Abstract
Commercially manufactured extracts of a variety of seaweeds have been used extensively for several decades for the relief of abiotic and biotic stresses for (terrestrial) agricultural crops and horticulture, to improve yield and quality. However, the use of seaweed extracts for their beneficial properties, as applied to marine macroalgae, only began in the mid to late 2000s. Kappaphycus alvarezii is an important red seaweed on the coasts of tropical to sub-tropical waters, mainly because of the various applications of kappa carrageenan, which is the major industrial colloid extracted from this extensively cultivated biomass. Cultivation of this seaweed has brought economic benefits to tens of thousands of seaweed farmers in Southeast Asia and other minor producing countries. Recently, Ascophyllum (aka. Acadian) Marine Plant Extract Powder (AMPEP), a commercial seaweed extract from the brown intertidal, macroalga A. nodosum was used in steps taken during the micropropagation and field cultivation of K. alvarezii. The reasons for utilizing this treatment included addressing the current problems facing the industry, such as decreased productivity, loss of vigor and diminished crop quality. This was brought about by shortages in the availability of good quality propagules (seedlings) and also disease and endo-epiphyte infestations, which affected the ability to grow and harvest saleable biomass, thus decreasing the income, and therefore the interest and participation of the potential seaweed farmers (based on the factor of repetitive, “drudge” labor and their derived income per unit effort). This paper reviews studies on Kappaphycus including the use of AMPEP specifically and also some alternative extracts to mitigate both biotic and abiotic stressors, using examples from micropropagation, field cultivation, endophyte mitigation and impacts on the resulting carrageenan qualities. Taken together, this body of evidence provides proof of concept and very promising results which may lead to further studies more specifically to identify the modes of action and the metabolic pathways by which the complex AMPEP extract might improve stress tolerances in K. alvarezii in order to obtain higher productivity and enhanced quality characteristics (i.e., exposure to increasing surface seawater temperature, salinity fluctuations and photo-inhibitory irradiance as well as attacks by pathogenic and opportunistic organisms).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kappaphycus is a red alga grown predominantly in the tropics (Parker 1974; Doty 1985; Doty and Norris 1985; Hurtado et al. 2014a, b, 2015) and to a lesser extent in sub-tropical waters (Hayashi et al. 2014; Msuya et al. 2014). This seaweed is the world’s main commercial source of kappa carrageenan (Bixler and Porse 2011; Porse and Rudolph 2017). This type of carrageenan is primarily used in a wide range of processed food applications, cosmetics, personal care products and pharmaceutical ingredients (Campbell and Hotchkiss 2017; Loureiro et al. 2017). Most recently, in attempts to diversify and add further value to the cultivated biomass, Kappaphycus has been used as a source of biostimulant itself and it has been tested on several agricultural crops (Zodape et al. 2010; Babu and Rengasamy 2012; Pramanick et al. 2014; Singh et al. 2016; Sharma et al. 2017) and also as feedstock for multiple stream processing (biorefinery; Ortiz-Tena et al. 2017) and for biofuel (Neish and Suryanarayan 2017).
Commercial cultivation of Kappaphycus began in the Philippines in 1970 (Doty 1973; Parker 1974; Doty and Alvarez 1981; Trono and Valdestamon 1994), from where its practice spread to neighboring Southeast Asian countries (Adnan and Porse 1987; Hurtado et al. 2014a), the western Indian Ocean, particularly Tanzania, Madagascar, India, and Sri Lanka (Msuya et al. 2014), and to Central and South America (Robledo et al. 2013; Hayashi et al. 2014, 2017). It is hard to imagine the availability of many more newly established cultivation sites, especially in view of tightened restrictions on the relocation of marine genetic material from one part of the world to another (Cottier-Cook et al. 2016).
Commercial extracts from different sources of seaweeds (also known as aquatic plants for registration purposes) have been manufactured at an industrial scale for several decades to be used extensively for improved agricultural crop production and harvest quality (see: USDA 2016). These aspects have been comprehensively reported on by Craigie (2011) and Bhattacharyya et al. (2015). Seaweeds extracts are noted to (1) enhance root vigor (Crouch and van Staden 1992), (2) increase leaf chlorophyll content (Blunden et al. 1996), (3) increase the number of leaves (Rayirath et al. 2008); (4) improve fruit yield (Arthur et al. 2003; Kumari et al. 2011; Kumar and Sahoo 2011), (5) enhance the flavonoid content of treated plants (Fan et al. 2011), and (6) enhance vegetative propagation (Leclerc et al. 2006). However, the most substantial improvements and value proposition to the farmers associated with their applications of seaweed extracts are improved tolerances towards abiotic stresses, including drought (Zhang and Ervin 2004; Spann and Little 2011; Martynenko et al. 2016; Santaniello et al. 2017; Shukla et al. 2017; Van Oosten et al. 2017), ion toxicity (Mancuso et al. 2006), freezing (Rayirath et al. 2009), and high temperatures (Zhang and Ervin 2008). Van Oosten et al. (2017) proposed the alternate term: “bioeffector” to encompass the wide range of claims related to biotic benefits of the category of biostimulants (including seaweed extract applications, Shekhar Sharma et al. 2014). Some performance improvements include enhanced tolerance to biotic stresses (i.e., pathogens and disease agents, for. For a number of extracts of seaweeds multiple biotic responses are reported, see: Jayaraman et al. 2011; Coronado and Dionisio Sese 2014; Mireya et al. 2014; Shekhar Sharma et al. 2014; Stadnik and de Freitas 2014; Le Lann et al. 2016; Esserti et al. 2017a, b, 2018), but these fall outside of the normally accepted biostimulant definition for regulatory purposes (du Jardin 2015).
As an aside, it is interesting to note the recent development of commercial, plant biostimulants from the expressed sap of Kappaphycus alvarezii. It is not the place of this review to investigate the efficacy of such extracts but Trivedi et al. (2017) should be cited demonstrating parallels in applications of biostimulants derived from red and brown seaweeds and improved salinity tolerance of treated plants. In this case, the role of quaternary ammonium compounds are considered in terms of mode of action for the red seaweed extract.
A considerable amount of iterative research has been reported for the improved cultivation of many eucheumatoids of commerce (e.g., Kappaphycus and Eucheuma), not only in the Philippines but in all countries to which their seedstock has been dispersed for commercial reasons. The activity of seaweed farming has transformed the lives of tens of thousands of seaweed farmers as a result of their economic (cash) gains derived from farming (Valderrama et al. 2013). Benefits provided to carrageenan processors have been substantial and demand for carrageenan has increased on average 3% year−1 and is currently worth US$ 758 M with predictions to reach US$ 1047 M by 2020 (Markets and Markets 2016; see also Campbell and Hotchkiss 2017; Neish and Suryanarayan 2017).
However, after four decades of good economic returns, the marine agronomy of eucheumatoids faces a number of new challenges (not unlike the history of terrestrial farming), see Buschmann et al. (2017) for a review of the bottlenecks and “vital needs” of the industry to support its perceived potential for growth. The reduced quality and yields of carrageenophytes per unit area of cultivated raw materials are of considerable concern, the primary causes of which have been due to the apparent loss of strain vigor and repeated disease and pest infestations of unprotected crops. These issues may be considered to be entirely predictable, yet the carrageenan industry, as a whole, paid little attention until crop shortages became evident in 2008, particularly noted first in the Philippines then spreading to other areas of SE Asia (Baricuatro, pers. comm.; Hurtado et al. 2014). Reasons ascribed to declining seaweed crop production included the intensive nature of the farming activities, poor farm management, introduction of non-indigenous species to new farm sites, and climate change (Cottier-Cook et al. 2016). The fact that the total reported volumes of cultivated eucheumatoid seaweeds continued to increase was due to yet more carrageenophyte farms being established around the world (Hayashi et al. 2017).
There remains a limited, yet important group of studies undertaken on Kappaphycus cultivation and the use of commercial seaweed extract from the brown seaweed Ascophyllum nodosum in its micropropagation, field cultivation, mitigation of various epibionts and enhancement of final carrageenan qualities (yield and physical properties) (Hurtado et al. 2009, 2012; Loureiro et al. 2010, 2012, 2014a, b; Borlongan et al. 2011; Yunque et al. 2011; Marroig et al. 2016; Ali et al. 2017a, b; Tibubos et al. 2017).
