″CS, Mitotuyo Corp., Japan), and the IZ values were recorded as the average (mm) of two diameter measurements per disc taken in perpendicular directions. Triplicates of the assay were performed for each strain.
Microdilution broth assays were carried out according to Sarker et al. (2007) with certain modifications. Different concentrations (100, 50, 25, 12.5, 10, 8 and 6 μg Ag mL−1) of Ag/AgCl NPs and AgNO3 solution were prepared, and each one was added to four wells of sterile polystyrene 96-well microtiter plates (3370 Costar, USA). Additionally, 100 μL of Mueller Hinton broth (MHB) with 2% NaCl and 50 μL of each bacterial strain suspension (1 × 106 UFC mL−1) were added to each well. Pure MHB with 2% NaCl, sterile water and bacterial suspension were used as a negative control, while sample blanks were the evaluated treatments and media without bacteria. After incubation at 29 °C for 20 h, 10 μL of 0.1% aqueous solution resazurine sodium salt were added and samples were incubated for four additional hours at the same temperature. Colour change was then assessed visually. Any colour changes from purple to pink or colourless were recorded as positive bacterial growth. The lowest concentration that produces a colour change was taken as the MIC value (Zhao and Stevens 1998). The lowest concentration at which no colour change occurred was registered to the MBC. Triplicates of the assay were performed for each strain.
Short-term evaluation in vivo tests
Two different experiments were conducted to examine effects in survival and growth performance, tissue bioaccumulation-distribution and depuration of the Ag/AgCl NPs by short-term dietary exposure in shrimp. The experiments were performed according to OECD recommendations for acute toxicity in aquatic arthropods (OECD 2005) and dietary exposure bioaccumulation-distribution in fish (OECD 2012).
Supplemented feed preparation
A commercial pelleted feed with 44% protein and 8% lipid contents (742 UANL, Nutrimar, Sinaloa, Mexico) was supplemented by aspersion with Ag/AgCl NPs stock solution in order to obtain Ag concentrations of 0, 10, 100, 1000 and 10,000 mg Ag kg−1. Different Ag/AgCl NP stock solutions were diluted or concentrated in the same final volume of distilled water (40 mL) and sprayed on 100 g of feed previously weighed and placed in aluminium trays. Diets were mixed for 5 min to ensure complete homogenization. Finally, the feed was oven-dried at 50 °C for 1 h. The final Ag concentration in Ag/AgCl NP-supplemented feeds was quantified by AAS as described above.
Estimation of NP losses from diets
The percentage of Ag/AgCl NPs lost from diets through leaching in seawater was estimated as described by Nieto-López et al. (2011) with certain modifications, including a correction in the % dry matter loss expression (%DML) for the marine salt absorbed by the immersed diets for 1 h in seawater (35 g L−1, 28 °C). Ag content in leaching diets and in the water of each sample was determined by AAS and expressed as mg Ag kg−1 dry matter or mg Ag L−1 water.
Short-term acute toxicity and tissue distribution in vivo test
The first in vivo test evaluated acute toxicity using behavioural, survival and growth performance parameters, and analysed tissue distribution of Ag/AgCl NPs after 7 days of feed consumption with low (0, 10 and 100 mg Ag kg−1) and high (1000, 10,000 mg Ag kg−1) dietary levels. During the experiment, 150 juvenile shrimp [Litopenaeus vannamei (Boone, 1931) provided by FITMAR, Sinaloa, Mexico (0.400 ± 0.02 g mean initial weight)] were randomly allocated in 15 rectangular fibreglass tanks of 10 L (10 shrimps per tank, five treatments and three replicates). Experimental tanks had constant individual aeration and were maintained daily with 100% manual exchange of artificial seawater (Pro Aquatics, Fritz Industrial Inc., Mesquite TX, USA) and natural conditions of photoperiod. Water temperature, salinity and pH, ammonia, nitrite, and dissolved oxygen contents were monitored daily and maintained under favourable limits for shrimp growth. The daily feed ration was 5% of total biomass per tank.
Acute toxicity parameters
After 7 days of feeding shrimp with the different treatments, their behavioural effects were registered. Zootechnical parameters such as survival (%), weight gain (%) and feed conversion ratio (FCR) were calculated according to Cruz-Suarez et al. (2009).
Tissue sampling for acute toxicity test
The hepatopancreas, cephalothorax cuticle and muscle from ten shrimps per treatment were dissected with tweezers before the beginning and at the end of the dietary exposure. The hepatopancreas for each shrimp was weighed individually, and the hepatosomatic index was determined using the formula shown in Eq. 2. The dissected shrimp samples of each tank were pooled and placed in pre-weighed and labeled Eppendorf tubes. Finally, all samples were oven-dried at 60 °C for 24 h and stored in a freezer at − 20 °C until Ag content analysis was performed by AAS. Results obtained were expressed in mg Ag kg−1 dry weight.
Depuration in vivo test
The second in vivo test was conducted to evaluate Ag/AgCl NP depuration using feeds supplemented at 0 and 100 mg Ag kg−1 nominal concentrations. The protocol was designed according to OECD recommendations which set uptake and depuration phases (OECD 2012; Connolly et al. 2016). Litopaneis vannnamei juvenile shrimp provided by FITMAR, Sinaloa, Mexico (1.9 ± 0.20 g mean initial weight) were randomly allocated in nine rectangular 10-L fibreglass tanks (19 shrimps per tank, two treatments and three replicates). Organisms were maintained in the same conditions as the first test. During the uptake phase (first 7 days), the organisms were fed the low-dose experimental diet, while the depuration phase (next 14 days) started on day 8 of the assay by switching to a diet without NPs supplement (0 mg Ag kg−1). The experiment duration was 21 days.
Tissue sampling for depuration test
The hepatopancreas, gills and muscle tissue from three shrimps per tank were randomly selected and dissected with tweezers at 0, 7, 16 years and 21 days of the assay. Dissected samples were pooled and placed in pre-weighed and labeled Eppendorf tubes. Finally, all the samples were oven-dried at 60 °C for 24 h and stored in a freezer at − 20 °C until Ag content analysis was performed by AAS. Results obtained were expressed in mg Ag kg−1 dry weight.
