Abstract
Manipulation of the nutrient concentration is an inexpensive and efficient method for increasing lipid and TAG accumulation in algal cells. However, high volumetric production requires finding a proper balance between the decrease of biomass production and the increase in the total lipid content. We isolated a strain of green microalga Bracteacoccus bullatus and increased its lipid content from 17 to 59% of biomass dry weight by manipulating of nitrogen and phosphorus content in the medium. The 10-fold reduction of the nitrogen and phosphorus concentration in the medium was the most efficient method of the lipid induction compared to nutrient deplete and high nutrient conditions. The oleic (48–64% mass of total fatty acids) and linoleic (14–24% mass of total fatty acids) acids dominated in the fatty acid profile, thus making this strain a suitable candidate for biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae have attracted attention as a promising source of lipids for biofuel production and human and animal nutrition (Bona et al. 2014; Fields et al. 2014; Maltsev et al. 2017a). Green microalgae can be successfully used for biodiesel production because of the fast growth rate at photoautotrophic conditions, high content of saturated and monounsaturated fatty acids, and ability to produce a large amount of triacylglycerides (TAGs) (Goncalves et al. 2016).
The fatty acid (FA) composition of lipids as well as a lipid content of microalgae can vary in a wide range depending on a multitude of factors (Khozin-Goldberg and Cohen 2006; Hu et al. 2008; Breuer et al. 2012; Guschina and Harwood 2013). The most common factors used for enhancing of lipid production are light stress and nutrient limitation. Nitrogen limitation is generally considered as the most effective method triggering the accumulation of lipids and fatty acids (Li et al. 2008; Lv et al. 2010; Li et al. 2011; Breuer et al. 2012; Bona et al. 2014; Fields et al. 2014). However, the highest biomass productivity and the high lipid content are usually obtained at different culturing conditions. Nitrogen limitation increases the lipid content but strongly reduces the growth rate. High lipid volumetric productivity is the outcome of these two factors and requires a balance between biomass production and lipid content (Griffiths and Harrison 2009; Lv et al. 2010).
Searching for new strains of algae with fast biomass accumulation rate, higher content of TAGs, and the optimal proportions of saturated and unsaturated fatty acids compared to the already known strains is one of the main directions of increasing efficiency of biodiesel production from algae biomass. The species of algae inhabiting biotopes with harsh environmental conditions can be very promising for this purpose. They are adapted to fluctuations of environmental conditions through the restructuring of physiological processes and changes in biochemical composition. Bracteacoccus Tereg (Chlorophyceae, Sphaeropleales) is a common terrestrial alga that occurs in a wide range of soil types and different climatic conditions (Fučíková et al. 2012; Scherbina et al. 2014; Maltseva et al. 2017; Maltsev et al. 2017b), often extreme, like Antarctica (Broady 1984), also in heavily polluted soils (Patova and Dorokhova 2008).
There is not much information about lipid metabolism of Bracteacoccus, but it has been shown that representatives of the genus Bracteacoccus contain up to 63% of lipids of biomass dry weight (Ratha et al. 2012; Minyuk et al. 2015). The aim of this paper was to study the influence of nitrogen and phosphorus limitation on the growth, lipid (total and TAGs), and fatty acid content of the recently described species Bracteacoccus bullatus Fuciková, Fletchner & L.A. Lewis. This paper also presents the results of the optimization of the growth conditions for high lipid production and high biomass content by a green microalga B. bullatus.