The present authors would like to highlight reports made for academia and industry in an effort to inspire further scientific endeavor by researchers and new entrants to the industry involved in open-water, extensive Kappaphycus and Eucheuma seaweed production. It is important to continue the quest and improve the sustainability of the carrageenophyte industry. This paper reviews the uses of Ascophyllum (Acadian) Marine Plant Extract Powder (AMPEP) as manufactured from A. nodosum particularly for the benefits of the genus Kappaphycus (and its variants) based on their present (colloid) and future potential (biological activity) uses of high economic value (Bixler and Porse 2011; Campbell and Hotchkiss 2017; Porse and Rudolph 2017). New publications of Garcia-Vaquero et al. (2016); Guan et al. (2017); and Pereira et al. (2017) make an encouraging departure including examinations of new methods of extraction and in particular production of seaweed polysaccharides for their, as yet untapped, value-added potential as biologically active molecules (i.e., not just fibers for their rheology).
The uses of seaweed extracts of various seaweed outside of their traditional range of commercial (biostimulant) applications in terrestrial agriculture/horticulture, such as marine agronomy are in their infancy. The reviewers are only aware of one other example where a commercial seaweed extract (i.e., Kelpak® manufactured from the subtidal kelp Ecklonia maxima) has been applied to benefit enhance the growth of another cultivated seaweed (i.e., Ulva sp.; Robertson-Andersson et al. 2006). Very recently, Shi et al. (2017) used published a first use of an A. nodosum extract to enhance the antioxidant and defense systems of two freshwater microalgae. Whilst the reviewed studies here pioneer the applications of AMPEP for the enhanced cultivation of eucheumatoids, the authors of this review would also like to encourage the applied phycological research community to examine applications of other biostimulant/bioeffector sources as inputs to a well-managed, marine agronomy program (see also Buschmann et al. 2017). The principles for AMPEP treatments could also be applied to other groups of commercially important, cultivated seaweeds, and, at present, some studies are underway for the use of AMPEP in various stages of nori and kelp cultivation (no data available); a case of “watch this space.”
Various effects of AMPEP on Kappaphycus
Micropropagation
The first report on the use of an A. nodosum extract, coined AMPEP (Acadian Marine Plant Extract Powder), was made by Hurtado et al. (2009). Table 1 shows a summary of the Kappaphycus varieties (and their abbreviations) which were used in micropropagation (Hurtado et al. 2009). The authors claimed that a shorter duration was observed for shoot formation when the explants were treated with AMPEP+Plant Growth Regulator (PGR = PAA + zeatin at 1 mg L−1; PAA = phenylacetic acid), as compared to AMPEP when used as a single treatment. However, the four explants responded differently when it came to the number of days required for shoot formation. Hence, there were varietal differences in responses. The authors further observed that amongst the four explants used, the purple and adik-adik cultivars initiated shoot formation with the use of AMPEP only at the higher concentrations tested (i.e., 3–5 mg L−1, Table 1) after a shorter period based on the findings of the authors, the use of AMPEP alone and/or in combination with a PGR as a cost-effective and reliable culture medium was highly encouraging for this tissue culture technique.
The results of Hurtado et al. (2009) led to further investigations by Yunque et al. (2011) on the use of AMPEP in the micropropagation of different varieties of Kappaphycus. The authors optimized the AMPEP concentrations required, and determined the effects of pH-temperature combinations and explant density. Positive results were obtained using a number of the commonly available cultivars.
Table 2 summarizes results from the addition of AMPEP and various plant growth regulators (PGRs). Performance was evaluated based on the shortest period required for new shoot emergence. The study showed that an addition of PGR to low concentrations of AMPEP was the most effective treatment to hasten shoot formation. When brown and purple color morphotypes of K. alvarezii var. tambalang and a green morphotype of K. striatum var. sacol were tested for pH-temperature combinations with 1.0 mg L−1 of AMPEP + PGR (PAA and zeatin at 1 mg L−1), the brown morphotype produced the greatest number of shoots at pH 7.7, at 20 °C, after as little as 20 days. Data presented in Table 3 clearly demonstrate that each color morphotype of K. alvarezii responded differently with regard to the number of explants and volume of culture media combinations. This study again reinforced the findings that AMPEP +PGRs were effective in the enhancement of shoot formation, since it took only 9 days for both the green and purple color morphotypes of Kappaphycus, and 21 days for the brown variety, to initiate shoot formation, in all the densities investigated. However, within the ranges tested, the density of explants did not have a significant effect on the rate of shoot formation, but it did influence the average number of shoots generated from the culture. The reasons for these different observations still remain unknown, further. Future studies are required on the physiology and metabolic pathways of the different strains and morphotypes and when available will undoubtedly provide useful information for enhanced cultivation practices.
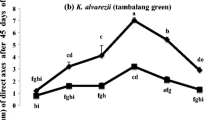
Hayashi et al. (2008) and Neves et al. (2015) reported on the use of spindle inhibitors such as colchicine and oryzalin, in combination with inorganic culture media such as von Stosch’s solution (VS 50) or 50% Guillard & Ryther solution (F/2 50), and synthetic ASP 12-NTA medium and PGRs on callus induction and direct shoot formation in K. alvarezii. The authors demonstrated that they were successful in regenerating microplantlets from their procedures; however, the report of Tibubos et al. (2017) using the same spindle inhibitors, with AMPEP K+ (a potassium fortified extract of A. nodosum) as the main culture medium, plus the addition of PGRs (i.e., IAA and kinetin at 1 mg L−1 each) was also highly encouraging. It should be noted that the former authors used inorganic culture media, while Tibubos et al. (2017) used an organic culture medium. The length of the direct axes formed, within 45 days of incubation, was the key indicator taken in determining the success rate of different combinations of AMPEP K+ with and without + PGRs and AMPEP K+ + PGRs + colchicine or oryzalin (Table 4). Table 4 summarizes the longest and shortest direct axes shoots formed with the corresponding combinations applied. Furthermore, significant differences were observed in the length of the newly formed shoots amongst the different concentrations of AMPEP K+, as used in combination with colchicine (P < 0.1) or oryzalin (P < 0.05). The use of either of the two spindle inhibitors resulted in more than 4–5 direct axis shoots per explant, as compared to control (those not treated with AMPEP K+ and PGRs only, which was 1–2 direct shoots). The efficacy of AMPEP was further reported by the recent findings of Ali et al. (2017a) on three strains of K. alvarezii and one strain of K. striatus in Sabah, Malaysia wherein the highest percentage of direct axes formed, K. alvarezii (tambalang brown), K. alvarezii (tambalang green), and K. striatus (sacol green) were recorded as follows: 100 ± 0.00, 99 ± 1.34, and 98 ± 2.66%, respectively. The authors further claimed that the shortest duration taken for the emergence of direct axes was observed in K. alvarezii (tambalang green) followed by tambalang brown and K. striatus (sacol green) on days 9, 10, and 15, respectively. All four studies reported on the use of AMPEP for the micropropagation of different color morphotypes K. alvarezii and K. striatus and concluded that the application of this brown seaweed extract could be used as a possible management protocol to mass produce robust and healthy plantlets for land-sea-based nursery cultivation operations for commercial purposes. Yong et al. (2014) and Luhan and Mateo (2017) also reviewed usage of inorganic and organic media (some including Ascophyllum seaweed extract) and additional PGRs with favorable responses for the clonal propagation of Kappaphycus for enhanced seedling production. Though the economics of using seaweed extracts for commercial purposes was beyond the scope of the earlier proof of concept studies, it would be worth determining that value in the near future. It is encouraging that other reports of using natural seaweed extracts (with and without additional PGRs) as media for micropropagation appears to be gaining interest, e.g., Sivanandhan et al. (2014) used simple water extracts of a red and a brown seaweed, Sargassum wightii and Gracilaria edulis, respectively, for both the micropropagation and metabolite enhancement of an important medicinal plant (Withania somnifera) producing steroidal lactones collectively called “withanolides.” In this evaluation, the red algal extract provided the most desired effects. Subsequently, Sharma et al. (2015) reported on using an extract from K. alvarezii for the mass production of Picrorhiza kurroa, which also enhanced production of bioactives in the treated medicinal herb. This extract was a commercial biostimulant (a pressed “sap” and not a chemically modified hydrolysate) provided by Sea6Energy Pvt. Ltd. (Bangalore, India). Esserti et al. (2017a, 2018) proposed growth media derived from extracts of Cystoseira myriophylloides and Fucus spiralis for in vitro plant tissue culture. Hence, it would seem seaweed extracts may play important multiple current and future, multiple roles in micropropagation of both land plants and seaweeds.
