Abstract
Cyanobacteria are prokaryotic organisms capable of oxygenic photosynthesis. H2 can be produced by cyanobacterial bidirectional hydrogenase, but mainly during anaerobic dark fermentation. Here, we screened H2 producing cyanobacteria isolated from marine environments in Thailand and optimized physiological conditions for maximizing H2 production of the selected isolate. Most of the 54 cyanobacterial strains isolated and purified from samples of seawater, stones, sand, and shells in the Gulf of Thailand and the Andaman Sea, southern part of Thailand produced H2 when cells were incubated in nitrogen-deprived medium under dark/anaerobic condition. The filamentous non-heterocystous cyanobacterium Geitlerinema sp. RMK-SH10 gave the highest H2 yield with highest H2 production rate found in 7-day grown cells. Geitlerinema sp. RMK-SH10 showed maximum H2 production rate of 0.271 ± 0.013 μmol H2 mg−1 dry weight h−1 when incubated in NaNO3-free ASN III medium containing 0.2 M NaCl, 18.9 mmol C-atom of glucose L−1, and 0.1 μM Ni2+. These results suggest that the marine filamentous cyanobacterium Geitlerinema sp. RMK-SH10 has high potential as a H2 producer amenable for further improvement by genetic manipulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, biological hydrogen production has attracted much attention as an efficient and alternative method for sustainable H2 production. Among H2 producing microorganisms, prokaryotic cyanobacteria provide a number of advantages such as cheap substrate for cultivation, easy manipulation, and simple mass cultivation method with high potential for industrial applications. Cyanobacteria are capable of producing H2 by hydrogenase activity using electrons derived from water oxidation via a direct biophotolysis process under light exposure (Prince and Kheshgi 2005; Allahverdiyeva et al. 2010); however, cyanobacterial hydrogenase activity is inhibited by O2 produced by a photolysis (Houchins 1984). Therefore, the main electron source for the reduction of proton to generate H2 by cyanobacteria is derived from the degradation of storage glycogen via a dark fermentation (Tamagnini et al. 2007; Ananyev et al. 2008). Some N2-fixing cyanobacteria can release H2 as a by-product by an action of nitrogenase via nitrogen fixation (Reddy et al. 1996; Chen et al. 2008).
Marine cyanobacteria ubiquitously found in marine habitats/environments show an ability to tolerate high salt concentrations (Thajuddin and Subramanian 2005). H2 production has been investigated in some strains of marine cyanobacteria; for example, Oscillatoria sp. Miami BG7 (Kumazawa and Mitsui 1981; Phlips and Mitsui 1983), Synechococcus sp. Miami BG 043511 (Luo and Mitsui 1994), Oscillatoria willei BDU 130511 (Saha et al. 2003), Phormidium valderianum BDU 20041 and Leptolyngbya valderiana BDU 20041 (Prabaharan and Subramanian 1996; Prabaharan et al. 2010), and the unicellular halotolerant cyanobacterium Aphanothece halophytica (Taikhao et al. 2013, 2015). Biodiversity among marine cyanobacteria for H2 production has not been paid much attention; only few studies have attempted to screen and characterize potential H2 producing cyanobacteria from marine natural environments (Kumar and Kumar 1992; Vyas and Kumar 1995; Allahverdiyeva et al. 2010; He et al. 2012; Kothari et al. 2012; Leino et al. 2014). In addition, several environmental factors, such as cultivation time, nutritional and mineral concentrations, play important roles in cyanobacterial H2 production (Dutta et al. 2005; Tiwari and Pandey 2012).
The aims of this study were to screen for high H2 producing marine cyanobacteria isolated from the two coastal waters of Thailand, the Gulf of Thailand, and the Andaman Sea and to optimize the conditions for H2 production by the selected high potential marine cyanobacterial isolate.
Materials and methods
Collection and isolation of cyanobacterial isolates
Cyanobacterial strains were isolated from seawater in both the Gulf of Thailand and the Andaman Sea of Thailand. Planktonic cyanobacterial strains were isolated from seawater, whereas benthic cyanobacterial strains were isolated from stones, sand, and shells. All samples from each environment were inoculated in flasks containing liquid ASN III medium (Rippka et al. 1979). The flasks were then incubated at 30 °C under light illumination of 30 μmol photons m−2 s−1 for 7–30 days. In case of planktonic cyanobacterial isolation, seawater samples were filtered through a 20-μm mesh plankton net and the biomass on the filter was subsequently inoculated in medium and cultivated under indicated conditions. Each filament or colony of cyanobacterial isolates was isolated and purified using a single cell isolation technique under a stereomicroscope (Nikon SMZ745T, Japan) (Hoshaw and Rosowki 1973). Bacterial contamination was examined by Gram staining technique (Gram 1884).
Cultivation of cyanobacterial isolates
Cyanobacterial isolates were cultivated in a 250-mL Erlenmeyer flask containing 100 mL of liquid ASN III medium and shaken at 120 rpm under a white-light illumination of 30 μmol photons m−2 s−1 at 30 °C for 14 days.