Depuration parameters
Depuration rate and assimilation efficiency were determined based on a simple two-compartment model. To calculate the depuration rate (k2), a linear regression of ln(concentration) versus time was performed. The slope of the regression line is an estimate of k2. The chemical assimilation efficiency (%) (α, absorption of test substance across the gut) was calculated with the formula in Eq. 3:
where C0,d is the measured concentration in shrimp at time zero of the depuration phase (mg Ag kg−1 of shrimp), k2 is the overall (not growth-corrected) depuration rate constant (day−1), Cfood is the measured concentration in food (mg Ag kg−1 feed), t is the duration of the feeding period (day), and Ig is the food ingestion rate constant adjusted for shrimp growth (g feed × shrimp−1 × day−1). During the uptake phase, Ig can be calculated as shown in Eq. 4:
where I is the gram feed per gram shrimp per day, W0 is the mean shrimp weight at the start of the experiment, and Wend of uptake is the mean weight on the last day of exposure.
Once the assimilation efficiency (α) is obtained, the biomagnifiction factor (BMF) can be calculated by multiplying α by the ingestion rate constant (Ig) and dividing by the (overall) depuration rate constant (k2). Estimation of the time (day) needed to reach the 50% depuration (t½) of Ag/AgCl NPs was estimated using Eq. 5.
Statistical analyses
Two-way ANOVA was used to analyse the significance on in vitro (diffusion disc assay), and one-way ANOVAs were used for in vivo assays (growth performance parameters, and final concentration of Ag in each tissue after 7 days of consumption), followed by Tukey post hoc tests. A Dunett post hoc test was used for the hepatosomatic index data (p < 0.05). All analyses were carried out using Statistica 8.0 (TIBCO Software, USA).
Results
NP synthesis and characterization
Reduction of Ag+ to Ag° in the sample was confirmed through a colour change from the initial colourless AgNO3 solution to reddish-brown after reacting with AEU (Fig. 1a, b). The ultraviolet-visible (UV-vis) absorbance spectrum for a purified Ag/AgCl NPs suspension presents a surface plasmon of resonance (SPR) peak centred at 435 nm; meanwhile, no peak was observed in the AEU spectrum (Fig. 1c). The final Ag concentration in the purified Ag/AgCl NPs solution was 535 μg Ag mL−1.
X-ray diffraction (XRD) studies
The XRD spectrum of the Ag/AgCl NPs shows a pattern displaying diffraction peaks at around 27.7°, 32.1°, 46.1°, 54.8°, 57.4°, 67.5°, 74.5° and 76.7° (Fig. 2). These peaks show a good match with the reference peak positions of face-centred cubic (fcc) structure of silver chloride (space group Fm3m, Joint Committee on Power Diffraction Standard (JCPDS) file 31-1238). The most intense of these peaks, which is centred at 32.1°, is associated to the (200) planes of AgCl and has a corresponding mean coherence length value of L200,AgCl = 41(1) nm. In addition, less intense and rather wider peaks were found at approximately 38.1°, 44.3°, 64.4° and 77.5° and were associated to the (111), (200), (220) and (311) crystalline planes of fcc metallic Ag structure, respectively (space group Fm3m, JCPDS file No. 04-0783). The average crystal size of this phase, estimated using the Scherrer equation and the position and width of the (111) peak, was L111, Ag = 18(1) nm.
Microstructural analyses
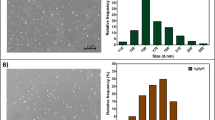
Transmission electron microscopy (TEM) images (Fig. 3a) revealed that the sample was composed of spherical and irregular particles with different sizes, 90% being in the range from 5 to 20 nm and the rest of the particles from 20 to 35 nm. Consequently, the particle size distribution of the sample was fitted to a log-normal distribution. Particle size best-fitting central value and log-normal standard deviation were 9.5 and 0.44 nm, respectively (see insert image of Fig. 3a).
a) Representative TEM image of Ag/AgCl NPs synthesized using Ulva aqueous extract. The insert image shows the related size distribution. Solid red line corresponds to the best log-normal distribution fit. b) SAED pattern. The expected positions of the diffraction rings associated to the crystallographic planes of fcc metallic silver (JCPDS 04-0783) and silver chloride (JCPDS 31-1238) are indicated. c) EDX spectrum
Selected area electron diffraction (SAED) patterns (Fig. 3b) exhibited spotty rings associated to both Ag and AgCl crystalline phases in agreement with the XRD results. Energy-dispersive X-ray (EDX) analysis (Fig. 3c) confirmed the presence of Ag, Cl and C in Ag/AgCl NPs (although the C peak partially comes from the lacey-carbon of the TEM grid). In addition, EDX spectrum often showed S peaks. The Cu peaks are due to background from the TEM grid. On the other hand, the presence of light elements, such as H, that are not detected using the EDX technique should not be discarded.
HRTEM images of the smallest nanoparticles (Figures S1a, S1c and S1e) displayed lattice fringes associated to a pure fcc Ag phase. Analysis of these micrographs by FFT (Figures S1b, S1d and S1f) revealed different microstructural characteristics in these particles, showing some to be polycrystalline (Figures S1a and S1e) with twinning boundaries and stacking faults (Figures S1e and S1f). However, nanoparticles with a rounded morphology and monocrystalline-like properties were also observed (Figures S1c and S1d), often along the [211] zone axis, and exhibited ½ {131} classically forbidden reflections.
The microstructural investigation of the largest particles by HRTEM (Figure S2a) revealed that they usually display both metallic Ag and AgCl crystals. An illustrative example is presented in Figures S2a–d. Figure S2a is an HRTEM image of two particles that display lattice fringes with d-spacing values associated to both crystalline phases detected by XRD and SAED, and that were determined analysing the spots of the corresponding FFT images (Figure S2b). Fourier filtering of HRTEM images selecting only reflections of AgCl (Figure S2c) or Ag (Figure S2d) showed the distribution of these crystalline phases in the particles, indicating that the crystals of both phases tend to coalesce to form particles of 20 to 35 of nanometres in size.