Field cultivation
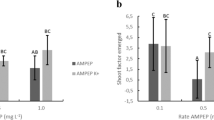
The efficacy of AMPEP at different concentrations (i.e., 0.01, 0.1, and 1.0 g L−1) and dipping duration (i.e., 30 and 60 min) was tested under field cultivation conditions, for optimization purposes, for three different color morphotypes of K. alvarezii, for a period of 3 months, which represented the wet season in the Philippines (Hurtado et al. 2012). During the period, from August to November, the three color morphotypes showed a distinct pattern of growth in response to the AMPEP treatments, i.e., the lower concentrations, irrespective of dip time, performed better. Significant differences in growth rate amongst the three color morphotypes were observed.
The efficacy of AMPEP was further evaluated in a commercial nursery using the yellowish-brown morphotype of K. alvarezii for a period of 12 months (Hurtado et al. 2012). Significant differences in growth rates were observed over the entire growth period. Expectedly, growth rates were much lower in July and August (the rainy months; lower SST and salinity) and they slowly increased thereafter. The proliferation of young, multiple shoots of the yellowish-brown K. alvarezii, grown in a commercial nursery, after 10–14 days of field growth was noteworthy to mention. This could be attributed to the presence of growth priming, promoting/signaling compounds within the Ascophyllum extract which stimulated auxin-like activity, which further promoted shoot growth (analogous to land plant, rooting responses, see Wally et al. 2013). The results of this study could be correlated with auxin-like, gibberellin-like (Rayorath et al. 2008a, b), cytokinin-like, precursors of ethylene and betaine stimulation (MacKinnon et al. 2010) which are individually and synergistically involved in growth and performance responses when land plants are treated with various seaweed extracts (Crouch and Van Staden 1993). Likewise, the treated eucheumatoids in this experiment were observed to be darkly pigmented and epiphyte-free, indicating the benefits of AMPEP in improving thallus pigmentation and enhancing resistance to epiphytism.
One further interesting result of the study of Hurtado et al. (2012) was that K. alvarezii (purple) and K. striatus (green) were analyzed for their phenolic content, and Fe+ chelating ability. Though no significant effects of AMPEP dipping were found, in regard to total phenolics in either K. alvarezii or K. striatum, the AMPEP-treated sacol green showed a significant increase (P < 0.05) in total antioxidant capacity during the October–November trial period. The tambalang purple strain with AMPEP treatment produced a sustained, high Fe2+ chelating ability from September to October through to January/February, which coincided with the enhanced growth of K. alvarezii in situ during that time frame. The increased free radical scavenging ability and transition metal chelating ability might be of interest from the point of view of resistance to pathogens and abiotic stresses (Dring 2006). The benefits ascribed to AMPEP are not necessarily exclusive to the processing of A. nodosum, as Catarino et al. (2017) reviewed the Fucaceae as being a resource for bioactive phlorotannins. Belda et al. (2016) provided evidence that polyphenols from the brown alga Himanthalia elongata provided protection from oxidative stresses. Further work is called for in this most important area of crop husbandry, and it will undoubtedly provide important insights to effective seedling production.
In the study of Loureiro et al. 2014a, Brazilian K. alvarezii was grown in tubular netting. However, there were no significant differences in the growth rates of treated seaweeds between the varied durations of dipping (p = 0.44). In another study (Marroig et al. 2016), also using K. alvarezii in tubular nets, the daily growth rates of treated seedlings were not significantly different between the cultivation period treatments applied (t = 0.92; p = 0.36, n = 15), with a total mean growth rate of 1.48 ± 0.84% day−1. However, the results did show a significant difference, when compared between sampling periods (F = 60.78, p < 0.001, n = 10).
The latest study to date, reporting on the use of AMPEP in the field cultivation of Kappaphycus seaweeds, was made by Ali et al. (2017b). The authors reported findings that the growth rate was species/variety specific, when grown for three consecutive, 45-day growth cycles (August–November) in Semporna, Sabah. Significant differences (P < 0.01) in growth rates between the species/variety and growth periods in relation to the treatment of AMPEP were observed.
Table 5 shows a summary of the daily growth rates of the different species/varieties grown in the field using AMPEP at different concentrations. The table illustrates that increased growth rates were obtained when Kappaphycus was dipped in AMPEP prior to out-planting in the sea.
Epiphyte mitigation
Kappaphycus cultivation in tropical and sub-tropical waters has seen marked increases (Hayashi et al. 2017) due to growing demand for its applications in the global market. It is utilized as an important structural ingredient in processed food applications, pharmaceuticals, cosmetics, and personal care products, and more recently as a source of raw biomass for biostimulant and biofuel production. However, the mass production of biomass may need to consider perhaps hitherto unforeseen ecological and societal consequences such disease outbreaks. The eucheumatoid seaweeds are typically produced extensively by vegetatively propagated monocrop systems. With the benefit of hindsight, it can be predicted that these types of farming methods are sensitive to the introduction of pests and pathogens; the reality is that there is a need to urgently change farm management practices (Cottier-Cook et al. 2016). The authors of this paper believe that greater societal benefits will be derived when the biological activity of multiple components of the cultivated biomass are fully appreciated and made available in a multi-use, zero effluent MUZE; biorefinery approach (Kapilkumar et al. 2017; Neish and Suryanarayan 2017; Ortiz-Tena et al. 2017).
“Ice-ice” malaise, epiphytes, and structurally damaging endophytes are detrimental to the health and growth of Kappaphycus. These problems are the primary causes of biomass loss in field cultivation which then had a “knock on” effect leading to shortages of dried seaweed for carrageenan processing, with an accompanying, considerable decrease in carrageenan yield and quality (i.e., average molecular weight of extract and almost elimination of both iota- and methylated- carrageenan content—see Mendoza et al. 2002). In addition, reduced biomass production also dramatically reduced the availability of good quality propagules (vegetative seedlings) for the next growth cycle and most importantly, represented an important loss of income and motivation for seaweed farmers, who may then be tempted to revert to less sustainable activities and alternative income generating activities out of sheer, economic necessity (Solante, per comm).
Though the ice-ice (whitening of the thallus) problem was first reported by Uyenco (1977) and Uyenco et al. (1981) in the Philippines, it was the epiphytic filamentous algae (EFA) (Ask 1999) infestation in Calatagan Is. Camarines Norte, Philippines, which came to be the most damaging (Largo 2002; Critchley et al. 2004; Hurtado et al. 2006). These reports were the earliest to provide an account of the incidence of EFA in Kappaphycus farms. EFA infestations (in terms of very large epiphytic loads on cultivated biomass) were later reported in other places such as Indonesia, Malaysia and Tanzania (Vairappan et al. 2008), China (Pang et al. 2012, 2015), and Madagascar (Ateweberhan et al. 2015; Tsiresy et al. 2016). Further studies showed that the culprits were species of Neosiphonia (i.e., N. apiculata and N. savatieri) which produced internal filaments within the eucheumatoid tissues and resulted in decay due to secondary bacterial infections and significant fragmentation of crops and losses from cultivation lines (Vairappan 2006; Vairappan et al. 2008). Tsiresy et al. (2016) stipulated their identification of the endophyte as a Polysiphonia sp. and suggested that Polysiphonia-Neosiphonia species exhibit different symptoms upon infecting various eucheumatoid species. However, the impacts of epiphyte/endophyte infestations are deleterious to crop production and the quality of the yield. In all cases, investigated so far, infestations have led to tissue disintegration and secondary bacterial infections of the carrageenophyte crops. Clearly, this threat is spreading within existing cultivation sites over an extremely wide geographical range, the likely remote vector being the international relocation of infected seedlings, and the marginal vectors for localized infections being the numerous reproductive spores of the polysiphonous epi/endophytes. There is an urgent need for dedicated efforts on these issues and it is fortunate that the GlobalSeaweedSTAR Initiative has projects to undertake further detailed research and provide well-founded interventions and best-practice management recommendations (Cottier-Cook et al. 2016).