Materials and methods
Isolation and cultivation
The strain Bracteacoccus bullatus MZ-Ch11 was isolated from the soil of the Robinia forest located in the steppe zone of Ukraine (48° 45′ 24″ N, 35° 27′ 40″ E), Dnipropetrovsk region. Monoclonal cultures were established by micropipetting of single cells under an inverted microscope. The culture was isolated and grown on the modified WC medium (Guillard and Lorenzen 1972) with a 10-fold amount of nitrate and phosphate (WC*10). The WC*10 medium contained (in g L−1) 0.85 NaNO3, 0.114 K2HPO4·3H2O, 0.0126 NaHCO3, 0.0368 CaCl2·2H2O, 0.0212 Na2SiO3·9H2O, 0.5 g Tris free base, 0.037 MgSO4·7H2O; trace elements (in mg L−1) (4.36 Na2EDTA; 3.15 FeCl3·6H2O; 0.01 CuSO4·5H2O; 0.022 ZnSO4·7H2O; 0.01 CoCl2·6H2O; 0.18 MnCl2·4H2O; 0.006 Na2MoO4·2H2O; 1.0 H3BO3); and vitamins (0.1 mg L−1 thiamine, 0.0005 mg L−1 biotin). Microalgae were cultured in laboratory incubator shaker Multitron (Infors HT) at 24 °C, constant shaking at 150 rpm and 5% CO2 in the air supply. Light intensity was 160 μmol photons m−2 s−1 with 16/8-h light/dark photoperiod. The culture was deposited in the BOROK WDCM602 collection of the Papanin Institute for Biology of Inland Waters of the Russian Academy of Sciences. Nitrate and phosphate concentrations were measured by nitrate UV screening method and Permachem PhosVer 3 Phosphate Reagent using UV-Vis spectrophotometer DR 6000 (HACH-Lange). To obtain dry mass data, the biomass was harvested at the 14th day of cultivation, centrifuged (2900×g, 15 min), and lyophilized. Dry weight was recorded, and biomass was stored in a freezer at − 70 °C until extraction.
Five types of cultivation conditions were used for analysis of induction of lipid synthesis: WC*10 medium (control), standard WC medium with a single amount of nitrogen and phosphorus (WC*1), WC*10 without nitrogen (−N), WC*10 without phosphorus (−P), and WC*10 without nitrogen and phosphorus simultaneously (−N−P). All experiments were conducted in three independent culture replicates. Figures show the mean values and standard errors.
Molecular analysis
Total DNA of monoclonal cultures was extracted using InstaGene Matrix according to the manufacturer’s protocol. Fragment (641 bp) of partial 18S ribosomal RNA gene, internal transcribed spacer 1 (ITS1), 5.8S ribosomal RNA gene, and internal transcribed spacer 2 (ITS2) was amplified using primers ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) from White et al. (1990). Amplification of the fragment 18S rRNA-ITS1-5.8S rRNA-ITS2 was carried out using the pre-made mix ScreenMix (Evrogen, Russia) for the polymerase chain reaction (PCR). The conditions of amplification were an initial denaturation of 5 min at 95 °C, followed by 35 cycles at 94 °C for denaturation (30 s), 60 °C for annealing (30 s) and 72 °C for extension (60 s), and a final extension of 5 min at 72 °C. Purification of DNA fragments was performed using ExoSAP-IT kit (Affymetrix, USA). Sequencing was performed using a Genetic Analyzer 3500 sequencer (Applied Biosystems). The obtained sequences were edited manually and assembled using BioEdit v7.1.3, Ridom TraceEdit (ver. 1.1.0), and Mega6 (Tamura et al. 2013). Newly determined sequence and sequences of 21 other representatives of green algae from GenBank were included in the alignments (taxa and accession numbers are given in the tree, Fig. 1). Two species of non-Bracteacoccus species (Neochloris aquatic Starr and Scenedesmus obliquus (Turpin) Kützing) were chosen as the outgroup (Fig. 1).
The nucleotide sequences were aligned using Mafft v7 based on the E-INS-i model with default parameters (Katoh and Toh 2010). Bayesian information criterion (BIC) implemented in jModelTest 2.1.1 (Posada 2006) indicated that the Kimura 2-parameter model of nucleotide substitution with gamma (G) distributed rates across sites was the most appropriate evolutionary model for the 18S rRNA-ITS1-5.8S rRNA-ITS2 alignment. Phylogenies of these sequences were constructed based on this model using Bayesian inference (BI) and maximum likelihood (ML) analysis. BI analysis was conducted with MrBayes 3.2.5 (Ronquist and Huelsenbeck 2003). Three “hot” and one “cold” Markov chains were run for 2 × 106 cycles in two repetitions with the selection of each 200th generated tree. The first 25% of the trees were discarded; the phylogenetic tree and posterior probabilities of its branching were obtained on the basis of the remaining trees, having stable estimates of the parameter models of nucleotide substitutions and likelihood. ML analysis was performed using the program RAxML (Stamatakis et al. 2008). The nonparametric bootstrap analysis with 1000 replicas was used. The statistical support values were visualized in FigTree v1.4.0 (http://tree.bio.ed.ac.uk/).