Fourier transform infrared spectrum (FTIR)
In the FTIR spectrum of the AEU (Fig. 4a), the most significant FTIR bands were found at 3258 cm−1, 1635 cm−1, 1539 cm−1, 1435 cm−1 and 1089 cm−1. The broad band observed in the wavenumber range of 3700–2800 cm−1 (centred at 3258 cm−1) corresponded to O–H stretching vibrations from polysaccharides, polyphenols or proteins (Shanmugam et al. 2014). The peaks located at 1635 cm−1 and 1539 cm−1 were associated to the stretching vibration of the C=O group in peptides bound (amide I) and the N–H bending vibration (amide II) in proteins, respectively (Mendoza-Reséndez et al. 2013; Luna et al. 2015a, 2015b). The band located at 1435 cm−1 could potentially be due to C–N stretching vibration in amine or amide groups. The sharp and intense peak at 1089 cm−1 is due to C–N stretching vibration in aliphatic amines (Shanmugam et al. 2014) and C–O–C alcoholic groups associated with polysaccharides (Kora et al. 2012; Kannan et al. 2013b).
The Ag/AgCl NPs, after the purification process, presented major absorption bands at 3350 cm−1, 2932 cm−1, 1599 cm−1, 1412 cm−1, 1081 cm−1 and 1039 cm−1. The broad band at 3350 cm−1, ascribed to –O–H stretching vibrations, appeared shifted with respect to the same band observed in the spectrum of the algae extract. The band located at 2932 cm−1 can be ascribed to –C–H stretching vibrations in alkanes (Kora et al. 2012). In comparison with the FTIR spectrum of algae extract, an enhanced and shifted absorbance band appeared at 1599 cm−1, suggesting modifications of the C=O and N–H stretching vibration in amine or amide groups due to these particles’ involvement in the reduction process (Kannan et al. 2013b). The band at 1412 cm−1 could be ascribed to C–N stretching vibration of amine or amide groups. Also, the C–N stretching vibration band of aliphatic amines and C–O–C stretching vibration of alcoholic groups associated to polysaccharides (Kumar et al. 2012) appeared shifted at 1081 cm−1 and 1039 cm−1, respectively.
Raman studies
The Raman spectra of the algae extract used in the biosynthesis and the final product can be observed in Fig. 5. The spectrum of Ag-based particles displays highly pronounced bands at 228 cm−1, 1370 cm−1 and 1595 cm−1 that are not observed in the algae extract spectrum. The sharp band at 228 cm−1 is attributed to stretching vibrations of Ag-N (Mukherjee et al. 2008; Kora et al. 2012) and Ag–O (Biswas et al. 2007; Kora et al. 2012) bonds, suggesting that proteins are bound to the particles’ surface though amino and/or carboxylate (Kora et al. 2012). Moreover, intense bands at 1370 cm−1 and 1595 cm−1 were observed, which were associated to symmetric and asymmetric C=O stretching vibrations in the COO− ions (Kora et al. 2012; Luna et al. 2015a, 2015b), respectively, and/or phenyl ring stretch (Biswas et al. 2007; Luna et al. 2015a, 2015b). Enhancement of the intensity of the C=O stretching vibrations bands indicates the direct binding of the COO− group with the Ag surface (Kora et al. 2012).
Antibacterial activity
Disc diffusion assay
The antibacterial test performed by disc diffusion showed significant differences in the growth inhibition zone diameter between the three V. parahaemolyticus strains (p = 0.001), for the products (p = 0.001) and in their interaction (p = 0.001) with a two-way ANOVA (Fig. 6). The discs impregnated with Ag/AgCl NPs produced an IZ of 9.2 ± 0.2, 5.3 ± 0.1 and 5.8 ± 0.2 mm for M5-28, M5-06 and M9-04 V. parahaemolyticus strains, respectively. The AgNO3 solution tested at same concentration produced lower IZ than Ag/AgCl NPs, with values of 6.5 ± 0.6, 4.6 ± 0.1 and 4.5 ± 0.2 mm for the three strains, respectively. In contrast, the AEU did not cause inhibition.
Average inhibition zone diameters (mm) observed with diffusion method tested at similar concentrations (10 μg Ag per disc for Ag/AgCl NPs or AgNO3). The error bars represent standard deviation (SD), and the data are means of three replicates (n = 3). The diameter of the paper disc is not included (6 mm). Different letters (a–d) represent significant differences according to a Fisher LSD post hoc multiple mean comparison test among the six treatments
Microdilution assay
The antibacterial test performed by microdilution assay was only done with the products that had obtained an IZ on the disc diffusion assay. The Ag/AgCl NP solution presented a minimum inhibitory concentration (MIC) of 3.2 μg mL−1 for all strains tested, while the AgNO3 solution showed a MIC of 1.6 μg mL−1 for V. parahaemolyticus M5-28 and M6-05 strains, and of 3.2 μg mL−1 for V. parahaemolyticus M9-04. The Ag/AgCl NP solution showed a minimum bactericide concentration (MBC) of 6.3 μg mL−1 for the three strains, while the AgNO3 solution presented an MBC of 3.2 μg mL−1 for M5-28 and M6-05 strains, and of 6.3 μg mL−1 for M9-04 (Table 1).
Short-term evaluation in vivo test
Stability of nanoparticles in supplemented feed
Ag contents in the experimental feeds are shown in Table 2. The leached feed had higher Ag content compared with the initial feed, and the nanoparticles lost from the feed surface to the seawater after 1 h were less than 5%.