The use of AMPEP was tested on Kappaphycus (both laboratory and field) in order to evaluate if dipping a red algal tissue in a brown seaweed extract could increase tolerances to biotic stressors. If so, this would be effect a response similar to that of a “bioeffector” as described for some land plants (Fan et. 2011; Rayirath et al. 2009; Van Oosten et al. 2017). Treatments evaluated if AMPEP had any consequences for associated pathogen and/or epi-endophytes. The presence of epiphytes such Cladophora sp. and Ulva sp. and Polysiphonia subtilissima on the apical tips of green, red, and brown variants of K. alvarezii (when dipping in to AMPEP solutions at 5, 10, 15, 20, 25, and 30 g L−1 for 1 h), was tested in Brazil (Loureiro et al. 2010. Of the associated epiphytes tested, Polysiphonia proved to be the most tolerant to AMPEP treatment since this red alga survived at the higher seaweed extract concentrations (i.e., 25–30 g L−1). The findings led to a more in-depth study on the in vitro relationships between red algal defense mechanisms and AMPEP treatments. It would be interesting to investigate if AMPEP treatments could be successful against the Polysiphonia sp. currently hindering eucheumatoid cultivation in Madagascar (Tsiresy et al. 2016); this may be tested in a future study. Loureiro et al. (2012) reported that the administration of the extract reduced the effects of a defensive, oxidative burst (i.e., production of hydrogen peroxide) by Kappaphycus which may be extremely aggressive both for both the host carrageenophyte and for its polysiphonous epiphytes. Bleaching of the non-corticated portions of P. subtilissima thalli which were cultivated as simulated epiphytes, confirmed that AMPEP protected K. alvarezii from the effects of its own superficial bursts of hydrogen peroxide. The same authors proposed that the use of AMPEP acted as a potential “vaccine,” eliciting the activation of the treated host’s natural defenses against pathogens and ameliorating the negative effects of self-harm due to long-term exposure to oxidative bursts. The modus operandi here seems to be not unlike the findings supporting Vacciplant®, a commercial biopesticide which was manufactured from Ascophyllum nodosum by Goëmar in France (Klarzynski et al. 2000). Further information on its applications for terrestrial crops affected by apple scab and fire blight are presented in Bernardon-Méy et al. (2013); mode of action including a patent were established based on the presence of laminarin in the extract and β-1,3 glucan activity (see also Klarzynski et al. 2000; Kadam et al. 2015; Raimundo et al. 2017). This vaccine-like effect also described for AMPEP-treated, Brazilian Kappaphycus was suggested to comprise a biochemically activated cascade of reactions initiated with the β-1,3 glucan (AMPEP)-β-1,3 glucanase (K. alvarezii) interaction (Fig. 1), thereby stimulating the production of volatile halogenated compounds which culminated in an oxidative pulse of self-cleansing peroxide (Loureiro et al. 2017). Esserti et al. (2017b, 2018) detailed further studies on the priming of antioxidant defense mechanisms of Lycopersicon (tomato, see also Stadnik and de Freitas 2014; Sangha et al. 2015) and Nicotiana (tobacco) using experimental extracts of Cystoseira myriophylloides, Laminaria digitata and Fucus spiralis which may provide new insights regarding modes of action of brown and red seaweed extracts, and new sources of raw materials and extracts for applications on plants and potentially, other seaweeds for the amelioration of biotic and abiotic stresses.
Suggested biochemical pathway for the beneficial effects of AMPEP on Kappaphycus alvarezii (after Loureiro et al. 2017)
AMPEP was also tested in the field for its ability to provide treated Kappaphycus with some level of tolerance to epiphytic infestations. Two varieties of Kappaphycus alvarezii from Sibugay, Zamboanga, Philippines, were tested using AMPEP dips, first as a source of nutrients for growth, and secondly to determine if such applications had any effect on the percentage occurrence of an epiphytic infestation of Neosiphonia sp., at four different depths in open-water cultivation. Borlongan et al. (2011) reported that an increased growth rate was significantly (P < 0.05) influenced by the pre-treatment of seedlings by dipping with AMPEP prior to out-planting, and likewise, a significantly reduced percentage occurrence of Neosiphonia sp. was found. A lower percentage occurrence (6–50%) of Neosiphonia sp. was assessed when K. alvarezii was dipped in AMPEP, as compared to control (10–75%). Their study further demonstrated that K. alvarezii had to be grown between 50 and 100 cm below the water surface in order to reduce the incidence of the damaging endophyte. Traditionally, K. alvarezii and K. striatus (although both normally subtidal in their natural habitat) are grown at, or near, to the surface of the water, exposing them to intense sunlight, this was considered a crucial factor in the huge Polysiphonia sp. incidence in Calaguas Is. Camarines Norte, Philippines (Hurtado et al. 2006) and possibly Madagascar (Tsiresy et al. 2016). The findings of Borlongan et al. (2011) were further supported by Ali et al. (2017b) where K. alvarezii (both Crocodile and Giant variants) and K. striatus were dipped in AMPEP at 0.1 g L−1 concentration for a duration of 45 min which resulted in a lower incidence of Neosiphonia sp., as compared to control, this study was carried out in the waters of Semporna, Sabah, Malaysia.
The work of Marroig et al. (2016) showed that when K. alvarezii was not dip-treated with AMPEP (i.e., the control), a higher epibiont biomass (i.e., 0.76 ± 0.52 g dwt tubular net−1) was recorded, whilst the AMPEP-treated samples had significantly (P < 0.002) less epibiont biomass (i.e., 0.26 ± 0.27 g dwt tubular net−1). Their findings demonstrated the prompt response of carrageenophyte tissues to AMPEP treatment and provided notable benefits against the early settlement of epibionts, especially during the first two growth periods. The rapid response of the seedlings with reduced, early epibiont spore settlement, and provision of ongoing protection, until the second sampling period can be explained by the defense-elicitation which AMPEP initiates in K. alvarezii (and a wide variety of its cultivars) which reverts to the possible vaccine-like, mode of action explanation as proposed by Loureiro et al. (2012). Though the epibionts measured and reported by Marroig et al. (2016) were not fully identified taxonomically, their results clearly demonstrated the positive and powerful effects of AMPEP in the mitigation of epi-endophytes, consequently, improving the growth and industrial quality of the treated host plants.
In the study of Ali et al. (2017b), the percentage occurrence of Neosiphonia apiculata on K. alvarezii (Crocodile and Giant) and K. striatus ranged from 35.6 ± 1.1–41.6 ± 2.4% which were treated with AMPEP prior to out-planting. Those in the control groups ranged from 44.4 ± 0.9–86.3 ± 2.2% over the same three, consecutive 45-day growth cycles and these were found to be significantly different from one another (P < 0.01). In general, a higher percentage occurrence of N. apiculata was observed in the controls. Amongst the three Kappaphycus tested, K. striatus seemed to be more tolerant to N. apiculata, especially during the Sept.–Oct. growth period. A positive growth rate (2 ± 0.4% day−1) and a lower percentage incidence of N. apiculata (53.8 ± 2.1%) were obtained, as compared to K. alvarezii (Crocodile and Giant, viz. growth of − 2.1 ± 0.35% and − 3.3 ± 0.91%, respectively) with concomitantly higher incidences of N. apiculata (75 ± 3.9% and 86.3 ± 2.2%, respectively).
Carrageenan quality
The global market demand for kappa carrageenan, as extracted from the cultivated biomass of Kappaphycus and its commercial varieties, has many valuable applications as a food texturizing ingredient. Growth in demand for these end-use markets has been steady over the ten-year period 2006–2015, with an estimated growth of 1.5–3% year−1, in each sector (Hotchkiss et al. 2016). This growth is reflective of the global trend and increasing demands for processed/convenience foods and was in response to both population growth and changes in regional economies and food aspirations/preferences. Hence, strong demand for the dried carrageenophytes is expected to continue (Campbell and Hotchkiss 2017).