The ITS2 secondary structure was estimated using the Mfold 2.3 web tool (Zuker 2003), the ITS2 database (Koetschan et al. 2010), and sequences and structures from Caisová et al. (2013). The secondary structures were viewed and edited using the software PseudoViewer2.5 and 3 (Byun and Han 2009).
Sequence from B. bullatus MZ-Ch11 obtained in this study was deposited to GenBank under accession number KY066480.
Determination of the total lipid content
Total lipid content was determined using method of Bligh and Dyer (1959) with modifications. Freeze-dried biomass portion of 200 mg collected at the 14 days of cultivation was mixed with 3.2 mL of methanol and homogenized. After homogenization, 4 mL of chloroform and 4 mL of methanol were added and mixed for 15 min. After mixing, 4 mL of chloroform and 4 mL of NaCl solution (0.3% w/v) were added. The upper fraction containing methanol and NaCl solution was discarded. Lower fraction containing chloroform and lipids was evaporated, and lipid content was determined gravimetrically.
Acylglyceride determination
Acylglycerides (TAGs, DAGs, MAGs) were extracted from 10 to 30 mg of freeze-dried biomass with 700 μL of methanol-dichloromethane (1:1) using Minilys homogenizer and glass beads (Bertin Technologies). The extract was centrifuged and the supernatant was collected. The extraction was repeated three times and three extracts were combined. Prior to analysis samples were filtered through a 0.22-μm PTFE filter. Samples were analyzed by HPLC in a gradient mode. HPLC analysis of the lipid extract was performed with Agilent 1260 Infinity series chromatograph with normal-phase column (250 mm × 4.6 mm, 5 μm Agilent HPLC column ZORBAX SIL) and evaporative light scattering detector (ELSD Agilent 4260). The temperature of the ELSD was kept at 50 °C, and the flow rate of the nebulizing gas N2 at 10 L min−1. The mobile phase was a mixture of a solvent A (a mixture of hexane 98% v/v and methyl tert-butyl ether 2% v/v) and a solvent B (a mixture of hexane 39.2% v/v, methyl tert-butyl ether 0.8% v/v, isopropanol 52% v/v, and water 8% v/v) at a gradient composition descending from 100% A to 100% B in 15 min and to 100% A in 25 min. The injection volumes of 10 μL and the flow rate of 1 mL min−1 were used in all experiments. The column temperature was held constant at 40 °C. The total analysis time was 25 min. The column and detector signal were calibrated with the mixture of standard samples of TAG, DAG, and MAG (1-monostearoyl-rac-glycerol C18:0, distearin C18:0, tristearin C18:0, all from Sigma-Aldrich). Individual peaks were identified by comparing their retention times with those of pure TAGs, DAGs, and MAGs in standard mixtures. The acylglycerides were quantified by peak area using calibration curve. Data acquisition was carried out by OpenLAB CDS ChemStation Edition software for LC and LC/MS Systems (Agilent Technologies). Statistical processing of HPLC and GC/MS data was performed in Microsoft Excel.
Fatty acid composition analysis
For fatty acid composition determination, 600 μL of nonadecanoic acid in hexane (375 ppm) used as the internal standard, three glass beads, and 2 mL of HCl methanol solution (1.25 M) were added to 5–10 mg of freeze-dried biomass. The sample was vortexed for 1 min and incubated for 2 h at 96.0 ± 0.1 °C in a water bath with vortexing every 30 min for 15 s. After 2 h of incubation, the sample was cooled to a room temperature and 1 mL of saturated NaCl solution was added to a mixture. After the phase separation, a 100 μL aliquot of the organic phase was sampled, diluted ten times with hexane, and analyzed by GC/MS system Thermo Scientific Trace GC Ultra DSQ II with temperature programming (140 °C for 5 min, heated to 280 °C at 10 °C min−1, isotherm 280 °C for 15 min; sample injection volume of 1 μL with 1:20 split; flow rate of helium > 99.9999% was 1.2 mL min−1; column was HP-5MS 30 m × 0.25 mm × 0.25 μm). The column was calibrated with fatty acid methyl ester standard samples (47885 and 47040, Supelco, USA). The fatty acid methyl esters were quantified by total ion current (EI, 70 eV) in the mass range 50–550 Da. Total fatty acid content was estimated as the sum of all detected fatty acids corrected by sensitivity of mass-selective detector according to Dodds et al. (2005).