Identification of cyanobacterial isolates by 16S rDNA sequencing analysis
Genomic DNA of all purified cyanobacterial isolates was isolated following the protocol previously described (Phunpruch et al. 2016). The fragment of 16S rDNA of cyanobacterial isolates was amplified by polymerase chain reaction using a primer pair, F16S rDNA Cyano (5’-GCTCAGGATGAACGCTGGCG-3’) and R16S rDNA Cyano (5’-CGGCTACCTTGTTACGACTCCA-3’). PCR reactions and conditions were as described previously (Phunpruch et al. 2006). Amplified PCR products were purified using the Gel/PCR DNA fragments extraction kit (Geneaid, Taiwan) and sequenced in both directions with Big-Dye terminator cycle sequencing ready reaction kit (Perkin Elmer, USA) using ABI PRISM 3700 DNA analyzer at First BASE Laboratories (Malaysia). The taxonomic identification of cyanobacterial isolates was assessed by comparison of the obtained sequences to other cyanobacterial 16S rRNA genes available in the NCBI database using the basic local alignment search tool (BLAST) (Altschul et al. 1990).
Screening of cyanobacterial isolates for H2 production
In this study, H2 production by two-stage cell culture was measured. In the first stage, cyanobacterial isolates were grown in ASN III medium for 14 days to accumulate biomass. In the second stage, cells were incubated in nitrogen deprived ASN III medium under the light for 24 h to stimulate the production of ATP, reducing powers and storage glycogen. Then, cells were transferred to vials, purged with argon to enter anaerobic condition, and incubated in the dark for 24 h before H2 production assay. For screening of H2 producing cyanobacterial isolates, the 14-day grown cells were subsequently harvested by centrifugation at 7000×g at 4 °C for 10 min. The cell pellet was subsequently washed twice with NaNO3-free ASN III medium and subsequently resuspended in 100 mL of NaNO3-free ASN III medium. The cell suspension was further incubated for 1 day before harvesting cells to determine H2 production. The harvested cells were resuspended in 5 mL of NaNO3-free medium and transferred to a 10-mL gas-tight vial. H2 production was allowed to proceed for 24 h under four conditions: (1) anaerobic/dark condition, (2) microaerobic/light condition, (3) aerobic/dark condition, and (4) aerobic/light condition. Under anaerobic/dark condition, cell suspension was purged with argon gas for 5 min to eliminate O2 in a vial before placing the vial on a shaker with a speed of 120 rpm at 30 °C for 24 h. All processes were performed under darkness. Under microaerobic/light condition, cell suspension in a vial was also purged with argon gas for 5 min and incubated under light intensity of 30 μmol photons m−2 s−1 for 24 h. O2 could be produced in a small amount from photosynthesis (0.42–1.82% (v/v) in air). Under aerobic condition, purging with argon gas was omitted. The vial was incubated at 30 °C either under the light intensity of 30 μmol photons m−2 s−1 (aerobic/light condition) or in the dark (aerobic/dark condition) for 24 h.
Determination of H2 production and bidirectional hydrogenase activity
To determine the amount of H2 produced after 24 h incubation, 500 μL of gas phase in a vial were withdrawn by a gas-tight syringe. The amount of H2 was analyzed by a gas chromatograph (Hewlett-Packard HP890A GC, Japan) equipped with a thermal conductivity detector as previously described (Taikhao et al. 2013). The in vivo bidirectional hydrogenase activity was determined by measuring H2 produced in the presence of dithionite-reduced methyl viologen. Two milliliters of reaction mixture contained 1 mL of cell suspension and 1 mL of 5 mM methyl viologen and 20 mM Na-dithionite (in 25 mM potassium phosphate buffer, pH 7.0). The reaction was incubated under argon for 15 min. H2 production was determined by analyzing 500 μL of the gas phase in a vial by gas chromatography (Taikhao et al. 2013).
Optimization of H2 production by the selected cyanobacterial isolate
To study the effect of cultivation time on H2 production, Geitlerinema sp. RMK-SH10 was grown in ASN III medium for 7, 14, and 21 days, harvested, washed twice with NaNO3-free ASN III medium. The cell pellet was then resuspended in NaNO3-free ASN III medium. The cell suspension was shaken at 30 °C under the light for 24 h, subsequently harvested, resuspended in 5 mL of NaNO3-free medium, and incubated under darkness for 24 h before H2 production measurement. To investigate the optimization of the nutrient and mineral concentrations in medium for H2 production, 7-day grown cells were harvested, washed twice, and resuspended in NaNO3-free ASN III medium containing various concentrations of NaNO3, MgSO4·7H2O, NaCl, Fe3+, and Ni2+. To investigate carbon sources and concentrations, Na2CO3 as a carbon source in ASN III medium was replaced by different types of carbon source, NaHCO3, glucose, fructose, sucrose, lactose, and maltose, with equimolar concentration of C-atom (0.189 mmol C-atom L−1). The concentrations of optimal sugar source were varied from 0 to 189 mmol C-atom L−1.
Dry cell weight determination
The growth measurement of Geitlerinema sp. RMK-SH10 was performed by determination of dry cell weight. Ten milliliters of culture were filtered through a filter paper no. 1 (55 mm diameter) (Whatman, UK). The filter containing cells was subsequently washed twice by 10 mL of distilled water and dried at 60 °C in an oven for 1–3 days. The filter containing cells was put in a desiccator for 1 h before measuring the weight.