Both AMPEP-treated and control Kappaphycus alvarezii were subject to lethal, low temperatures (e.g., 16–18 °C) under laboratory conditions, in order to determine their response, growth rate, and carrageenan quality. Though lower carrageenan yield and viscosity were measured when K. alvarezii was treated with AMPEP, at the three temperatures tested, the yield values obtained for the treated material (ca. 30%) was within industry standards (Hayashi et al. 2007; Hung et al. 2009; Góes and Reis Goes and Reis 2011, Góes and Reis 2012; Loureiro et al. 2014a). However, in addition, an increased gel strength was measured in the AMPEP treatments. These authors concluded that the use of AMPEP as a treatment for K. alvarezii seedlings in Brazil could be used as a farmer-mediated response to the effects of the seasonally lower SST, which is a factor delaying out-planting of field material due to the detrimental effects of “colder water” on the production of eucheumatoid crops.
Table 6 shows a summary of carrageenan quality characteristics of the different Kappaphycus species/varieties when dipped with AMPEP. When AMPEP-treated and control K. alvarezii were grown on commercial floating rafts in Brazil, the carrageenan yield was highest in the treated samples (p < 0.001) after 20 days of growth. Likewise, after 40 days, the carrageenan yield in the treated samples was significantly higher (p = 0.009) than the control. However, the extracted carrageenan gel strength was higher (p = 0.03) and the viscosity was highest in the samples which were not treated with AMPEP (i.e., control; p < 0.001; Loureiro et al. 2014b). The same authors postulated that the higher carrageenan yields obtained in the AMPEP-treated K. alvarezii may be explained in the light of the fact that the biostimulant/bioeffector can act as both elicitor or primer of responses to abiotic stress conditions, thereby prompting the seaweed’s defenses resulting in greater production of carrageenan, which is a structural component of the thalli to provide strength and flexibility in its natural habitat (Loureiro et al. 2012). This might explain the higher carrageenan yield results of K. alvarezii samples exposed to AMPEP as early as 20 days after the initiation of cultivation, when compared to the control samples. However, the findings of Marroig et al. (2016) using AMPEP, also on K. alvarezii on commercial floating rafts was the opposite to those findings of Loureiro et al. (2014a), since the former authors obtained higher gel strengths than those of the control (no AMPEP treatment), but a lower gel viscosity. However, higher carrageenan yields were obtained in AMPEP-treated K. alvarezii. The carrageenan yield (44.65 ± 7.09%) showed no significant differences between the treatments (t = 0.46; p = 0.64), with larger percentages by the end of the third sampling period (F = 130.96, p < 0.001). The findings of Loureiro et al. (2014b) and Marroig et al. (2016) agreed with the findings of Ali et al. (2017b) in that, relative to control, there were higher carrageenan yields for both refined carrageenan (RC) and semi-refined carrageenan (SRC) when two varieties of K. alvarezii (Crocodile and Giant) and K. striatus were pre-dipped in AMPEP and out-planted on long-lines in Semporna, Sabah, Malaysia. However, between the RC and SRC formats, in the treated samples, the carrageenan yield and viscosity were higher in the SRC with the gel strength of RC being much higher.
Conclusions
AMPEP, a commercial extract from the brown seaweed Ascophyllum nodosum, was used singly or in combination with PGRs (e.g., IAA and Kinetin) and spindle inhibitors (e.g., colchicine and oryzalin). It was demonstrated to be an effective culture medium for the mass generation of microplantlets which are increasingly, desperately required for nursery purposes and which ultimately enhance field cultivation through biomass production and colloid quality. The use of AMPEP has been demonstrated as a suitable biostimulant for the benefit of early production of these red seaweed seedlings, providing levels of protection against abiotic stresses. The ability of AMPEP to act as a potential vaccine, eliciting/priming activation of eucheumatoid seaweeds’ endogenous, natural defenses against pathogens, and ameliorating the negative effects of self-imposed, long-term exposure to oxidative bursts is another noteworthy finding for the multiple modes of action of AMPEP as a bioeffector. Taken together, these findings suggest the AMPEP treatment of seedlings prior to out-planting can play an important, economic role in reducing superficial epiphytes and effects of damaging endophyte settlement and development to the point of dramatic crop losses.
There are many parallels to be drawn between land plant and selected red seaweed responses. One other account of AMPEP benefits can be found using the green alga, Ulva (Kumar et al. 2013). The authors showed that supplementation of AMPEP (150 μg mL−1) to their culture medium significantly reduced the accumulation of ROS and lipid peroxidation together with the inhibition of lipoxygenase (LOX) activity specially LOX-2 and LOX-3 isoforms. Likewise, the positive results of AMPEP treatment on economically important carrageenan quality characteristics such as yield, viscosity, and gel strength are not to be under-estimated.
Clearly, further work is required on concentrations and application timings of AMPEP and treatments for multiple groups of other cultivated seaweeds (e.g., agarophytes and alginophytes) should be investigated. The protocols required would be very similar to the ongoing work where the product is used for its biostimulant/bioeffector properties, with multiple benefits for terrestrial crops. AMPEP when constituted as a solution is a complex mixture of hydrolysed components of the Ascophyllum nodosum thalli and does not provide a “single chemistry” (i.e., one compound/one defined response), as with many agrochemicals or pharmaceuticals. To date, there has been very little work undertaken on the role extracts of seaweed may play in the future of marine agronomy. It is quite plain to see that there will be many needs for inputs such as fertilizers, biostimulants, and bio-pesticides for the future, sustainable practices of seaweed husbandry on a massive scale. The time has come for this situation to change if seaweed agronomy is to reach its fullest global potential (Cottier-Cook et al. 2016; Buschmann et al. 2017). It is patently evident that further work is called for to examine which components of a commercial extract of the brown seaweed such as Ascophyllum effects the priming and signaling physiological pathways of a red seaweed such as Kappaphycus.
The fundamental question remains, why would a brown seaweed extract have such significant impacts on the growth and productivity of land plants and some selected seaweeds? The extract is produced by hydrolysis (in the case of AMPEP, open atmosphere and KOH). AMPEP is only one commercial extract, and there are others from a number of brown seaweeds (Shekhar Sharma et al. 2014), different methods of hydrolysis are employed by different manufacturers, as in atmospheric pressure and various pH; see United States Department of Agriculture (USDA) regulations and definitions related to the organic uses of aquatic plant extracts (USDA 2016). The interactions of different biomass and different extraction procedures results in pools of different hydrolysates; Shekhar Sharma et al. (2014) reviewed a range of plant biostimulants and extraction methods in relation to abiotic and biotic properties of the extracts. Although Ascophyllum processed over different seasons and from different geographies had “remarkably similar extract profiles” (Craigie et al. 2007), the same species of different macroalgae processed by different techniques resulted in different biostimulant characteristics (Craigie 2011; Sangha et al. 2015; Goñi et al. 2016; Lötze and Hoffman 2016; Olivares-Molina and Fernández 2016; Urbani et al. 2016; Moreira et al. 2017) therefore expressing different efficacies and properties when applied in bioassays and field trials. Whilst the extracts are described as being “crude,” they indeed contain many products of hydrolysis not normally found in nature. The processing is not merely liberation of active compounds within the seaweed biomass, bioactivity is created as a result of the chemical reactions taking place during hydrolysis, yet even to date, these chemical reactions remain to be characterized. In essence, such extracts comprise new compounds resulting from the interaction of algal thalli and the hydrolytic process applied. Most seaweed (aquatic plant) extracts are sold for applications in agriculture/horticulture on the basis of their beneficial effects on plants and their alleviation of abiotic and biotic stresses (Craigie 2011; Bhattacharyya et al. 2015), yet very few modes of action have been elucidated. It would be extremely germane if the nature of the signaling/priming compounds within a seaweed extract could be ascertained and then to understand how these might have conserved/analogous functions across terrestrial plants and seaweed physiological pathways in general.
From this review, it would seem that there is an urgent need to elucidate the biochemical and physiological pathways behind the observations of positive benefits and current applications and learn to use extracts of Ascophyllum (and potentially other seaweeds) strategically and surgically (i.e., effective rates and timings of applications) for its most cost-effective benefits. In particular, it is anticipated that AMPEP can make further significant contributions to the successful and sustainable production of global seaweed raw material requirements.