Flow cytometry and fluorescent microscopy
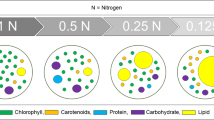
Neutral lipids in cells were stained by Nile Red (stock solution 500 mg L−1 in DMSO, Sigma-Aldrich) at the concentration of 7.85 μM for 15 min (Chen et al. 2009). The image of Nile Red-stained cells was captured by Nikon A1 confocal microscope with argon laser excitation and spectral detection (ex. BP 450/90 nm, em. LP 520 nm). The red color corresponds to chloroplasts and polar lipids. Lipid droplets with nonpolar lipids have a yellow color. The number of Nile Red-stained cells was estimated using Guava EasyCyte flow cytometer at the Yellow Channel (ex. 488 nm, em. 583/26 nm).
Data analysis
Results were obtained from three independent culture replicates. All results are presented as means ± SE. The significant differences between different groups were analyzed using a one-way ANOVA analysis with Tukey’s post hoc test. A significant difference between two groups was declared if P < 0.05.
Results
Taxonomic affiliation of the strain
Braceteacoccus bullatus MZ-Ch11 is a unicellular coccoid soil green microalga (Fig. 1). Vegetative cells are spherical, rarely ovoid, and 7.0–22.7 μm in diameter. Cell wall is thin or occasionally slightly thickening after the age of 6 months (up to 1.5 μm). Asexual reproduction is by aplanospore. Sexual reproduction is not observed.
According to the modern concept of taxonomy of Chlorophyta, B. bullatus belongs to Bracteacoccaceae family within Sphaeropleales (Fučíková et al. 2012). Phylogenetic analysis (ML and BI methods) of the partial 18S rRNA-ITS1-5.8S rRNA-ITS2 region showed that the strain B. bullatus MZ-Ch11 was closely related to other B. bullatus strains including the type strain SAG 2032 (Fig. 2). These species form a single clade (B) with sufficient statistical support (ML 99; BI 100).
Phylogenetic position of Bracteacoccus bullatus MZ-Ch11 constructed from an alignment with 21 sequences and 438 characters (18S rRNA-ITS1-5.8S rRNA-ITS2). Values above horizontal lines are bootstrap support as RAxML analyses (< 50 are not shown); values below horizontal lines are Bayesian posterior probabilities (< 80 are not shown). Model of nucleotide substitution—K2+G
The examination of the secondary structure of the ITS2 and the identification of compensatory base changes (CBCs) and hemi-CBCs (HCBCs) are used in the species-level taxonomy of green algae (Coleman 2003). The typical secondary structure of ITS2 of the representatives of the genus Bracteacoccus has four helixes and forked helix I (Fig. 3) (Keller et al. 2008; Fučíková et al. 2012). We performed analysis of the ITS2 secondary structure of strain MZ-Ch11 and the type strain of B. bullatus SAG 2032 and found no CBCs in the most conserved regions of helixes 1, 2, and 3 (Fig. 3). These results confirm the identification of the strain MZ-Ch11 as B. bullatus.