Statistical analysis
All H2 production values were presented as means ± SD from three independent biological replicates. Differences among treatments were analyzed by Duncan’s multiple range test with the P < 0.05 significance level using one-way ANOVA of the SPSS 24 software (IBM Corp., USA).
Results
Screening of potential H2 producing cyanobacterial isolates
Several cyanobacterial strains were isolated from samples of seawater, stones, sand, and shells in the Gulf of Thailand and the Andaman Sea in Thailand. Only 54 cyanobacterial isolates could be purified devoid of bacterial contamination. They were genetically identified by 16S rDNA sequencing and morphological analyses. Among them, 35 isolates were classified in genus Geitlerinema, 9 isolates were classified in genus Leptolyngbya, and 3 isolates each were classified in genus Phormidium and genus Synechococcus, respectively. The remaining four isolates were each classified in genus Chroococcus, Cyanothece, Pseudanabaena, and Synechocystis (Table 1). The cyanobacterial isolates were grown in ASN III medium for 14 days before incubation in NaNO3-free ASN III medium under the light at 30 °C for 24 h. The cultures were subsequently harvested and resuspended in 5 mL of NaNO3-free ASN III medium. The cell suspensions were transferred to a glass vial, and their H2 production was measured after incubation under four conditions for 24 h. The results showed that all cyanobacterial isolates except Leptolyngbya sp. PKR-ST1 could produce H2 under anaerobic/dark condition (Table 1). The filamentous cyanobacterium Geitlerinema sp. RMK-SH10 showed the highest H2 production of 273.701 ± 7.451 and 141.252 ± 11.845 μmol H2 g−1 dry weight under anaerobic/dark and microaerobic/light conditions, respectively, whereas low H2 production was detected under aerobic/dark and aerobic/light conditions, with more than half of the tested isolates had no H2 production (Table 1). Under aerobic/dark condition, Cyanothece sp. P-SH8.2.1 gave the highest H2 production of 142.422 ± 0.806 μmol H2 g−1 dry weight, whereas under aerobic/light condition, very low H2 production was detected whereby no cyanobacterial isolates showed H2 production higher than 10 μmol H2 g−1 dry weight (Table 1). From screening results, the Geitlerinema sp. RMK-SH10 (the description and values shown in italic) was chosen for further optimization for H2 production under anaerobic/dark condition.
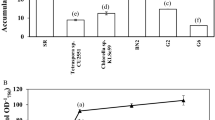
Effect of cultivation time on H2 production by Geitlerinema sp. RMK-SH10
Geitlerinema sp. RMK-SH10 was cultivated in ASN III medium, and its growth by dry cell weight measurement was monitored every 2 days. Figure 1 shows that Geitlerinema sp. RMK-SH10 could grow well in ASN III medium. To study the effect of growth phase on H2 production, cells were grown in ASN III medium for 7, 14, and 21 days before harvesting cells. Cells were then processed for H2 production measurement as described in materials and methods. The 7-day old cells of Geitlerinema sp. RMK-SH10 gave the highest H2 production rate of 13.382 ± 0.600 μmol H2 g−1 dry weight h−1 (Fig. 1), after which the H2 production was decreased. Therefore, further H2 production optimization study used 7-day grown Geitlerinema sp. RMK-SH10.
Biomass of Geitlerinema sp. RMK-SH10 cultivated in ASN III medium for 21 days and dark fermentative H2 production rate of 7-, 14-, and 21-day grown cells under nitrogen deprived condition. Data are means ± SD (n = 3). Different letters on columns indicate the significant difference according to Duncan’s multiple range test at P < 0.05
Effect of nutrient and mineral concentrations on H2 production by Geitlerinema sp. RMK-SH10
To investigate the effect of nitrate, sulfate, NaCl, Fe3+, and Ni2+ concentrations on H2 production, Geitlerinema sp. RMK-SH10 grown in ASN III medium for 7 days was harvested, washed twice, and resuspended in medium containing various concentrations of NaNO3, SO42−, NaCl, Fe3+, Ni2+, and glucose. The cultures were shaken at 30 °C under the light for 24 h, subsequently harvested, resuspended in 5 mL of the corresponding media, and incubated under anaerobic/dark condition for 24 h before analyzing H2 production. The highest H2 production of 318.237 ± 23.405 μmol H2 g−1 dry weight was found in cells incubated in NaNO3-free medium, an approximately 10-fold increase compared to that of cells incubated in the normal ASN III medium containing 8.8 mM NaNO3 (Fig. S1, Supplementary file). All following experiments for measurement of H2 production in this study were performed under N-deprivation. Under various SO42− concentrations, the highest H2 production of 387.383 ± 17.378 μmol H2 g−1 dry weight was found in cells incubated in NaNO3-free ASN III medium containing 1.4 mM MgSO4·7H2O, whereas at 0 and 14 mM MgSO4·7H2O, there were slight differences of H2 production (Table 2). Under various NaCl concentrations, maximum H2 production of 434.705 ± 25.337 μmol H2 g−1 dry weight was detected in cells incubated in N-free ASN III medium containing 0.2 M NaCl, an approximately 1.2-fold increase compared to that of cells incubated in the normal medium containing 0.4 M NaCl (Table 2). For Fe3+ and Ni2+, cells gave the highest H2 production of 391.501 ± 8.278 and 359.826 ± 6.836 μmol H2 g−1 dry weight when incubated under 2 μM Fe3+ and 0.1 μM Ni2+, respectively (Table 2). H2 production was enhanced with an increase of glucose concentrations up to 18.9 mmol C-atom L−1 where the maximum H2 production of 1458.094 ± 34.751 μmol H2 g−1 dry weight was observed (Table 2).