References
Adnan H, Porse H (1987) Culture of Eucheuma cottonii and Eucheuma spinosum in Indonesia. Hydrobiologia 151/152:355–358
Ali MM, Sani MZB, Hi KK, SMd Y, Critchley AT, Hurtado AQ (2017a) The comparative efficiency of a brown algal-derived biostimulant extract (AMPEP), with and without supplemented PGRs: the induction of direct, axis shoots as applied to the propagation of vegetative seedlings for the successful mass cultivation of three commercial strains of Kappaphycus in Sabah, Malaysia. J Appl Phycol. https://doi.org/10.1007/s10811-017-1366-1
Ali MM, Yashir S, Critchley AT, Hurtado AQ (2017b) Impacts of Ascophyllum Marine Plant Extract Powder (AMPEP) on the growth, incidence of the endophyte Neosiphonia apiculata and associated carrageenan quality of three, commercial cultivars of Kappaphycus. J Appl Phycol. https://doi.org/10.1007/s10811-017-1312-2
Arthur GD, Stirk WA, van Staden J (2003) Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S Afr J Bot 69:207–211
Ask E (1999) Cottonii and spinosum cultivation handbook. FMC Food Ingredients Division, Philadelphia 52pp
Ateweberhan M, Rougier A, Rakotomahazo C (2015) Influence of environmental factors and farming technique on growth and health of farmed Kappaphycus alvarezii (cottonii) in southwest Madagascar. J Appl Phycol 27:923–934
Babu S, Rengasamy R (2012) Effect of Kappaphycus alvarezii SLF treatment on seed germination, growth and development of seedling in some crop plants. J Acad Ind Res 1:186–195
Belda M, Sanchez D, Bover E, Prieto B, Padrón C, Cejalvo D, Lloris JM (2016) Extraction of polyphenols in Himanthalia elongata and determination by high performance liquid chromatography with diode array detector prior to its potential use against oxidative stress. J Chromatogr B 1033-1043:334–341
Bernardon-Mery A, Joubert J-M, Hoareau A (2013) Laminarin used against apple scab. Phytoma 662:4
Bhattacharyya D, Zamani M, Pramod B, Prithiviraj R (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Blunden G, Jenkins T, Liu YW (1996) Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol 8:535–543
Borlongan IAG, Tibubos KR, Yunque DAT, Hurtado AQ, Critchley AT (2011) Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J Appl Phycol 23:615–621
Buschmann AH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MC, Pereda SV, Gomez-Pinchetti J-L, Golberg A, Tadmor-Shalev N, Critchley AT (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Campbell R, Hotchkiss S (2017) Carrageenan industry market overview. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on spinosum and cottonii of commerce. Springer, Dodrecht, pp 193–205
Catarino MD, Silva AMS, Cardosa SM (2017) Fucaceae: a source of bioactive phlorotannins. Int J Mol Sci 18(6). doi:https://doi.org/10.3390/ijms18061327
Coronado AS, Dionisio Sese ML (2014) Antimicrobial property of crude ethanolic extract from Sargassum crassifolium J.G. Agardh. Asian J Microbiol Biotechnol Environ Sci 16:471–474
Cottier-Cook EJ, Nagabhatla N, Badis Y, Campbell ML, Chopin T, Dai W, Fang J, He P, Hewitt CL, Kim GH, Huo Y, Jiang Z, Kema G, Li X, Liu F, Liu H, Liu Y, Lu Q, Luo Q, MaoY MFE, Rebours C, Shen H, Stentiford GD, Yarish C, Wu H, Yang X, Zhang J, Zhou Y, CMM G (2016) Safeguarding the future of the global seaweed aquaculture industry. UNU-INWEH and SAMS, South Hamilton, 12 pp
Craigie J (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Craigie JS, MacKinnon SL, Walter JA (2007) Liquid seaweed extracts identified using 1H NMR profiles. J Appl Phycol 20:665–671
Critchley AT, Largo D, Wee W, Bleicher L’honneur G, Hurtado AQ, Schubert J (2004) A preliminary summary on Kappaphycus farming and the impact of epiphytes. Jpn J Phycol (Supplement) 52:231–232
Crouch IJ, van Staden J (1992) Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J Appl Phycol 4:291–296
Crouch IJ, van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Doty MS (1973) Farming the red seaweed, Eucheuma, for carrageenans. Micron 9:59–73
Doty MS (1985) Eucheuma alvarezii sp. novum (Gigartinales, Rhodophyta) from Malaysia. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweeds: with reference to some Pacific and Caribbean species. California Sea Grant College Program, La Jolla, pp 37–45
Doty MS, Alvarez VB (1981) Eucheuma farm productivity. Int Seaweed Symp 8:688–691
Doty MS, Norris JN (1985) Eucheuma species (Solieriaceae, Rhodophyta) that are major sources of carrageenan. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweeds: with reference to some Pacific and Caribbean species. California Sea Grant College Program, La Jolla, pp 47–61
Dring M (2006) Stress resistance and disease resistance in seaweeds: the role of reactive oxygen metabolism. Adv Bot Res 43:175–207
du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
Esserti S, Smaili A, Rifai LA, Koussa T, Makroum K, Belfaiza M, Kabil EM, Faize L, Burgos L, Alburquerque N, Faize M (2017a) Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J Appl Phycol 29:1081–1093
Esserti S, Faize M, Rifai LA, Smaili A, Belfaiza M, Alburquerque N, Burgos L, Koussa T, Makroum K (2017b) Media derived from brown seaweeds Cystoseira myriophylloides and Fucus spiralis for in vitro plant tissue culture. Plant Cell Tissue Organ Cult 128:437–446
Esserti S, Smaili A, Makroum K, Belfaiza M, Rifai LA, Koussa T, Kasmi I, Faize M (2018) Priming of Nicotiana benthamiana antioxidant defences using brown seaweed extracts. J Phytopathol 166:86–94
Fan D, Hodges DM, Zhang JZ, Kirby CW, Ji XH, Locke SJ, Critchley AT, Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Garcia-Vaquero M, Rajaura G, O’Doherty JV, Sweeney T (2016) Polysaccharides from macroalgae: recent advances, innovative technologies and challenges in extraction and purification. Food Res Int 99:1011–1020
Goes HG, Reis RP (2011) An initial comparison of tubular netting versus tie-tie methods of cultivation for Kappaphycus alvarezii (Rhodophyta, Solieriaceae) on the south coast of Rio de Janeiro State, Brazil. J Appl Phycol 23:607–613
Góes HG, Reis RP (2012) Temporal variation of the growth, carrageenan yield and quality of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultivated at Sepetiba Bay, southeastern Brazilian coast. J Appl Phycol 24:173–180
Goñi O, Fort A, Quille P, McKeown PC, Spillane C, O’Connell SO (2016) Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: same seaweed but different. J Agric Food Chem 64:2980–2989
Guan J, Liang L, Mao S (2017) Applications of carrageenan in advanced drug delivery. In: Venkatesan J, Anil S, Kim SK (eds) Seaweed polysaccharides, isolation, biological and biomedical applications. Elsevier, Amsterdam, pp 283–298
Hayashi L, Oliveira EC, Bleicher-L'honneur G, Boulenguer P, Pereira RTL, von Seckendorff R, Shimoda VT, Leflamand A, Vallée P, Critchley AT (2007) The effects of selected cultivation conditions on the carrageenan characteristics of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Ubatuba Bay, São Paulo, Brazil. J Appl Phycol 19:505–511
Hayashi L, Yokoya NS, Kikuchi DM, Oliveira EC (2008) Callus induction and micropropagation improved by colchicines and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J Appl Phycol 20:653–659
Hayashi L, Bulboa C, Kradolfer P, Soriano G, Robledo D (2014) Cultivation of red seaweeds: a Latin American perspective. J Appl Phycol 26:719–727
Hayashi L, Reis RP, Alves dos Santos AA, Castelar B, Robledo D, de Vega GB, Msuya FE, Eswaran K, Yasir S, Ali MJ, Hurtado AQ (2017) The cultivation of Kappaphycus and Eucheuma in tropical and sub-tropical waters. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on spinosum and cottonii of commerce. Springer, Dordrecht, pp 55–90
