Abstract
Difficulties and cost of suspended microalgal biomass harvest and processing can be overcome by cultivating microalgae as biofilms. In the present work, a new photoautotrophic biofilm photobioreactor, the rotating flat plate photobioreactor (RFPPB), was developed aiming at a cost-effective production of Chlorella vulgaris (SAG 211-12), a strain not frequently referred in the literature but promising for biofuel production. Protocols were developed for evaluating initial adhesion to different materials and testing the conditions for biofilm formation. Polyvinyl chloride substrate promoted higher adhesion and biofilm production, followed by polypropylene, polyethylene, and stainless steel. The new RFPPB was tested, aiming at optimizing incident light utilization, minimizing footprint area and simplifying biomass harvesting. Tests show that the photobioreactor is robust, promotes biofilm development, and has simple operation, small footprint, and easy biomass harvest. Biomass production (dry weight) under non-optimized conditions was 3.35 g m−2, and areal productivity was 2.99 g m−2 day−1. Lipid content was 10.3% (dw), with high PUFA content. These results are promising and can be improved by optimizing some operational parameters, together with evaluation of long-term photobioreactor maximum productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae have a multiplicity of uses, from biochemical to biofuel production, and high-density culture is of primary interest to the algal industry (Mata et al. 2010). Microalgae are predominantly cultivated under photoautotrophic conditions either in open algal ponds or closed photobioreactors (PBRs) (Borowitzka 2013). Although having advantages, PBRs present many problems, including high production costs, low light utilization, and difficulty in scale-up (Wang et al. 2014). Thus, research aiming at the maximization of photobioreactor performance is of utmost importance. New designs, combining improved production with more performing operation, can boost microalgal exploitation. Among the factors of concern, light collection, land occupation, and harvesting process are crucial for optimization (Mata et al. 2010; Chini Zittelli et al. 2013). In fact, one of the most expensive steps in large-scale microalgal production relates to harvesting and dewatering, as cells are usually cultivated in planktonic form, producing relatively diluted suspensions (Irving and Allen 2011). One way to reduce harvesting costs is to change to attached biomass production, as cells are already concentrated and easier to collect (Ozkan et al. 2012). Biofilm formation is common in nature, with microalgae being important components (Sekar et al. 2004). The utilization of microalgae biofilms can be seen in some wastewater treatment technologies (Craggs et al. 1996; Travieso et al. 2002; Shi et al. 2007; Kesaano and Sims 2014), but the lack of control over the cultivated species is disadvantageous for other utilizations (Blanken et al. 2014). An alternative could be cultivation of single species biofilms in photobioreactors, but the literature is still limited on this subject. Li et al. (2016) tested the effect of light on the growth of Halochlorella rubescens in a porous substrate photobioreactor and concluded that the dark respiration could explain growth rate decrease of biofilm over cultivation time. Yin et al. (2015) discovered that the water footprint of biofilm cultivation of Haematococcus pluvialis could be greatly decreased by using sealed narrow chambers combined with slow aeration rate. An algal biofilm membrane photobioreactor (BMPBR) equipped with solid carriers and submerged membrane module was developed for attached growth of Chlorella vulgaris and secondary effluent treatment (Gao et al. 2015). However, the harvesting of microalgae biofilm adhered to the carriers had to be done by ultrasound followed by centrifugation. Multi-layers photobioreactors were used to produce attached culture of Botryococcus braunii, aiming at the production of extracellular polymeric substances (EPS) (Shen et al. 2015).The fatty acid composition in the stimulated biofilms was more simple and stable than in suspended biomass, showing that this kind of reactors is potentially attractive.