Effect of carbon sources on H2 production by Geitlerinema sp. RMK-SH10
Geitlerinema sp. RMK-SH10 grown in ASN III medium for 7 days was harvested, washed twice, and resuspended in N-free ASN III medium containing various carbon sources such as Na2CO3, NaHCO3, glucose, fructose, sucrose, lactose, and maltose with concentration of 0.189 mmol C-atom L−1. The cultures were shaken at 30 °C under the light for 24 h, subsequently harvested, resuspended in 5 mL of the corresponding media, and incubated under darkness for further 24 h before analyzing H2 production. The highest H2 production of 609.076 ± 41.562 μmol H2 g−1 dry weight was obtained in cells incubated in NaNO3-free ASN III medium containing glucose in place of Na2CO3 (Fig. 2).
Effect of different carbon sources on H2 production rate by Geitlerinema sp. RMK-SH10. Cells grown in ASN III medium for 7 days were incubated under different carbon sources with fixed concentration at 0.189 mmol C-atom L−1 for 24 h before measurement of H2 under dark/anaerobic condition. Data are means ± SD (n = 3). Different letters on columns indicate the significant difference, and the same letter indicates no significant difference according to Duncan’s multiple range test at P < 0.05
Maximum H2 production and bidirectional hydrogenase activity of Geitlerinema sp. RMK-SH10 under various conditions
In order to maximize dark fermentative H2 production, the effects of combined factors on H2 production and hydrogenase activity of Geitlerinema sp. RMK-SH10 incubated in various media containing different nutrients were investigated. The results showed that Geitlerinema sp. RMK-SH10 gave the highest H2 production rate of 0.271 ± 0.013 μmol H2 mg−1 dry weight h−1 and the highest hydrogenase activity of 0.389 ± 0.012 μmol H2 mg−1 dry weight min−1 when incubated in NaNO3-free ASN III medium containing 0.2 M NaCl, 18.9 mmol C-atom glucose L−1, and 0.1 μM Ni2+ for 4 h (Table 3). It should be noted that the maximum production of H2 at 2.083 ± 0.107 μmol H2 mg dry weight−1 was obtained by cells incubated in the same medium for 24 h (Table 3). This H2 production was approximately 70- and 6-folds higher than that of cells incubated in ASN III and NaNO3-free ASN III media, respectively.
Discussion
In this study, most of cyanobacterial isolates were identified as Geitlerinema which is a predominant benthic cyanobacterium found attached to shells and stones. Geitlerinema sp. could be found in both two coastlines of Thailand, the Gulf of Thailand connected to the Pacific Ocean, and the Andaman Sea connected to the Indian Ocean. All 54 purified cyanobacterial isolates were screened for H2 production under N-deprived four conditions. Most cyanobacterial isolates produced high amount of H2 under both anaerobic/dark and anaerobic/light conditions, with higher H2 production seen under the former condition (Table 1). Under N-deprivation, the selected Geitlerinema sp. RMK-SH10 was found to accumulate glycogen in the cells (data not shown). The increase of glycogen has also been reported for Synechocystis sp. PCC 6803 grown in nitrogen deficient medium under light (Monshupanee and Incharoensakdi 2014). During anaerobic fermentation the glycogen is catabolized via glycolytic pathway to provide sufficient amounts of ATP and NAD(P)H for H2 production by an activity of bidirectional hydrogenase (Troshina et al. 2002). The primary screening process for H2 production by cyanobacteria was mostly performed under N-deprivation (Ramana et al. 1990; Schütz et al. 2004; Allahverdiyeva et al. 2010; He et al. 2012). However, the deprivation of other compositions might also play an important role in cyanobacterial H2 production (Antal and Lindblad 2005; Raksajit et al. 2012). Cyanobacteria in this study generally produced less H2 in the light than in the dark because during light exposure cyanobacteria were able to split water to generate O2 as a main product of photolysis. The generated O2 inhibited the activity of bidirectional hydrogenase and nitrogenase (Fay 1992; Tamagnini et al. 2000) resulting in a decrease in H2 production.