Short-term acute toxicity in vivo (first test) and Ag distribution in tissues
During the first short-term feed exposure test, the organisms showed no behavioural changes and survival was 100% for all treatments. The consumption of supplemented feed for 7 days did not cause significant changes in feed conversion but did affect weight gain (%), which was slightly improved in shrimp consuming feeds supplemented with 10 and 100 mg Ag kg−1 feed, and slightly (but not significantly) depressed in those consuming feeds with 10,000 mg Ag kg−1 (Table 3). In contrast, the hepatosomatic index (HPI) showed a significant reduction with the consumption of feeds supplemented with high doses (1000 and 10,000 mg Ag kg−1; Fig. 7a).
a) Hepatosomatic index (HPI) box diagram (n = 3) and Ag bioaccumulation in shrimp tissues, b) hepatopancreas (n = 12), c) cephalothorax cuticle (n = 4) and d) muscle (n = 4) after consumption for 7 days of feeds supplemented with Ag/AgCl NPs at graded levels (from 0 to 10,000 mg Ag kg−1 feed). a) The white line represents the average HPI value (n = 3), letters on top of the bars indicate the absence (a) or presence (b) of a significant difference to the control group (Dunnet test). b)–d) Letters on top of the bars indicate different homogeneous subsets as determined by a Tukey multiple means comparison test. Error bars represent SD for all figures
Average concentration values of Ag were reported for different organ pools analysed at the end of 7 days of supplemented feed consumption (Fig. 1; hepatopancreas 7b, cephalothorax cuticle 7c and muscle 7d). A greater bioaccumulation of Ag was observed in hepatopancreas showing a dose-dependent relation of up to 1500 mg kg−1 dry weight (dw), while concentrations in cuticle and muscle were lower than 8 mg kg−1dw. The hepatopancreas and cuticle Ag content became significantly different when diets contained 1000 and 10,000 ppm Ag. In contrast, muscle Ag contents did not change significantly with any treatment.
Depuration parameters (second test)
The depuration rate (k2), assimilation efficiency (α), half-life time (t1/2) and biomagnification factor (BMF) could be determined for hepatopancreas and gills only (Fig. 8). The model used for calculating depuration parameters was not applicable in muscle tissue due to low Ag concentrations. However, results indicate that the hepatopancreas has higher k2 and α values (0.156 day−1 and 33.053%, respectively) than gills (0.072 day−1 and 0.169%). The 50% depuration time (or t1/2) was shorter in gills than in hepatopancreas, at 4.4 and 9.5 days, respectively. The hepatopancreas showed a high food biomagnification factor (BMF) value of 10.553, while the gills had a 0.116 value. Finally, depuration of Ag accumulated in hepatopancreas and gills was already 89 and 99%, respectively, after only 14 days of depuration (i.e. consuming the feed without Ag/AgCl NPs).
Discussion
AgNPs exhibit an intense absorption peak in the UV-vis spectrum due to the SPR. According to Mie’s theory, the form, width, height and the number of SPR peaks are strongly related with shape, particle size, concentration and distribution of the particles, respectively (Sun and Xia 2002; Dehnavi et al. 2013). The location and form of the single SPR peak observed for the purified Ag/AgCl NPs demonstrate the presence of spherical particles smaller than 100 nm, with a log-normal distribution. These results are in agreement with the SPR spectra reported by other authors for AgNPs or AgCl NPs (Choi et al. 2008; Mendoza-Reséndez et al. 2013; Dhas et al. 2014; Pal et al. 2015).
Ethanol is frequently used as an antisolvent to help recover the particles selectively (McLeod et al. 2005; Liu et al. 2009), in combination with centrifugation, in order to eliminate the ionic silver that had not reacted during the synthesis; additionally, centrifugation may be used to fraction particles by size (Novak et al. 2001; Pal et al. 2015). The final Ag concentration after the particle purification process was lower than the initial concentration due to elimination of Ag from the initial mixture.
The signals from the crystallographic planes on the XRD analysis indicate the presence of Ag and AgCl particles. The formation of these particles could be due to the interaction of the silver ions with chloride ions present in the Ulva extract. The presence of Na+, K+ and Cl− is typical in macroalgae given that they are the main ions responsible for maintaining internal osmotic pressure (Lee and Liu 1999). Similar results have been reported by Dhas et al. (2014) and Venkatesan et al. (2016) regarding the biosynthesis of AgNPs with aqueous extracts of the brown algae Sargassum plagiophyllum and Ecklonia cava.
Synthesized NPs mediated by natural extracts also present special and unique morphological and microstructural characteristics, which are strongly linked to the reducing agent’s composition. Ag/AgCl NPs exhibited a heterogeneous morphology, with a predominance of spherically and irregularly shaped particles. This is in agreement with reports from several authors of spherical, irregular and triangular particle shapes of NPs synthesized with green algae extracts (Yousefzadi et al. 2014; Sharma et al. 2016; Vijayan et al. 2016). Ag/AgCl NPs’ sizes, as measured by TEM, are also similar to that of other AgNPs synthesized with green algae extracts. These authors report a range from 5 to 210 nm (Devi and Bhimba 2012; Kannan et al. 2013a; Abirami and Kowsalya 2015; Sharma et al. 2016; Ramkumar et al. 2017). Particle size is relevant in this study given that the biological activity of NPs is strongly associated to their size distribution (Dehnavi et al. 2013).
Microstructural studies by SAED and EDX confirmed that particles are composed mostly of Ag and Cl, followed by C, S and O. These signals can likely be attributed to adsorbed proteins and sulphated polysaccharides (ulvan) coming from the extract composition. HRTEM studies exhibited two types of particles: The first are small, spherical and monocrystalline composed by only Ag phase, while the second are large, irregular and polycrystalline composed of Ag and AgCl phases. In addition, stacking faults were found in the morphology of large particles. This property and the irregular particle form are due to a growth in particle size by coalescence of smaller particles that seek to stabilize within the medium. This phenomenon has been reported briefly in AgNPs synthesized using natural extracts (Mendoza-Reséndez et al. 2013, 2014). There is only one previous report of gold particles with stacking faults. In that study, particles were synthesized using an aqueous extract of a unicellular green alga (Chlorella vulgaris), and the faults were attributed to particle growth and stabilization (Xie et al. 2007).