Growth parameters of Bracteacoccus bullatus
The highest dry weight (2.4 g L−1) of B. bullatus was reached on WC*10 medium with 10 mM nitrate and 0.5 mM phosphate after 14 days of cultivation (Table 1). In the phosphorus-depleted conditions the dry weight was 2.3 g L−1. On the WC*1 medium with reduced concentrations of nitrate and phosphate (1.0 and 0.05 mM, respectively), the biomass dry weight was lower and reached 2.1 g L−1. In the nitrogen-depleted conditions (−N), the dry weight was 1.9 g L−1. At the simultaneous nitrogen and phosphorus depletion (−N−P), the dry weight was 1.7 g L−1 that is 29% below than WC*10. The post hoc Tukey tests showed statistically significant (P < 0.05) difference between nitrogen-depleted (−N and −N−P) conditions and conditions with nitrogen (WC*10, WC*1, and −P).
Total lipid content and acylglyceride accumulation
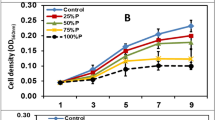
At the conditions with high nitrogen concentration (WC*10), the total lipid content was lowest and reached 17% of dry weight (Fig. 4, Table 1). The reduction of the nitrogen concentration led to increasing of total lipid content to 49–59% of dry weight while phosphorus-depleted cells contained 30% of lipids. Statistically significant (P < 0.05) differences were found between concentrations of the total lipids at reduced nitrogen concentrations (WC*1, −N, −N−P) from one side and conditions with high nitrogen concentration (WC*10 and −P). The increasing of total lipids in phosphorus-depleted cells (−P) comparing to WC*10 medium was statistically insignificant (P = 0.45).
Characteristics of the Bracteacoccus bullatus MZ-Ch11 grown in WC*10 and WC*1 medium, nitrogen-depleted conditions (−N), phosphorus-depleted conditions (−P), and nitrogen and phosphorus-depleted conditions (−N−P). a Biomass dry weight (g L−1). b Total lipid content (% of biomass). c Total lipid content (g L−1). d TAG content (% of biomass). e TAG content (g L−1). f Percentage of Nile Red-positive cells (% of total cell number). Data are mean ± standard error from three independent biological replicates
To assess the neutral lipid accumulation, the cells were examined by flow cytometry after Nile Red staining. Nile Red is a commonly used probe for neutral lipids that have a good correlation with other methods of lipid analysis such as gravimetric method (Chen et al. 2009). The number of Nile Red-positive cells was higher in the samples with nutrient deplete (58–66% of cells) comparing to nutrient replete conditions (14% in WC*10 medium, P < 0.05) (Fig. 4).
The lowest TAG content and the highest DAG content were observed in the cells grown in WC*10 medium (1.4 and 5.4% of dry weight, respectively). The cells that were grown at reduced concentrations of nutrients (WC*1, −N, −P, and −N−P) conditions contained much higher amount of TAGs (8.3 to 10.9%) and lower amount of DAGs (2.7 to 3.7%) (Table 1). MAGs were not detected in the studied samples. The low TAG content in comparison with total lipids may be caused by incomplete TAG extraction from biomass and also by the accumulation of other lipids, especially in nutrient-rich media.
Fatty acid composition
The fatty acid profile of the strain MZ-Ch11 was dominated by oleic acid (C18:1, from 48 to 64% mass of total fatty acids at different growth conditions), linoleic acid (C18:2, 14–24%), and palmitic acid (C16:0, 9–13%) (Table 2). The composition of fatty acids differed according to growth conditions. The fatty acid profile of B. bullatus grown on WC*10 medium was dominated by oleic acid (48%) followed by linoleic acid (24%) and palmitic acid (13%). In the WC*1 medium with reduced concentrations of nitrogen and phosphorus, the oleic acid content increased up to 64%. The content of other fatty acids (C16:0, 16:2, and 18:2) decreased with reduced concentrations of nitrogen and phosphorus starvation. Estimated total fatty acid content correlated with total lipids and varied from 13 to 63% of biomass dry weight (see Table 1). However, this estimation may not be precise because of the possible deviations of GC-MS sensitivity from the values reported by Dodds et al. (2005), especially for the polyunsaturated fatty acids.