Hotchkiss S, Brooks M, Campbell R, Philip K, Trius A (2016) The use of carrageenan in food. In: Pereira L (ed) Carrageenans: sources and extraction methods, Molecular Structure, Bioactive Properties and Health Effects. Nova Science Publications Inc., New York
Hung LD, Hori K, Nang HQ, Kha T, Hoa LT (2009) Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam. J Appl Phycol 21:265–272
Hurtado AQ, Critchley AT, Trespoey A, Bleicher-L’honneur G (2006) Occurrence of Polysiphonia epiphytes in Kappaphycus farms at Calaguas Is. Camarines Norte, Philippines. J Appl Phycol 18:301–306
Hurtado AQ, Yunque DA, Tibubos KT, Critchley AT (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Hurtado AQ, Joe M, Sanares RC, Fan D, Prithiviraj B, Critchley AT (2012) Investigation of the application of Acadian Marine Plant Extract Powder (AMPEP) to enhance the growth, phenolic content, free radical scavenging, and iron chelating activities of Kappaphycus Doty (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 24:601–611
Hurtado AQ, Gerung GS, Critchley AT YS (2014a) Cultivation of tropical red seaweeds in the BIMP-EAGA region. J Appl Phycol 26:702–718
Hurtado AQ, Neish IC, Critchley AT (2014b) Farm productivity of seaweed carrageenan farming in the ASEAN countries. Paper presented at the International Seaweed Congress, 19–21 Nov 2014, Waterfront Hotel, Cebu
Hurtado AQ, Neish IC, Critchley AT (2015) Developments in production technology of Kappaphycus in the Philippines: more than four decades of farming. J Appl Phycol 27:1945–1961
Jayaraman J, Norrie J, Punja ZK (2011) Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol 23:353–361
Kadam SU, O’Donnell CP, Rai DK, Hossain MB, Burgess CM, Walsh D, Tiwari BK (2015) Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: ultrasound assisted extraction, characterization and bioactivity. Mar Drugs 13:4270–4280
Kapilkumar I, Vitkin E, Robin A, Yakhini Z, Mishori D, Golberg A (2017) Macroalgal biorefinery from Kappaphycus alvarezii: conversion modeling and performance prediction for India and the Philippines as examples. Bioenerg Res. https://doi.org/10.1007/s12155-017-9874-z
Klarzynski O, Plesse B, Joubert J, Yvin JC, Kopp M, Kloareg B, Fritig B (2000) Linear β -1,3 glucans are elicitors of defense responses in Tobacco-France. Plant Physiol 124:1027–1037
Kumar G, Sahoo D (2011) Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J Appl Phycol 23:251–255
Kumar M, Reddy CRK, Jha B (2013) The ameliorating effect of Acadian marine plant extract against ionic liquids-induced oxidative stress and DNA damage in marine macroalga Ulva lactuca. J Appl Phycol 25:369–378
Kumari R, Kaur I, Bhatnagar A (2011) Effect of aqueous extract of Sargassum johnstonii Setchell on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633
Largo DB (2002) Recent development in seaweed diseases. In: Hurtado AQ, Guanzon NG Jr, de Castro-Mallare TR, Luhan MRJ (eds). Proc Nat Seaweed Planning Workshop. Tigbauan, Iloilo, pp 35–42
Le Lann K, Surget G, Couteau C, Coiffard L, Cérantola S, Gaillard F, Larnicol M, Zubia M, Guérard F, Poupart N, Stiger-Pouvreau V (2016) Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J Appl Phycol 28:3547–3559
Leclerc M, Caldwell CD, Lada RR, Norrie J (2006) Effect of plant growth regulators on propagule formation in Hemerocallis spp. and Hosta spp. Hortscience 41:651–653
Lötze E, Hoffman E (2016) Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J Appl Phycol 28:1379–1386
Loureiro RR, Reis RP, Critchley AT (2010) In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J Appl Phycol 22:101–104
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2012) Extract powder from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (AMPEP): a ‘vaccine-like; effect on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva. J Appl Phycol 24:427–432
Loureiro RR, Reis R, Marroig R (2014a) Effect of the commercial extract of the brown alga Ascophyllum nodosum Mont. on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva in situ submitted to lethal temperatures. J Appl Phycol 26:629–634
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2014b) Effects of a commercial extract of the brown alga Ascophyllum nodosum on the biomass production of Kappaphycus alvarezii (Doty) Doty ex P.C. Silva and its carrageenan yield and gel quality cultivated in Brazil. J Appl Phycol 26:763–768
Loureiro RR, Hurtado AQ, Critchley AT (2017) Impacts of AMPEP on epiphytes and diseases in Kappaphycus and Eucheuma cultivation. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on spinosum and cottonii of commerce. Springer, Dodrecht, pp 111–119
Luhan MRJ, Mateo JP (2017) Clonal production of Kappaphycus alvarezii (Doty) Doty in vitro. J Appl Phycol 29:2339–2344
MacKinnon SL, Hiltz D, Ugarte R, Craft CA (2010) Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Mancuso S, Azzarello E, Mugnai S, Briand X (2006) Marine bioactive substances (IPA extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv Hortic Sci 20:156–161
Markets and Markets (2016) Hydrocolloids market global forecast by type (gelatin, xanthan, carrageenan, alginate, agar, pectin, guar, locust bean, gum arabic, and CMC), function (thickener, stabilizer, gelling, fat replacer, and coating), source, application, and by region—Global forecast to 2020 Markets and Markets, February 2016
Marroig RG, Loureiro RR, Reis RP (2016) The effect of Ascophyllum nodosum (Ochrophyta) extract powder on the epibiosis of Kappaphycus alvarezii (Rhodophyta) commercially cultivated on floating rafts. J Appl Phycol 28:2471–2477
Martynenko A, Shotton K, Astatkie T, Petrash G, Fowler C, Neily W, Critchley AT (2016) Thermal imaging of soybean response to drought stress: the effect of Ascophyllum nodosum seaweed extract. SpringerPlus 5:1393
Mendoza WG, Montaño NE, Ganzon-Fortes ET, Villanueva RD (2002) Chemical and gelling profile of ice-ice infected carrageenan from Kappaphycus striatum (Schmitz) Doty ‘sacol’ strain (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 14:409–418
Mireya R, Vigen-Calleros G, Ruiz-López M, Zañudo-Hernández J, Délano-Frier JP, Sánchez-Hernández C, Hernández-Herrera A (2014) Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani. J Appl Phycol 26:1607–1614
Moreira R, Sineiro J, Chenlo F, Arufe S, Díaz-Varela D (2017) Aqueous extracts of Ascophyllum nodosum obtained by ultrasound-assisted extraction: effects of drying temperature of seaweed on the properties of extracts. J Appl Phycol 29:3191–3200
Msuya FE, Buriyo A, Omar I, Pascal B, Narrain K, Ravina JJM, Mrabu E, Wakibia JG (2014) Cultivation and utilisation of red seaweeds in the Western Indian Ocean (WIO) region. J Appl Phycol 26:699–705
Neish IC, Suryanarayan S (2017) Development of eucheumatoid seaweed value-chains through carrageenan and beyond. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on spinosum and cottonii of commerce. Springer, Dordrecht, pp 173–192
Neves FAS, Simioni C, Bouzon ZL, Hayashi L (2015) Effects of spindle inhibitors and phytoregulators on the micropropagation of Kappaphycus alvarezii (Rhodophyta, Gigartinales). J Appl Phycol 27:437–445
Olivares-Molina A, Fernández K (2016) Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J Appl Phycol 28:1295–1302
Ortiz-Tena JG, Shieder D, Sieber V (2017) Carrageenan and more: biorefinery approaches with special references to the processing of Kappaphycus. In: Hurtado AQ, Critchley AT, Neish IC (eds) Tropical seaweed farming trends, problems and opportunities: focus on spinosum and cottonii of commerce. Springer, Dordrecht, pp 155–164
Pang T, Liu J, Liu Q, Zhang L, Lin W (2012) Impacts of glyphosate on photosynthetic behaviors in Kappaphycus alvarezii and Neosiphonia savatieri detected by JIP-test. J Appl Phycol 24:467–473
Pang T, Liu J, Liu Q, Li H, Li J (2015) Observations on pests and diseases affecting a eucheumatoid farm in China. J Appl Phycol 27:1975–1984