Seaweeds constitute an inexhaustible source of bioactive compounds and therefore are considered an important resource in biotechnology (Rauwel et al. 2015). Some biomolecules extracted from green algae, such as polysaccharides, peptides, phenolic compounds and pigments, are responsible for metal bioreduction during NP manufacture, because these compounds contain in their structure reducing functional groups like hydroxyl (–OH), carbonyl (–COOH), primary and secondary amines (–NH, –NH2), alcohol and aldehydes (–CHO) (El-Kassas et al. 2014; Yousefzadi et al. 2014; Ramkumar et al. 2017). FTIR and Raman analyses confirmed the presence in the AEU of functional groups from biomolecules, mainly proteins, sulphated polysaccharides, and polyphenols that are adsorbed to the surface and microstructure of Ag/AgCl NPs. According to several authors who reported similar vibrations in FTIR studies of NPs synthesized with green algae extracts, these molecules are the main compounds responsible for reducing and buffering nanoparticles (El-Kassas et al. 2014; Ramkumar et al. 2017), making them more stable, and for a longer time, than those chemically synthesized (Vijayan et al. 2016). Additionally, these compounds may contribute novel and unique properties, as well as potential applications, for the corresponding NPs (Nezamdoost et al. 2014).
The antibacterial activity of the Ag/AgCl NPs obtained in the present study agreed with several authors who reported IZ with AgNPs or colloidal Ag in the range of 4.0 to 16.5 mm against V. parahaemolyticus AHPND+ (Morales-Covarrubias et al. 2016; Sivaramasamy and Zhiwei 2016; Sivaramasamy et al. 2017) and 10 to 14.27 mm against Vibrio harveyi (Kandasamy et al. 2012; Sivaramasamy and Zhiwei 2016).
Sivaramasamy et al. (2017) reported similar results for AgNO3 IZ ranging from < 1 to 4.6 mm for a strain of V. parahaemolyticus that causes AHPND (the diameter of the paper disc was not included for all results and comparisons in this paper). This result is interesting because Ag+ ions arising from solutions of AgNO3 usually exhibit more prominent effects in growth inhibition against bacteria than AgNPs (Choi et al. 2008). Some authors have reported antibacterial activity of Ulva sp. extracts (made with solvents of different polarities) against Vibrio and other important strains for human health (Rebecca et al. 2012; Saritha et al. 2013). However, in our case, AEU antibacterial activity was not observed. This result may be due to a difference in bacteria sensibility, Ulva species or the extraction method. Nevertheless, the compounds extracted with water from U. clathrata had an excellent reducing and stabilizing activity for the formation of NPs. Among the V. parahaemolyticus strains evaluated, M5-28 presented the highest susceptibility.
The minimum inhibitory concentration (MIC) represents the lowest concentration of antibacterial solution inhibiting bacterial growth. In contrast, the minimum bactericidal concentration (MBC) represents the lowest concentration of antibacterial solution required to kill more than 99.9% of the initial bacterial population (Petrus et al. 2011). The MIC and MBC values obtained for Ag/AgCl NP were below those reported by Petrus et al. (2011), where colloidal Ag was evaluated against other Vibrio strains reporting a MIC of 9.64 μg mL−1 and a MBC ˃ 100 μg mL−1. In contrast, MIC and MBC values for colloidal Ag against a strain of Vibrio causing AHPND reported by Morales-Covarrubias et al. (2016) were lower (2 μg mL−1).
The differences between theoretical and measured Ag concentrations in supplemented feeds could be due to the manipulation during the preparation process, coupled with the analytical error. However, these differences with respect to nominal concentrations were acceptable. The increase shown in the Ag concentrations in feed after leaching can be attributed to the loss of soluble nutriments such as proteins (amino acids) and sugars, while less soluble ingredients, or those that do not leach in the water, were actually concentrated in the immersed feed. This result confirms that dietary exposure is a good and stable way to deliver NPs and to evaluate their effect in shrimp. Nevertheless, further studies regarding the residual content in shrimp feces and their final disposal are needed to inform future applications in shrimp aquaculture.
During the first in vivo test, consumption of feeds supplemented at low concentrations (10 and 100 ppm) had a positive effect on growth. This result coincides with survival and growth results reported by Sivaramasamy and Zhiwei (2016), who fed L. vannamei shrimp (6.82 ± 2.16 g) with a concentration of 10,000 ppm Bacillus subtilis-synthesized AgNPs for 65 days. In contrast, feeds supplemented at high concentrations (1000 and 10,000 ppm) likely resulted in moderate liver damage (non-visible), as suggested by a significant reduction in HPI. This negative effect on HPI, followed by histological damage, has been reported in trout after 8 weeks of being exposed to water with NPs at concentrations of 3300 and 1000 mg Ag L−1 (Monfared and Soltani 2013). However, there are no similar studies in shrimp allowing a comparison with our results in terms of HPI reduction and toxicity of dietary AgNPs.
In terms of bioaccumulation and biodistribution, the highest Ag concentrations found in hepatopancreas were not surprising, since the crustacean hepatopancreas gland can be considered a ‘target organ’ for the accumulation of many types of substances. In addition to performing important functions, such as secreting digestive enzymes, serving as the main site for food adsorption, and storing metabolic reserves, this organ also contains vesicles with metal-binding proteins that allow it to sequester heavy metals and other types of substances (Carvalho et al. 1999). In contrast, muscle showed practically no bioaccumulation, which is desirable given that it represents the most edible portion of the shrimp. However, medium-intensity bioaccumulation in the cephalothorax cuticle suggests that shell-on shrimp tails could contain certain levels of residual Ag. In this regard, several studies have found that the biodistribution and toxicity of nanoparticles are strongly influenced by particle size, shape, surface charge, surface coating and solubility (Sivaramasamy and Zhiwei 2016).
In addition, results suggest that shrimp have a fast Ag depuration capacity. This is mostly carried out by the hepatopancreas, which showed the highest depuration rates, similar to what was previously seen in the assimilation efficiency. The BMF value indicates how many times the Ag feed content can be biomagnified by hepatopancreas, which in this case indicates an Ag biomagnification capacity of ten times more than that administered by feed exposure.
Ag half-life times of 4 to 9 days were found in the tissues sampled during the depuration study (hepatopancreas, gills and muscle). This means that waiting an additional half-life period can eliminate 50% of the residual Ag. These values are lower than (but rather congruent with) that previously reported by Metian et al. (2010), who showed a t½ value of 10.8 ± 2 days for the depuration of Litopenaeus stylirostris after dietary exposure to radiolabeled Ag+.