Some promising examples of biofilm photobioreactors are those based on rotating systems (Gross et al. 2013; Orandi and Lewis 2013; Blanken et al. 2014; Gross et al. 2015b). The rotating biological contactor (RBC), originally designed for wastewater treatment, has advantages such as the small footprint, low energy consumption, large surface area, and low operational costs. Microalgal systems based on the RBC principle exist in the literature (e.g., PRBC—photorotating biological contactor (Orandi and Lewis 2013) or Algadisk reactor (Blanken et al. 2014)), with all of them using disks placed in a horizontal shaft, entering vertically in the tank fluid. A slight exception is the RAB (rotating algal biofilm) reactor described by Gross et al. (2013). One of the problems with RBC-type systems for microalgal production is the light intensity received per disk, which has been regulated changing disk size, distances between disks, or illuminating both sides of the disks. The harvest process, usually by scraping, can also be complicated. Different disk surfaces and coatings were also tested for better biofilm formation (Ozkan et al. 2012; Orandi and Lewis 2013; Blanken et al. 2014). Nevertheless, further work is needed for future application of this type of reactors by the microalgae industry. The implementation of such reactors in an industrial scale is still a challenge due to the difficulty in detaching and collecting microalgae biofilm in an automated way. Furthermore, the reactor footprint should be decreased to allow a much higher areal productivity, which is of utmost importance when land is becoming scarce for agricultural purposes.
Among the microalgae of interest, the genus Chlorella, and more specifically C. vulgaris, is one of the most extensively employed and has biofilm formation ability (Sekar et al. 2004; Johnson and Wen 2010; Feng et al. 2011). The potential of the strain C. vulgaris SAG 211-12 for enhanced lipid production was characterized at pilot plant using flat panel airlift technology (Münkel et al. 2013). The possibility of cultivating this strain attached to a substrate in biofilm reactors, to the best of our knowledge, has not yet been referred in the literature and could be important in the context of the production system optimization for future integration in a biorefinery system (Seth and Wangikar 2015). Moreover, the utilization of a simple methodology to assess the effectiveness of the adhesion and biofilm development process, similarly to what is used in bacterial biofilm studies (An and Friedman 1997), is of practical interest.
As a contribution towards increasing our knowledge on microalgal biofilm photobioreactors, in the present work, it was tested the capacity of C. vulgaris SAG 211-12 adhesion to different substrates and of biofilm formation and development. To the best of the author’s knowledge, there are no recommended procedures in the literature to evaluate microalgae attachment and biofilm growth; therefore, simple protocols based on bacterial biofilm studies were developed. Latter, the best substrate found was used to build and operate a new type of rotating biofilm photobioreactor, based on flat plates. Special attention was given to a cost-effective and scalable type of construction, with reduced footprint and improved light and labor utilization, thus facilitating the operation and the harvest procedure.
Materials and methods
Microalgal culture maintenance, acclimation, and kinetic studies
The microalga used in this work, Chlorella vulgaris f. viridis Chodat (strain SAG 211-12), belongs to the Division Chlorophyta and the Class Trebouxiophyceae. It was purchased from the Algal Culture Collection of the University of Göttingen (SAG, Germany) and was selected due to its lipid production capacity.
Cultures originating from agar slants were transferred to Bold’s basal medium (BBM), with stock maintenance performed with standard procedures (Stein 1973). Kinetic studies were done in triplicate or quadruplicate with increasing culture volumes, using as inoculum 1.5 × 106 cells mL−1. Whenever needed, cultures were also acclimated to BBM/2 or BBM/10 (BBM diluted to 1/2 or 1/10 the original strength). The microalgae were kept at room temperature, without shaking or with shaking at 40–50 rpm in a rocking platform (Stuart Scientific, model 5STR8) for volumes up to 500 mL. Higher volumes were continuously aerated by filtered air (Millipore FG membranes, 0.2 μm), using an aquarium pump (Resun–Air Pump, AC-9904), at 11.4 to 17.5 mL s−1 of air. A special illuminated incubator was built to enable easy culture manipulation at the laboratory bench, using fluorescent lights (two 18 W, T12 Cool White Sylvania lamps initially and two more 18 W Duralight tld (T8), later). A photoperiod of 12 h L:12 h D was fixed using a timer. Photosynthetically active radiation (PAR) was evaluated using a Universal Light Meter (ULM-500, Walz), with spherical micro quantum sensor US-SQS/L and average recorded values were 80 ± 10 and 110 ± 20 μmol photons m−2 s−1.