In the preliminary screening, Geitlerinema sp. RMK-SH10 produced the highest H2 under anaerobic/dark condition when cells were incubated in NaNO3-free ASN III medium for 24 h (Table 1). Geitlerinema is a filamentous non-heterocystous cyanobacterium belonging to the order Oscillatoriales (Castenholz et al. 2001). All Geitlerinema isolates were single filamentous trichomes with a flexuous or straight shape. Each individual trichome contained a blue-green cylindrical filament with one or more granules of cyanophycin, one of which was close to the cross wall. Until now, no H2 production by marine or freshwater Geitlerinema has been investigated. Although the diazotrophic cyanobacterium Geitlerinema sp. PCC 9228 (formerly Oscillatoria limnetica “Solar lake”) was shown to be capable of N2 fixation in pure culture (Stal and Krumbein 1981) and contains a nitrogen fixation operon (nifHDK) and a bidirectional hydrogenase gene cluster (hoxEFUYH) but does not possess an uptake hydrogenase genes (hupSL) (Grim and Dick 2016), Geitlerinema sp. RMK-SH10 isolated from seawater in this study showed no detectable nitrogenase activity (data not shown). It is possible that H2 production of Geitlerinema sp. RMK-SH10 might be dependent on the bidirectional hydrogenase activity rather than nitrogenase activity.
The marine Geitlerinema sp. RMK-SH10 could grow in liquid ASN III medium (Fig. 1), because the synthetic seawater ASN III medium contains nutritional and mineral elements including NaCl which are required for marine cyanobacterial growth (Rippka et al. 1979). On the other hand, Geitlerinema sp. RMK-SH10 was not able to grow well in BG11 medium lacking NaCl, a common medium for freshwater cyanobacteria (data not shown).
The highest H2 production rate of 13.382 ± 0.600 μmol H2 g−1 dry weight h−1 was found in 7-day grown cells with a decreased production at a later growth phase (Fig. 1). This suggested that H2 production by Geitlerinema sp. RMK-SH10 is growth phase dependent. This result is similar to those previously reported in freshwater unicellular cyanobacterium Synechocystis sp. PCC 6803 (Baebprasert et al. 2010) and halotolerant unicellular cyanobacterium A. halophytica (Taikhao et al. 2013). The growth phase dependent H2 production has also been shown in a marine filamentous cyanobacterium Oscillatoria sp. Miami BG7 (Phlips and Mitsui 1983) and a marine unicellular cyanobacterium Synechococcus sp. Miami 04351 (Luo and Mitsui 1994) with highest production observed at the beginning of stationary growth phase and at the early log phase, respectively. Thus, H2 production in cyanobacteria is dependent on species and their growth phase.
Nutrients and microelements have been reported to play an important role in cyanobacterial H2 production (Datta et al. 2000; Carrieri et al. 2008). In Geitlerinema sp. RMK-SH10, the highest H2 production of 318.237 ± 23.405 μmol H2 g−1 dry weight was obtained in cells incubated in NaNO3-free ASN III medium, an approximately 10-fold increase compared to that of cells incubated in the normal ASN III medium containing 8.8 mM NaNO3 (Fig. S1, Supplementary file). The increase of H2 production under N-deprivation condition is ascribed to an increased electrons flow towards hydrogenase accompanying a degradation of the fermentative glycogen accumulated during photoautotrophic growth. This result is in agreement with previous studies in freshwater cyanobacteria Gloeocapsa alpicola (Serebryakova et al. 1998; Troshina et al. 2002) and Arthrospira maxima (Ananyev et al. 2008) as well as in marine cyanobacteria Oscillatoria sp. Miami BG7 (Kumazawa and Mitsui 1981), P. valderianum BDU 20041 (Prabaharan and Subramanian 1996), L. valderiana BDU 20041 (Prabaharan et al. 2010), and A. halophytica (Taikhao et al. 2013).
Sulfur deprivation does not appear to affect H2 production by Geitlerinema sp. RMK-SH10, since only slight differences of H2 production were observed in the absence and in the presence of 1.4 and 14 mM MgSO4·7H2O (Table 2). However, sulfur deprivation has been found to enhance the rate of H2 production in other cyanobacterial species such as G. alpicola, Synechocystis sp. PCC 6803, and A. halophytica (Antal and Lindblad 2005; Taikhao et al. 2013). Sulfur is a very important component in the photosystem II repair cycle (Wykoff et al. 1998). Lack of sulfur causes an inhibition of the oxygenic photosynthesis resulting in a decrease of O2 and thus leads to an enhancement of H2 production by G. alpicola and Synechocystis sp. PCC 6803 (Antal and Lindblad 2005).
The H2 production by Geitlerinema sp. RMK-SH10 was stimulated by low concentration of NaCl with the maximum of 434.705 ± 25.337 μmol H2 g−1 dry weight observed in cells incubated in NaNO3-free ASN III medium containing 0.2 M or 1.2% (w/v) NaCl, and at higher than 0.4 M NaCl, the production was decreased (Table 2). Salinity affects H2 production depending on the type of cyanobacterial species. In freshwater cyanobacteria, H2 production was highest in cells incubated in NaCl-free medium but it is decreased when NaCl concentration is increased (Shah et al. 2003). On the contrary, marine cyanobacteria produced the highest H2 at their optimal level of NaCl concentration; for example, marine cyanobacterium Lyngbya sp. strain 108 showed the highest H2 production in medium containing 3% (w/v) NaCl (Kuwada and Ohta 1989). Too high NaCl concentration reduces H2 production in all types of cyanobacteria, because the needed energy for H2 production was used to combat salinity stress by extrusion of Na+ ions out of cells or by prevention of Na+ influx into the cells (Tel-Or and Melhamed-Harel 1981; Rai and Abraham 1995).