Several authors have studied the bioaccumulation and purification of noble and heavy metals including ionic, radiolabeled or complexed silver in crustaceans. Their purpose was to establish the current state of accumulation, distribution and migration within the trophic chain of metals that were a product of contamination in the ecosystem. The main objective of our study is not centered around metal contamination in aquatic systems but rather aims to provide information on next-generation antibacterial products manufactured using nanotechnology, and to promote appropriate doses that would have minimal impact on biological systems.
Conclusions
In vitro antibacterial activity studies herein suggest that Ag/AgCl NPs synthesized for the first time with aqueous extract of cultivated U. clathrata seaweed have a potential use for the management of Vp AHPND+. In addition, consumption of Ag/AgCl NPs through dietary exposure for 7 days had no lethal effects on shrimp. Finally, shrimp hepatopancreas has a rapid capacity for bioaccumulation and purification, while Ag accumulation was lower in cuticles and insignificant in muscle tissue. This suggests that headless, peeled shrimp could be safer than shell-on presentations. Results also indicate that Ag/AgCl NPs could be an effective treatment against AHPND+ in farms. However, further studies are required to properly establish safe parameters when using Ag/AgCl NPs against V. parahaemolyticus AHPND+ infections, as well as to investigate the specific effects of Ag/AgCl NPs on shrimp immune system and histology. Through additional research, we hope to provide a broader picture of the necessary considerations for the application of NPs as an antibacterial additive in shrimp feeds.
References
Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM (2017) Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem 10:S3029–S3039
Abirami RG, Kowsalya S (2015) Ulva fasciata nanoparticles characterization and its anticancer activity. World J Pharm Pharm Sci 4:1164–1175
Aguirre-Guzmán G, Ruíz HM, Ascencio F (2004) A review of extracellular virulence product of Vibrio species important in diseases of cultivated shrimp. Aquac Res 35:1395–1404
AOAC (ed) (1997) Official Method of Analysis, 16th edn. AOAC International, Gaithersburg
Biswas N, Kapoor S, Mahal HS, Mukherjee T (2007) Adsorption of CGA on colloidal silver particles: DFT and SERS study. Chem Phys Lett 444:338–345
Carvalho RA, Benfield MC, Santschi PH (1999) Comparative bioaccumulation studies of colloidally complexed and free-ionic heavy metals in juvenile brown shrimp Penaeus aztecus (Crustacea: Decapoda: Penaeidae). Limnol Oceanogr 44:403–414
Choi O, Deng KK, Kim NJ, Ross L Jr, Surampallie RY, Hu Z (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res 42:3066–3074
Conapesca (2017) Anuario estadístico de acuacultura y pesca. Comisión Nacional de Acuacultura y Pesca, Mazatlan, Sinaloa, Mexico. pp 28 https://www.conapesca.gob.mx/work/sites/cona/dgppe/2017/ANUARIO_ESTADISTICO_2017.pdf. Access 26 Oct 2019
Connolly M, Fernández M, Conde E, Torrent F, Navas JM, Fernandez-Cruz ML (2016) Tissue distribution of zinc and subtle oxidative stress effects after dietary administration of ZnO nanoparticles to rainbow trout. Sci Total Environ 551–552:334–343
Cruz-Suarez LE, Tapia-Salazar M, Nieto-Lopez MG, Guajardo-Barbosa C, Ricque-Marie D (2009) Comparison of Ulva clathrata and the kelps Macrocystis pyrifera and Ascophyllum nodosum as ingredients in shrimp feeds. Aquac Nutr 15:421–430
Cruz-Suarez LE, León A, Peña-Rodríguez A, Rodríguez-Peña G, Moll B, Ricque-Marie D (2010) Shrimp/Ulva Co-Culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 301:64–68
Cruz-Suarez LE, Nieto-Lopez M, Tapia-Salazar M, Maldonado-Muñiz M, Villarreal-Cavazos D, Ricque-Marie D (2013) Avances en la valoración de macroalgas del género Ulva como nutracéutico en Litopenaeus vannamei. In: Cruz-Suárez LE, Ricque-Marie D, Tapia-Salazar M, Nieto-Lopez MG, Villarreal-Cavazos DA, Gamboa-delgado J, Alvarez-Gonzalez C (eds) Contribuciones recientes en alimentacion acuicola. Universidad Autonoma de Nuevo León, San Nicolas de los Garza, pp 553–598 https://www.uanl.mx/utilerias/nutricion_acuicola/xii/LibroCRANA-ISBN.pdf. Access 08 July 2018
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258
Dehnavi AS, Raisi A, Aroujalian A (2013) Control size and stability of colloidal silver nanoparticles with antibacterial activity prepared by a green synthesis method. Synth React Inorganic Met Nano-Metal Chem 43:543–551
Devi JS, Bhimba BV (2012) Anticancer activity of silver nanoparticles synthesized by the seaweed Ulva lactuca In vitro. J Nanomedine Biotherapeutic Discov 1:1–5
Dhas TS, Kumar VG, Karthick V, Jini AK, Govindaraju K (2014) Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim Acta A 120:416–420
El-Kassas EK, El-Kassas H, E-K MM (2014) Biogenic silver nanoparticles using seaweed Ulva rigida and their fungicidal and cytotoxic effects. JKAU Mar Sci 25:3–20
Ibañez E, Herrero M, Mendiola JA, Castro-Puyana M (2012) Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M (ed) Marine Bioactive Compounds: Sources, Characterization and Applications. Springer US, Boston, pp 55–98
Joshi J, Srisala J, Truong VH, Chen I-T, Nuangsaeng B, Suthienkul O, Lo CF, Flegel TW, Sritunyalucksana K, Thitamadee S (2014) Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture 428:297–302