Parker HS (1974) The culture of the red algal genus Eucheuma in the Philippines. Aquaculture 3:425–439
Pereira L, Soares F, Freitas AC, Duarte AC, Ribeiro-Claro P (2017) Extraction, characterization and uses of carrageenan. In: Sudha PN (ed) Industrial applications of marine biopolymers. CRC Press, Boca Raton, pp 37–90
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Pramanick B, Brahmachari K, Ghosh A, Zodape ST (2014) Foliar nutrient management through Kappaphycus and Gracilaria saps in rice-potato-green gram crop sequence. J Sci Ind Res 73:613–617
Raimundo S, Pattathil S, Eberhard S, Hahn MG, Popper ZA (2017) β-1,3-glucans are components of brown seaweed (Phaeophyceae) cell walls. Protoplasma 254:997–1016. https://doi.org/10.3389/fpls.2017.01362
Rayirath P, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429
Rayirath P, Benkel B, Hodges DM, Allan-Wojtas P, MacKinnon S, Critchley AT, Prithiviraj B (2009) Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 230:135–147
Rayorath PM, Khan W, Palanisamy R, MacKinnon SL, Stefanova R, Hankins SD, Critchley AT, Prithiviraj B (2008a) Extracts of the brown seaweed Ascophyllum nodosum induce gibberellic acid (GA3)-independent amylase activity in barley. J Plant Growth Regul 27:370–379
Rayorath PM, Narayanan JM, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008b) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana. J Appl Phycol 20:423–429
Robertson-Andersson DV, Leitao D, Bolton JJ, Anderson RJ, Njobeni A, Ruck K (2006) Can kelp extract (Kekpak®) be useful in seaweed mariculture? J Appl Phycol 18:315–321
Robledo D, Gasca-Leyva E, Fraga J (2013) Social and economic dimensions of carrageenan seaweed farming in Mexico. In: Valderrama D, Cai J, Hishamunda N, Ridler N (eds) Social and economic dimensions of carrageenan seaweed farming. Fish aqua tech paper 580. FAO, Rome, pp 185–204
Sangha JS, Kandasamy S, Khan W, Singh Bahia W, Singh RP, Critchley AT, Prithiviraj B (2015) λ-carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Mar Drugs 13:2875–2889
Santaniello A, Scartazza A, Gresta F, Loreta E, Iasone A, Di Tommaso D, Piaggesi A, Perata P (2017) Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Protoplasma 254:997–1016
Sharma N, Chauhan RS, Sood H (2015) Seaweed extract as a novel elicitor and medium for mass propagation and picroside-I production in an endangered medicinal herb Picrorhiza kurroa. Plant Cell Tissue Organ Cult 122:57–65
Sharma L, Banerjee M, Malik GC, Gopalakrishnan VAK, Zodape ST, Arup Ghosh A (2017) Sustainable agro-technology for enhancement of rice production in the red and lateritic soils using seaweed based biostimulants. J Clean Prod 149:968–975
Shekhar Sharma HS, Fleming C, Selby C, Rao JR, Martin T (2014) Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J Appl Phycol 26:465–490
Shi P, Geng S, Feng T, Wu H (2017) Effects of Ascophyllum nodosum extract on growth and antioxidant defense systems of two freshwater microalgae. J Appl Phycol. https://doi.org/10.1007/s10811-017-1287-z
Shukla PS, Shotton K, Norman E, Neily W, Critchley AT, Prithiviraj B (2017) Seaweed extract improves drought tolerance of soybean by regulating stress-response genes. AoB Plants 10:10
Singh S, Singh MK, Pal SK, Trivedi K, Yesuraj D, Singh CS, Vijay Anand KG, Chandramohan M, Patidar R, Kubavat D, Zodape ST, Ghosh A (2016) Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J Appl Phycol 28:2099–2112
Sivanandhan S, Selvaraj N, Ganapathi A, Manickavasagam M (2014) Improved production of withanolides in shoot suspension culture of Withania somnifera (L.) Dunal by seaweed extracts. Plant Cell Tissue Organ Cult 119:221–225
Spann TM, Little HA (2011) Applications of a commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown ‘Hamlin’ Sweet Orange nursery trees. Hort Sci 46:577–582
Stadnik J, de Freitas MB (2014) Algal polysaccharides as sources of plant resistance inducers. Trop Plant Pathol 39:111–118
Tibubos K, Hurtado AQ, Critchley AT (2017) Direct formation of axes in new plantlets of Kappaphycus alvarezii (Doty) Doty, as influenced by the use of AMPEP K+, spindle inhibitors and plant growth hormones. J Appl Phycol 29:2345–2349
Trivedi K, Vijay Anand KG, Kubavat D, Patidar R, Ghosh A (2017) Drought alleviatory potential of Kappaphycus seaweed extract and the role of the quaternary ammonium compounds as its constituents towards imparting drought tolerance in Zea mays L. J Appl Phycol. https://doi.org/10.1007/s10811-017-1375-0
Trono GC, Valdestamon RG (1994) New aspects in the ecology and culture of Kappaphycus and Eucheuma. Korean J Phycol 9:205–216
Tsiresy G, Preux J, Lavitra T, Dubois P, Lepoint G, Eeckhaut I (2016) Phenology of farmed seaweed Kappaphycus alvarezii infestation by the parasitic epiphyte Polysiphonia sp. in Madagascar. J Appl Phycol 28:2903–2914
Urbani S, Ziosi V, MacKinnon S, Henderson D, Ratcliffe J, Manda A, Pirondi A (2016) Testing the bioactivity of kelp extracts developed via chemical and physical separation techniques using bioassays. Acta Hortic 1148:109–114
USDA (2016) https://www.ams.usda.gov/sites/default/files/media/Aquatic%20Plant%20Extract%20Tech%20Review.pdf. Accessed Jan 2018
Uyenco FR (1977) Microbiological studies of diseased Eucheuma sp. and other seaweeds. Nat Seaweeds Symp. Metro Manila, Philippines
Uyenco FR, Saniel LS, Jacinto GS (1981) The ‘ice-ice’ problem in seaweed farming. Proc Int Seaweed Symp 10:625–630
Vairappan CS (2006) Seasonal occurrences of epiphytic algae on the commercially cultivated red alga Kappaphycus alvarezii (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 18:611–617
Vairappan CS, Chung CS, Hurtado AQ, Msuya FE, Bleicher L’honneur G, Critchley AT (2008) Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J Appl Phycol 20:477–483
Valderrama D, Cai J, Hishamunda N, Ridler N (2013) Social and economic dimensions of carrageenan seaweed farming. Fish Aqua Tech Paper 580. FAO, Rome
Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric 4:5
Wally OSD, Critchley AT, Hiltz D, Craigie JS, Han X, Zaharia LI, Abrams SR, Prithiviraj B (2013) Regulation of phytohormone bio-synthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J Plant Growth Regul 32:324–339
Yong WTL, Ting SH, Yong YS, Thien VY, Wong SH, Chin WL, Rodrigues KF, Anton A (2014) Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J Appl Phycol 26:1597–1606
Yunque DAT, Tibubos KR, Hurtado AQ, Critchley AT (2011) Optimization of culture conditions for tissue culture production of young plantlets of carrageenophyte Kappaphycus. J Appl Phycol 23:433–438
Zhang XZ, Ervin EH (2004) Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci 44:1737–1745
Zhang XZ, Ervin EH (2008) Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci 48:364–370
Zodape ST, Mukhopagyay S, Eswaran K, Reddy MP, Chikara J (2010) Enhanced yield and nutritional quality in green gram (Phaseolus radiata L) treated with seaweed (Kappaphycus alvarezii) extract. J Sci Ind Res 69:468–471
Acknowledgments
The senior author would like to thank ASL, Canada for the continued donations of AMPEP samples provided in order to conduct these studies. The authors are most grateful to the constructive feedback of two anonymous reviewers and also to Lynn Cornish for the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hurtado, A.Q., Critchley, A.T. A review of multiple biostimulant and bioeffector benefits of AMPEP, an extract of the brown alga Ascophyllum nodosum, as applied to the enhanced cultivation and micropropagation of the commercially important red algal carrageenophyte Kappaphycus alvarezii and its selected cultivars. J Appl Phycol 30, 2859–2873 (2018). https://doi.org/10.1007/s10811-018-1407-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1407-4