Discussion
The ideal conditions for lipid production by algae are high biomass production and high lipid content. However, significant lipid production in algal cells usually occurs under stress conditions where biomass production is low. Oleaginous green algae show an average total lipid content of 25.5% dry weight at normal conditions, whereas at stressed conditions, the average content of total lipids increases to 45.7% dry weight (Hu et al. 2008). Experiments performed with B. bullatus MZ-Ch11 showed that nitrogen concentration in the medium strongly influenced the biomass accumulation. Tukey’s honest significance test showed a statistically significant decrease of biomass dry weight in the nitrogen and nitrogen-phosphorus-depleted conditions while phosphorus depletion or 10-fold reduction of nitrogen concentration did not decrease the biomass dry weight comparing to WC*10 medium.
The decrease of nitrogen concentration also strongly influenced the total lipid content. Tenfold reduced nitrogen, nitrogen-depleted, nitrogen and phosphorus-depleted conditions caused a 3-fold increase of total lipids from 17 to 49–59% of biomass dry weight. These values were higher than in the other strains of oleaginous green algae like Desmodesmus sp. WC08 (31.3% of biomass dry weight) (Zhang et al. 2014), Scenedesmus obliquus SAG 276-3 after N-limitation (43% of biomass dry weight) (Mandal and Mallick 2009), and comparable with the other top-performing oleaginous strains such as the eustigmatophyte alga Nannochloropsis oceanica (64.3% of biomass dry weight) (Wan et al. 2013).
The highest total lipid content, estimated total fatty acid content, and the highest biomass dry weight were observed at 10-fold reduced nitrogen concentration that resulted in the highest volumetric content of lipids in B. bullatus culture. It shows that the complete removal of nitrogen from the medium or alternatively high concentration of nitrogen (10 mM) negatively influences the lipid production by B. bullatus and optimal concentration of nitrogen in the medium should be 1 mM.
Contrary to biomass dry weight and total lipid content that were mostly determined by nitrogen concentration, the production of TAGs was stimulated by both nitrogen and phosphorus manipulation. The decrease of nutrient concentration increased TAG content in B. bullatus biomass from 1.4% in WC*10 medium to 8.3–10.9% at nutrient-depleted conditions. The number of Nile Red-positive cells also increased from 14% of cells in WC*10 medium to 61–66% at nutrient-depleted conditions. Nutrient limitation is widely used for increasing of TAG content in algae. Nitrogen depletion can induce TAG accumulation from 5% to more than 40% in Chlorella vulgaris, Neochloris oleoabundans, and Scenedesmus obliquus (Breuer et al. 2012). Phosphorus depletion increased TAG content from 6.5% under optimal conditions to 39.3% in eustigmatophyte Monodus subterraneus (Khozin-Goldberg and Cohen 2006). On the other hand, the highest biomass dry weight and lipid content of a green alga Rhopalosolen saccatus (Filarsky) Fott was observed under the standard growth conditions, and phosphorus limitation did not significantly increase both biomass dry weight and lipid content (Challagulla et al. 2015).
TAG content per cell of B. bullatus MZ-Ch11 strain was relatively high compared to other strains of microalgae. It varied from 1.6 pg cell−1 in WC*10 medium to 17.4 pg cell−1 under the nitrogen-deficient conditions. This is higher than the data obtained for M. subterraneus which accumulated 0.1 fg cell−1 of TAGs under optimal conditions and 1.7 fg cell−1 during phosphorus starvation (Khozin-Goldberg and Cohen 2006). TAG concentration in MZ-Ch11 is comparable with diatom Thalassiosira pseudonana Hasle & Heimdal (12.4 pg cell−1) and dinoflagellate Heterocapsa sp. (47.8 pg cell−1) (Mansour et al. 2005).