Kandasamy K, Alikunhi NM, Manickaswami G, Nabikhan A, Ayyavu G (2012) Synthesis of silver nanoparticles by coastal plant Prosopis chilensis (L.) and their efficacy in controlling vibriosis in shrimp Penaeus monodon. Appl Nanosci 3:65–73
Kannan RRR, Arumugam R, Ramya D, Manivannan K, Anantharaman P (2013a) Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl Nanosci 3:229–233
Kannan RRR, Stirk WA, Van Staden J (2013b) Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S Afr J Bot 86:1–4
Kora AJ, Sashidhar RB, Arunachalam J (2012) Aqueous extract of gum olibanum (Boswellia serrata): A reductant and stabilizer for the biosynthesis of antibacterial silver nanoparticles. Process Biochem 47:1516–1520
Kumar P, Selvi SS, Prabha AL, Kumar KP, Ganeshkumar RS, Govindaraju M (2012) Synthesis of silver nanoparticles from Sargassum tenerrimum and screening phytochemicals for its antibacterial activity. Nano Biomed Eng 4:12–16
Laramore S, Baptiste R, Wills PS, Hanisak MD (2018) Utilization of IMTA-produced Ulva lactuca to supplement or partially replace pelleted diets in shrimp (Litopenaeus vannamei) reared in a clear water production system. J Appl Phycol 30:3603–3610
Lee T-M, Liu C-H (1999) Correlation of decreased calcium contents with proline accumulation in the marine green macroalga Ulva fasciata exposed to elevated NaCl contents in seawater. J Exp Bot 50:1855–1862
Liu J, He F, Gunn TM, Zhao D, Roberts CB (2009) Precise seed-mediated growth and size-controlled synthesis of palladium nanoparticles using a green chemistry approach. Langmuir 25:7116–7128
Luna C, Cruz-Suarez LE, Mendoza-Resendez R, Ricque-Marie D, Gonzalez-Dueñas C, Maldonado-Muñiz M (2015a) Instituto Mexicano de la Propiedad Intelectual (IMPI) Solicitud de Patente MX/a/2015/002621 https://siga.impi.gob.mx/newSIGA/content/common/principal.jsf. Access 26 Oct 2019
Luna C, Chávez VHG, Barriga-Castro ED, Nuñez N, Mendoza-Resendez R (2015b) Biosynthesis of silver fine particles and particles decorated with nanoparticles using the extract of Illicium verum (star anise) seeds. Spectrochim Acta A 141:43–50
McLeod MC, Kitchens CL, Roberts CB (2005) CO2-expanded liquid deposition of ligand-stabilized nanoparticles as uniform, wide-area nanoparticle films. Langmuir 21:2414–2418
Mendoza-Reséndez R, Núñez NO, Barriga-Castro ED, Luna C (2013) Synthesis of metallic silver nanoparticles and silver organometallic nanodisks mediated by extracts of Capsicum annuum var. aviculare (piquin) fruits. RSC Adv 3:20765–20771
Mendoza-Reséndez R, Gómez-Treviño A, Barriga-Castro ED, Nuñez N, Luna C (2014) Synthesis of antibacterial silver-based nanodisks and dendritic structures mediated by royal jelly. RSC Adv 4:1650–1658
Metian M, Hédouin L, Eltayeb MM, Lacoue-Labarthe T, Teyssié JL, Mugnier C, Bustamante P, Warnau M (2010) Metal and metalloid bioaccumulation in the Pacific blue shrimp Litopenaeus stylirostris (Stimpson) from New Caledonia: Laboratory and field studies. Mar Pollut Bull 61:576–584
Monfared AL, Soltani S (2013) Effects of silver nanoparticles administration on the liver of rainbow trout ( Oncorhynchus mykiss ): histological and biochemical studies. Pelagia Res Libr 3:285–289
Morales-Covarrubias MS, García-Aguilar N, Bolan-Mejía MDC, Puello-Cruz AC (2016) Evaluation of medicinal plants and colloidal silver efficiency against Vibrio parahaemolyticus infection in Litopenaeus vannamei cultured at low salinity. Dis Aquat Org 122:57–65
Mukherjee P, Roy M, Mandal BP, Dey GK, Mukherjee PK, Ghatak J, Tyagi AK, Kale SP (2008) Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 19:1–7
Nezamdoost T, Bagherieh-Najjar MB, Aghdasi M (2014) Biogenic synthesis of stable bioactive silver chloride nanoparticles using Onosma dichroantha Boiss. root extract. Mater Lett 137:225–228
Nieto-López M, Tapia-Salazar M, Ricque-Marie D, Villarreal-Cavazos D, Lemme A, Cruz-Suarez LE (2011) Digestibility of different wheat products in white shrimp Litopenaeus vannamei juveniles. Aquaculture 319:369–376
Novak JP, Nickerson C, Franzen S, Feldheim DL (2001) Purification of molecularly bridged metal nanoparticle arrays by centrifugation and size exclusion chromatography. Anal Chem 73:5758–5761
OECD (2005) OECD Detailed review paper on aquatic arthropods in life cycle and two-generation toxicity tests. OECD Environment Health and Safety Publications, OECD Publishing, Paris, http://www.oecd.org/chemicalsafety/testing/34241659.pdf. Accessed 15 Feb 2017
OECD (2012) Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris https://doi.org/10.1787/9789264185296-en
OIE (2014)Antimicrobial use in aquatic animals. In: Aquatic animal health code, Seventeent. World organisation for animal health, Paris, pp 109-117
Pal S, Tak YK, Song JM (2015) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. J Biol Chem 290:1712–1720
Peña-Rodríguez A, León A, Moll B, Tapia-Salazar M, Nieto-López MG, Villarreal-Cavazos D, Ricque-Marie D, Cruz-Suárez LE (2010) Uso de Ulva clathrata en la nutrición del camarón blanco: revisión. En: Cruz-Suarez LE, Ricque-Marie D, TapiaSalazar M, Nieto-López MG, Villarreal-Cavazos A, Gamboa-Delgado J (Eds), Avances en Nutrición Acuícola X - Memorias del X Simposio Internacional de Nutrición Acuícola, 8-10 de Noviembre, San Nicolás de los Garza, N. L., México.Universidad Autónoma de Nuevo León, Monterrey, México, pp 700–712
Peña-Rodríguez A, Mawhinney TP, Ricque-Marie D, Cruz-Suárez LE (2011) Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem 129:491–498
Petrus EM, Tinakumari S, Chai LC, Ubong A, Tunung R, Elexson N, Chai LF, Son R (2011) A study on the minimum inhibitory concentration and minimum bactericidal concentration of nano colloidal silver on food-borne pathogens. Int Food Res J 18:55–66