Under various Fe3+ concentrations, the highest H2 production was found in cells incubated in NaNO3-free ASN III medium containing 2 μM Fe3+ (Table 2). ASN III medium contains Fe3+concentration at 2 μM that is suitable for cyanobacterial growth. This Fe3+ concentration is suitable for H2 production by Geitlerinema sp. RMK-SH10 because iron is required for hydrogenase activity to produce H2. Iron is normally a cofactor of NiFe-hydrogenase enzyme, and it enhances the electron transport process towards hydrogenase to evolve H2 (Lin and Stewart 1997). In addition, iron is also involved in the electron transport system in cyanobacterial cells, such as photosynthesis and respiration (Raven et al. 1999) and nitrogen fixation (Küpper et al. 2008). However, too high iron concentration at 200 μM drastically reduced H2 production, which might be due to the diversion of the energy and reducing power for the extrusion of excess iron out of the cells to alleviate iron toxicity.
Apart from iron, a trace amount of nickel also stimulated H2 production by Geitlerinema sp. RMK-SH10, with maximum production observed at 0.1 μM Ni2+ (Table 2). Nickel is known as a metal cofactor of NiFe–hydrogenase in cyanobacteria; therefore, nickel is required for hydrogenase activity (Axelsson and Lindblad 2002; Gutekunst et al. 2006). In addition, nickel may have other roles in cell function other than hydrogen metabolism (Daday et al. 1985). However, too high concentrations of Ni2+ resulted in a decrease of H2 production (Table 2). It is suggested that the optimal nickel ion concentration can maximize H2 production by Geitlerinema sp. RMK-SH10.
Among different types of carbon source, glucose seemed to be an optimal carbon source for H2 production by Geitlerinema sp. RMK-SH10 (Fig. 2) and the highest H2 production of 1458.094 ± 34.751 μmol H2 g−1 dry weight was found when glucose concentration was 18.9 mmol C-atom L−1 (Table 2). In Geitlerinema sp. RMK-SH10, the type of carbon source and its concentration had much greater influence on H2 production than that by other nutrients and minerals. When glucose catabolism occurs, it leads to an increase of NAD(P)H and ATP which are used for H2 production aided by nitrogenase or hydrogenase activity (Datta et al. 2000; Chen et al. 2008). However, in the case of Geitlerinema sp. RMK-SH10, only hydrogenase appeared to be responsible for H2 production since no nitrogenase activity was detected. The results of the present study are consistent with those of the previous reports where the halophilic cyanobacterium A. halophytica and a marine cyanobacterium Synechococcus sp. Miami BG 043511 used glucose as a carbon source for H2 production (Luo and Mitsui 1994; Taikhao et al. 2013).
In this study, the maximum H2 production rate, hydrogenase activity, and H2 accumulation were obtained in Geitlerinema sp. RMK-SH10 incubated in NaNO3-free ASN III medium containing 0.2 M NaCl, 18.9 mmol C-atom glucose L−1 and 0.1 μM Ni2+ (Table 3). H2 production by cells in each type of media corresponded well with hydrogenase activity. The results confirm that the absence of nitrogen source and the presence of optimal concentrations of NaCl, glucose, and Ni2+ in media promote hydrogenase activity and H2 production. Table 4 shows dark fermentative H2 production rates and conditions of various marine filamentous cyanobacterial strains in comparison with Geitlerinema sp. RMK-SH10 reported in this study. Geitlerinema sp. RMK-SH10 gave the highest H2 production rate with 0.271 μmol H2 g−1 dry weight h−1 or 6.072 mL H2 g−1 dry weight h−1 (Table 4). This rate is slightly higher than those of Oscillatoria sp. Miami BG7 and Phormidium valderianum BDU 20041, but much higher than those of other filamentous cyanobacteria. Thus, Geitlerinema sp. RMK-SH10 represents a filamentous cyanobacterium with high potential for H2 production.
In conclusion, several marine cyanobacterial strains isolated from seawater environments in the Gulf of Thailand and the Andaman Sea of Thailand were screened for H2 production. Among them, a marine cyanobacterium Geitlerinema sp. RMK-SH10 shows a high potential for H2 production under anaerobic/dark condition. It produces the maximum H2 yield and hydrogenase activity under the optimal conditions: no addition of NaNO3 but with an addition of 0.2 M NaCl, 18.9 mmol C-atom L−1 glucose, and 0.1 μM Ni2+.