Volumetric concentration is one of the most important parameters for biotechnology. The highest biomass production and the highest lipid content are often obtained at the different culture conditions (Griffiths and Harrison 2009; Lv et al. 2010). For B. bullatus MZ-Ch11, we found that the volumetric TAG concentration under phosphorus depletion was higher than under nitrogen depletion (250.9 and 190.8 mg L−1, respectively). Tukey’s honest significance test also showed statistically significant difference between phosphorus and nitrogen-depleted conditions (P < 0.05). Nitrogen starvation reduced the biomass dry weight in medium much more than the phosphorus starvation (1.9 and 2.3 g L−1, respectively) although the increase of TAG content was comparable under nitrogen or phosphorus starvation (10.1 and 10.9%, respectively). It should be noted that discrepancies in total lipid content, TAG content, and total fatty acid content measured for strain MZ-Ch11 could be explained by various reasons. For example, total lipids could be overestimated by gravimetric methods, TAGs could be underestimated because of incomplete extraction or degradation, and total fatty acid values are estimated only indirectly. Nevertheless, well-correlated data on total lipids and TAGs show that they both increase with the change from WC*10 to WC*1 medium and remain approximately the same under nitrogen and combined nitrogen and phosphorus starvation. Phosphorus starvation alone decreases total lipids and total fatty acids, but slightly increases TAG content. These observations may be explained by possible decrease in polar lipids (particularly, phospholipids) accumulation.
The quality of biodiesel fuel is determined by the fatty acid composition. The saturated fatty acids increase the cetane number and resistance of biodiesel to degradation while unsaturated fatty acids enhance the cold flow of biodiesel (Talebi et al. 2013). B. bullatus MZ-Ch11 fatty acids contain 48–64% mass of oleic acid and 14–24% mass of linoleic acid (Table 2). Plant oils used for biodiesel production often contain oleic and linoleic acids as a major component. For example, rapeseed oil contains up to 64% of oleic and 12–22% of linoleic acid, soybean oil—21.8 and 54.9%, jatropha oil—34–45 and 29–44% (Kumar et al. 2003). Therefore, fatty acid composition of B. bullatus MZ-Ch11 is suitable for biodiesel production.
Conclusion
We studied lipid production by a new strain of green alga Bracteacoccus bullatus MZ-Ch11 at different concentrations of nitrogen and phosphorus. The highest volumetric production of lipids was observed at 1 mM nitrogen and 0.05 mM phosphorus concentration. The increase of nutrient concentration to 10 mM of nitrogen and 0.5 mM phosphorus resulted in higher biomass dry weight but decreased lipid content. Complete nitrogen depletion was less effective in terms of volumetric lipid production and biomass dry weight. The highest volumetric production of TAGs was obtained in the phosphorus-depleted medium with 10 mM nitrogen and no phosphorus. Considering that the highest production of biomass, lipids, and TAGs occurs under different conditions, further research is necessary in order to optimize the culture growth conditions.
References
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bona F, Capuzzo A, Franchino M, Maffei ME (2014) Semicontinuous nitrogen limitation as convenient operation strategy to maximize fatty acid production in Neochloris oleoabundans. Algal Res 5:1–6
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Broady PA (1984) Taxonomic and ecological investigations of algae on steam-warmed soil on Mt. Erebus, Ross Island, Antarctica. Phycologia 23:257–271
Byun Y, Han K (2009) PseudoViewer3: generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 25:1435–1437
Caisová L, Marin B, Melkonian M (2013) A consensus secondary structure of ITS2 in the Chlorophyta identified by phylogenetic reconstruction. Protist 164:482–496
Challagulla V, Fabbro L, Nayar S (2015) Biomass, lipid productivity and fatty acid composition of fresh water microalga Rhopalosolen saccatus cultivated under phosphorus limited conditions. Algal Res 8:69–75
Chen W, Zhang CH, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile Red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47
Coleman AW (2003) ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet 19:370–375
Dodds ED, McCoy MR, Rea LD, Kennish JM (2005) Gas chromatographic quantification of fatty acid methyl esters: flame ionization detection vs. electron impact mass spectrometry. Lipids 40:419–428
Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, Corredor L, Moll K, Peyton BM, Characklis GW, Gerlach R (2014) Sources and resources: importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. Appl Microbiol Biotechnol 98:4805–4816