Prasad TNVKV, Kambala VSR, Naidu R (2013) Phyconanotechnology: synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J Appl Phycol 25:177–182
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, Sivagurunathan P, Saratale GD, Dung TNB, Kannapiranf K (2017) Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol Rep 14:1–7
Rauwel P, Küünal S, Ferdov S, Rauwel E (2015) A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mater Sci Eng 2015:1–9
Rebecca LJ, Dhanalakshmi V, Sharmila S (2012) Effect of the extract of Ulva sp on pathogenic microorganisms. J Chem Pharm Res 4:4875–4878
Saritha K, Mani AE, Priyalaxmi M, Patterson J (2013) Antibacterial activity and biochemical constituents of seaweed Ulva lactuca. Glob J Pharmacol 7:276–282
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Shanmugam N, Rajkamal P, Cholan S, Kannadasan N, Sathishukumar K, Viruthagiri G, Sundaramanickam A (2014) Biosynthesis of silver nanoparticles from the marine seaweed Sargassum wightii and their antibacterial activity against some human pathogens. Appl Nanosci 4:881–888
Sharma A, Sharma S, Sharma K, Chetri S, Vashishtha A, Singh P, Kumar R, Rathi B, Agrawa V (2016) Algae as crucial organisms in advancing nanotechnology: a systematic review. J Appl Phycol 28:1759–1774
Singh CR, Kathiresan K, Anandhan S (2015) A review on marine based nanoparticles and their potential applications. Afr J Biotechnol 14:1525–1532
Sirvakumar K, Kannappan S, Masilamani D, Prasanna KP (2014) Evaluation of marine macro alga, Ulva fasciata against bio-luminescent causing Vibrio harveyi during Penaeus monodon larviculture. Afr J Microbiol Res 8:803–813
Sivaramasamy E, Zhiwei W (2016) Enhancement of vibriosis resistance in Litopenaeus vannamei by supplementation of biomastered silver nanoparticles by Bacillus subtilis. J Nanomed Nanotechnol 7:1–10
Sivaramasamy E, Zagorsek K, Li F, Xiang J (2017) In situ synthesis of silver nanoparticles into TEMPO-mediated oxidized bacterial cellulose and their antivibriocidal activity against shrimp pathogens. Carbohydr Polym 166:329–337
Soto-Rodriguez SA, Gomez-Gil B, Lozano-Olvera R, Betancourt-Lozano M, Morales-Covarrubias MS (2015) Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl Environ Microbiol 81:1689–1699
Sun Y, Xia Y (2002) Shape-controlled synthesis of gold and silver nanoparticles. Science 298:2176–2179
Swain P, Sasmal A, Nayak SK, Barik S, Mishra SS, Mohapatra KD, Swain SK, Saha JN, Sen AK, Jayasankar P (2014) Evaluation of selected metal nanoparticles on hatching and survival of larvae and fry of Indian major carp, rohu ( Labeo rohita ). Aquac Res 47:498–511
Venkatesan J, Kim S-K, Shim M (2016) Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 6:1–18
Vieira AP, Stein EM, Andreguetti DX, Colepicolo P, da Costa Ferreira AM (2016) Preparation of silver nanoparticles using aqueous extracts of the red algae Laurencia aldingensis and Laurenciella sp. and their cytotoxic activities. J Appl Phycol 28:2615–2622
Vijayan SR, Santhiyagu P, Ramasamy R, Aravalagan P, Kumar G, Ethiraj K, Ramaswamy BR (2016) Seaweeds: A resource for marine bionanotechnology. Enzym Microb Technol 95:45–57
Xie J, Lee JY, Wang DIC, Ting YP (2007) Identification of active biomolecules in the high-yield synthesis of single-crystalline gold nanoplates in algal solutions. Small 3:672–682
Yang YT, Chen IT, Lee CT, Chen CY, Lin SS, Hor LI, Tseng TC, Huang YT, Sritunyalucksana K, Thitamadee S, Wang HC, Lo CF (2014) Draft genome sequences of four strains of Vibrio parahaemolyticus, three of which cause Early Mortality Syndrome/Acute Hepatopancreatic Necrosis Disease in shrimp in China and Thailand. Genome Announc 2:e00816–e00814
Yousefzadi M, Rahimi Z, Ghafori V (2014) The green synthesis , characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (Wulfen). J Agardh Mater Lett 137:1–4
Zhao G, Stevens SE (1998) Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. BioMetals 11:27–32
Acknowledgements
The authors sincerely thank the company FITMAR for their support with the organisms used during the experimental tests in this research project.
Author contributions statement
MM-M synthetized the particles, performed the experiments, analysed the results and wrote the manuscript. CL conceived the synthesis and characterization of particles. RM-R conceived the synthesis and characterization of particles. EDB-C performed the characterization studies. SS-R provided the bacterial strains and conceived the microbiological studies. DR-M performed statistical and mathematical analyses, and wrote the manuscript. LEC-S designed and conceived all the experiments and wrote the manuscript. All authors reviewed the manuscript.
Funding
This study was part of the project ‘Evaluación multidisciplinaria del potencial nutraceutico de macroalgas en camarones y murinos y su repercusión en el manejo de enfermedades bacterianas y metabólicas’, which received financial support from the Consejo Nacional de Ciencia y Tecnología (CONACyT) (Ciencia Basica #2014-238458).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 965 kb)
Rights and permissions
About this article
Cite this article
Maldonado-Muñiz, M., Luna, C., Mendoza-Reséndez, R. et al. Silver nanoparticles against acute hepatopancreatic necrosis disease (AHPND) in shrimp and their depuration kinetics. J Appl Phycol 32, 2431–2445 (2020). https://doi.org/10.1007/s10811-019-01948-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01948-w