References
Allahverdiyeva Y, Leino H, Saari L, Fewer DP, Shunmugam S, Sivonen K, Aro EM (2010) Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int J Hydrog Energy 35:1117–1127
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Ananyev G, Carrieri D, Dismukes GC (2008) Optimization of metabolic capacity and flux through environmental cues to maximize hydrogen production by the cyanobacterium “Arthrospira maxima”. Appl Environ Microbiol 74:6102–6113
Antal TK, Lindblad P (2005) Production of H2 by sulphur-deprived cells of the unicellular cyanobacteria Gloeocapsa alpicola and Synechocystis sp. PCC 6803 during dark incubation with methane or at various extracellular pH. J Appl Microbiol 98:114–120
Axelsson R, Lindblad P (2002) Transcriptional regulation of Nostoc hydrogenases: effects of oxygen, hydrogen, and nickel. Appl Environ Microbiol 68:444–447
Baebprasert W, Lindblad P, Incharoensakdi A (2010) Response of H2 production and Hox-hydrogenase activity to external factors in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Int J Hydrog Energy 35:6611–6616
Carrieri D, Ananyev G, Garcia Costas AM, Bryant DA, Dismukes GC (2008) Renewable hydrogen production by cyanobacteria: nickel requirements for optimal hydrogenase activity. Int J Hydrog Energy 33:2014–2022
Castenholz RW, Rippka R, Herdman M (2001) Phylum BX. Cyanobacteria, oxygenic photosynthetic bacteria. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey’s manual of systematic bacteriology. Springer, Berlin, pp 473–599
Chen PC, Fan SH, Chiang CL, Lee CM (2008) Effect of growth conditions on the hydrogen production with cyanobacterium Anabaena sp. strain CH3. Int J Hydrog Energy 33:1460–1464
Daday A, Mackerras AH, Smith GD (1985) The effect of nickel on hydrogen metabolism and nitrogen fixation in the cyanobacterium Anabaena cylindrica. J Gen Microbiol 131:231–238
Datta M, Nikki G, Shah V (2000) Cyanobacterial hydrogen production. World J Microbiol Biotechnol 16:8–9
Dutta D, De D, Chaudhuri S, Bhattacharya SK (2005) Hydrogen production by cyanobacteria. Microb Cell Factories 4:36–46
Fay P (1992) Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 56:340–373
Gram HC (1884) Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten. Fortschr Med 2:185–189
Grim SL, Dick GJ (2016) Photosynthetic versatility in the genome of Geitlerinema sp. PCC 9228 (formerly Oscillatoria limnetica ‘Solar Lake’), a model anoxygenic photosynthetic cyanobacterium. Front Microbiol 7:1546
Gutekunst K, Hoffmann D, Lommer M, Egert M, Suzuki I, Schulz-Friedrich R, Appel J (2006) Metal dependence and intracellular regulation of the bidirectional NiFe-hydrogenase in Synechocystis sp. PCC 6803. Int J Hydrog Energy 31:1452–1459
He M, Li L, Liu J (2012) Isolation of wild microalgae from natural water bodies for high hydrogen producing strains. Int J Hydrog Energy 37:4046–4056
Hoshaw RW, Rosowki JR (1973) Methods for microscopic algae. In: Stein (ed) Handbook of phycological methods, culture methods and growth measurements. Cambridge University Press, New York, pp 54–66
Houchins JP (1984) The physiology and biochemistry of hydrogen metabolism in cyanobacteria. Biochim Biophys Acta 768:227–255
Kothari A, Potrafka R, Garcia-Pichel F (2012) Diversity in hydrogen evolution from bidirectional hydrogenases in cyanobacteria from terrestrial, freshwater and marine intertidal environments. J Biotechnol 162:105–114
Kumar D, Kumar HD (1992) Hydrogen production by several cyanobacteria. Int J Hydrog Energy 17:847–852
Kumazawa S, Mitsui A (1981) Characterization and optimization of hydrogen photoproduction by saltwater blue-green algae, Oscillatoria sp. Miami BG7. I. Enhancement through limiting the supply of nitrogen nutrients. Int J Hydrog Energy 6:339–348
Küpper H, Šetlík I, Seibert S et al (2008) Iron limitation in the marine cyanobacterium Trichodesmium reveals new insights into regulation of photosynthesis and nitrogen fixation. New Phytol 179:784–798
Kuwada Y, Ohta Y (1989) Hydrogen production and carbohydrate consumption by Lyngbya sp. (No. 108). Agric Biol Chem 53:2847–2851
Lambert GR, Smith GD (1977) Hydrogen formation by marine blue-green algae. FEBS Lett 83:159–162
Leino H, Shunmugam S, Isojärvi J, Oliveira P, Mulo P, Saari L, Battchikova N, Sivonen K, Lindblad P, Aro EM, Allahverdiyeva Y (2014) Characterization of ten H2 producing cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int J Hydrog Energy 39:8983–8991
Lin JT, Stewart V (1997) Nitrate assimilation by bacteria. Adv Microb Physiol 39:1–30
Luo YH, Mitsui A (1994) Hydrogen production from organic substrates in an aerobic nitrogen-fixing marine unicellular cyanobacterium Synechococcus sp. strain Miami BG 043511. J Biotechnol Bioeng 44:1255–1260
Monshupanee T, Incharoensakdi A (2014) Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J Appl Microbiol 116:830–838
Phlips EJ, Mitsui A (1983) Role of light intensity and temperature in the regulation of hydrogen photoproduction by the marine cyanobacterium Oscillatoria sp. strain Miami BG7. Appl Environ Microbiol 45:1212–1220