Fučíková K, Flechtner VR, Lewis LA (2012) Revision of the genus Bracteacoccus Tereg (Chlorophyceae, Chlorophyta) based on a phylogenetic approach. Nova Hedwigia 96:15–59
Goncalves EC, Wilkie AC, Kirst M, Rathinasabapathi B (2016) Metabolic regulation of triacylglycerol accumulation in the green algae: identification of potential targets for engineering to improve oil yield. Plant Biotechnol J 14:1649–1660
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RR, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14
Guschina IA, Harwood JL (2013) Algal lipids and their metabolism. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 17–36
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Katoh K, Toh H (2010) Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26:1899–1900
Keller A, Schleicher T, Förster F, Ruderisch B, Dandekar T, Müller T, Wolf M (2008) ITS2 data corroborate a monophyletic chlorophycean DO-group (Sphaeropleales). BMC Evol Biol 8:218
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Koetschan C, Förster F, Keller A, Schleicher T, Ruderisch B, Schwarz R, Müller T, Wolf M, Schultz J (2010) The ITS2 Database III—sequences and structures for phylogeny. Nucleic Acids Res 38:D275–D279
Kumar MS, Ramesh A, Nagalingam B (2003) An experimental comparison of methods to use methanol and Jatropha oil in a compression ignition engine. Biomass Bioenergy 25:309–318
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Maltsev YI, Konovalenko TV, Barantsova IA, Maltseva IA, Maltseva KI (2017a) Prospects of using algae in biofuel production. Regul Mech Biosyst 8:455–460
Maltsev YI, Pakhomov AY, Maltseva IA (2017b) Specific features of algal communities in forest litter of forest biogeocenoses of the steppe zone. Contemp Probl Ecol 10:71–76
Maltseva IA, Maltsev YI, Solonenko AN (2017) Soil algae of the oak groves of the steppe zone of Ukraine. Int J Algae 19:215–226
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Mansour MP, Frampton DMF, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24–C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300
Minyuk GS, Chelebieva ES, Chubchikova IN (2015) Secondary carotenogenesis of the green microalga Bracteacoccus minor (Chlorophyta) in a two-stage culture. Int J Algae 25:21–34
Patova EN, Dorokhova MF (2008) Green algae in tundra soils affected by coal mine pollutions. Biologia 63:831–835
Posada D (2006) Modeltest server: a web-based tool for the statistical selection of models of nucleotide substitution online. Nucleic Acids Res 34:700–703
Ratha SK, Babu S, Renuka N, Prasanna R, Prasad RBN, Saxena AK (2012) Exploring nutritional modes of cultivation for enhancing lipid accumulation in microalgae. J Basic Microbiol 53:440–450
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Scherbina VV, Maltseva IA, Solonenko AN (2014) Peculiarities of postpyrogene development of algae in steppe biocenoses at Askania Nova Biospheric national park. Contemp Probl Ecol 7:187–191
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol 57:758–771
Talebi AF, Mohtashami SK, Tabatabaei M, Tohidfar M, Bagheri A, Zeinalabedini М, Mirzaei HH, Mirzajanzadeh M, Shafaroudi SM, Bakhtiari S (2013) Fatty acids profiling: a selective criterion for screening microalgae strains for biodiesel production. Algal Res 2:258–267
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Wan C, Bai FW, Zhao XQ (2013) Effects of nitrogen concentration and media replacement on cell growth and lipid production of oleaginous marine microalga Nannochloropsis oceanica DUT01. Biochem Eng J 78:32–38
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: A guide to methods and applications. Academic Press, New York, pp 315–322
Zhang S, Liu PH, Yang X, Hao ZD, Zhang L, Luo N, Shi J (2014) Isolation and identification by 18S rDNA sequence of high lipid potential microalgal species for fuel production in Hainan Dao. Biomass Bioenergy 66:197–203
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgements
All authors contributed equally to this work. In general, LLC “Solixant” is interested in the potential of microalgae as an alternative sustainable source of lipids. This does not alter the authors’ adherence to the Journal of Applied Phycology policies on sharing data and materials. This work was supported by Ministry of Education and Science of the Russian Federation (project 14.574.21.0137, identifier RFMEFI57417X0137).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mamaeva, A., Namsaraev, Z., Maltsev, Y. et al. Simultaneous increase in cellular content and volumetric concentration of lipids in Bracteacoccus bullatus cultivated at reduced nitrogen and phosphorus concentrations. J Appl Phycol 30, 2237–2246 (2018). https://doi.org/10.1007/s10811-018-1471-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1471-9