Phunpruch S, Baebprasert W, Thongpeng C, Incharoensakdi A (2006) Nucleotide sequencing and transcriptional analysis of uptake hydrogenase genes in the filamentous N2-fixing cyanobacterium Anabaena siamensis. J Appl Phycol 18:713–722
Phunpruch S, Taikhao S, Incharoensakdi A (2016) Identification of bidirectional hydrogenase genes and their co-transcription in unicellular halotolerant cyanobcterium Aphanothece halophytica. J Appl Phycol 28:967–978
Prabaharan D, Subramanian G (1996) Oxygen-free hydrogen production by the marine cyanobacterium Phormidium valderianum BDU 20041. Bioresour Technol 57:111–116
Prabaharan D, Arun-Kumar D, Uma L, Subramanian G (2010) Dark hydrogen production in nitrogen atmosphere—an approach for sustainability by marine cyanobacterium Leptolyngbya valderiana BDU 20041. Int J Hydrog Energy 35:10725–10730
Prince RC, Kheshgi HS (2005) The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit Rev Microbiol 31:19–31
Rai AK, Abraham G (1995) Relationship of combined nitrogen sources to salt tolerance in freshwater cyanobacterium Anabaena doliolum. J Appl Bacteriol 78:501–506
Raksajit W, Satchasataporn K, Lehto K, Mäenpää P, Incharoensakdi A (2012) Enhancement of hydrogen production by the filamentous non-heterocystous Arthrospira sp. PCC 8005. Int J Hydrog Energy 37:18791–18797
Ramana CHV, Sasikala K, Rao PR, Subramanyam M (1990) Hydrogen production by cyanobacteria. I. Screening of unicellular and filamentous forms. Proc Indian Natl Sci Acad B56:361–366
Raven JA, Evans MCW, Korbs RE (1999) The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth Res 60:111–149
Reddy PM, Spiller H, Albrecht SL, Shanmugam KT (1996) Photo-dissimilation of fructose to H2 and CO2 by a dinitrogen-fixing cyanobacterium, Anabaena variabilis. Appl Environ Microbiol 62:1220–1226
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 1979:1–61
Saha SK, Uma L, Subramanian G (2003) Nitrogen stress induced changes in the marine cyanobacterium Oscillatoria willei BDU 130511. FEMS Microbiol Ecol 45:263–272
Schütz K, Happe T, Troshina O, Lindblad P, Leitão E, Oliveira P, Tamagnini P (2004) Cyanobacterial H2 production—a comparative analysis. Planta 218:350–359
Serebryakova LT, Sheremetieva M, Tsygankov AA (1998) Reversible hydrogenase activity of Gloeocapsa alpicola in continuous culture. FEMS Microbiol Lett 166:89–94
Shah V, Garg N, Madamwar D (2003) Ultrastructure of the cyanobacterium Nostoc muscorum and exploitation of the culture for hydrogen production. Folia Microbiol 48:65–70
Stal LJ, Krumbein WE (1981) Aerobic nitrogen fixation in pure cultures of a benthic marine Oscillatoria (cyanobacteria). FEMS Microbiol Lett 11:295–298
Taikhao S, Junyapoon S, Incharoensakdi A, Phunpruch S (2013) Factors affecting biohydrogen production by unicellular halotolerant cyanobacterium Aphanothece halophytica. J Appl Phycol 25:575–585
Taikhao S, Incharoensakdi A, Phunpruch S (2015) Dark fermentative hydrogen production by the unicellular halotolerant cyanobacterium Aphanothece halophytica grown in seawater. J Appl Phycol 27:187–196
Tamagnini P, Costa J-L, Almeida L, Oliveira M-J, Salema R, Lindblad P (2000) Diversity of cyanobacterial hydrogenases, a molecular approach. Curr Microbiol 40:356–361
Tamagnini P, Leitão E, Oliveira P, Ferreira D, Pinto F, Harris DJ, Heidorn T, Lindblad P (2007) Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol Rev 31:692–720
Tel-Or E, Melhamed-Harel H (1981) Adaptation to salt of photosynthetic apparatus in cyanobacteria. In: Akoyunoglou G (ed) Photosynthesis. Bablan International, Philadelphia, pp 455–462
Thajuddin N, Subramanian G (2005) Cyanobacterial biodiversity and potential applications in biotechnology. Curr Sci 89:47–57
Tiwari A, Pandey A (2012) Cyanobacterial hydrogen production—a step towards clean environment. Int J Hydrog Energy 37:139–150
Troshina O, Serebryakova LT, Sheremetieva M, Lindblad P (2002) Production of H2 by the unicellular cyanobacterium Gloeocapsa alpicola CALU743 during fermentation. Int J Hydrog Energy 27:1283–1289
Vyas D, Kumar HD (1995) Nitrogen fixation and hydrogen uptake in four cyanobacteria. Int J Hydrog Energy 20:163–168
Wykoff DD, Davies JP, Melis A, Grossman AR (1998) The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117:129–130
Funding
This study was financially supported by a research grant from the Faculty of Science, King Mongkut’s Institute of Technology Ladkrabang. A.I. thanks the Chulalongkorn University for the research grant on the Frontier Research Energy Cluster (CU-59-048-EN).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 19kb)
Rights and permissions
About this article
Cite this article
Tinpranee, N., Incharoensakdi, A. & Phunpruch, S. Screening cyanobacteria from marine coastal waters of Thailand for biohydrogen production. J Appl Phycol 30, 3471–3481 (2018). https://doi.org/10.1007/s10811-018-1490-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1490-6