To avoid contamination, all the laboratory material and glassware were washed and autoclaved at 121 °C for 20 min.
Substrate materials
Materials chosen for the study of adhesion included borosilicate glass (VD, colorless, transparent, smooth), polyurethane foam (PU, white, opaque, porous), polyvinyl chloride (PVC, dark gray, opaque, smooth), stainless steel (INOX plate 0.5, 25 × 50 FM, opaque, brilliant, smooth), polyethylene (PE, colorless, transparent, smooth), and polypropylene (PP, colorless, transparent, smooth). This selection was based on reported hydrophilic/hydrophobic behavior (Sekar et al. 2004; Irving and Allen 2011), non-toxicity, availability, and ease of manipulation. With the exception of glass (microscope slides, standard dimensions of 7.6 cm × 2.6 cm × 0.1 cm), all the other materials were cut as coupons with 2 cm × 2 cm and thickness from 0.05 (INOX) to 0.4 cm (PVC). These materials were thoroughly washed to remove grease and sterilized with alcohol at 70% (v/v) (An and Friedman 1997). With the exception of Inox, which was purchased, as it was referred in the literature (Percival et al. 1998; Sekar et al. 2004), all the other substrates were either simple laboratory consumables (slides, Petri dishes, pipes) or reused packaging material. The purpose was to test simple, cheap, non-toxic, easy handling material for possible utilization in a biodisk-like bioreactor.
Chlorella vulgaris adhesion and biofilm formation tests
Preliminary substrate adhesion tests
In the first series of preliminary tests, glass slides and PVC pieces were used, in duplicate covered glass containers with BBM, inoculated with exponential phase cells, and placed with agitation in the illuminated incubator as above. The assays lasted 30 days and adhesion was evaluated visually (Leica MZ75 stereomicroscope). In a second series of preliminary tests, strips of glass, PVC, and PU materials were fixed individually to the glass containers with transparent silicone (Soudal, for aquariology). The medium used was BBM/10, to limit growth and favor adhesion (Sekar et al. 2004), inoculated at an initial optical density at 680 nm (OD680) of 0.03 (to have a cell density near 6 × 105 cells mL−1 (Sekar et al. 2004)), and the containers were placed under the illumination and temperature conditions described in the “Microalgal culture maintenance, acclimation, and kinetic studies” section. A control without substrate was also prepared. The assay duration was 6 h and adhesion was evaluated by direct visualization and by OD readings of the culture media (1 mL samples and readings at 680 nm with 10 mm cuvettes and a Genesys 6 Thermo Scientific spectrophotometer). Biofilm development was assessed by keeping the same trays with the colonized substrates for 6 days under the same conditions.
Controlled evaluation of adhesion capacity
Batch tests under controlled conditions were performed with PVC, INOX, PE, and PP coupons, using a protocol adapted from An and Friedman (1997), Sekar et al. (2004), Irving and Allen (2011), and Johnson and Wen (2010). Briefly, for each material, four coupons previously degreased and disinfected were fixed with silicone to the bottom of sterilized Petri dishes (10 cm Ø, 1.5 cm depth), in duplicate per treatment. Then 30 mL of sterile BBM/10 were aseptically poured and inoculated with 1 × 106 cells mL−1 (initial OD680 of 0.05), which is a cell density value intermediate between Sekar et al. (2004) and Irving and Allen (2011), and the dishes were covered and placed randomly in the rocking platform. Blanks without coupons were also prepared. Three different methodologies were used to assess adhesion after 48 h (only for PE coupons), 72 h, and 144 h (all substrata) of incubation: changes in OD680 in the culture media, visual observation (optical microscope Leitz, Germany, at 400× magnification), and cell counting (Neubauer hemocytometer). These timings were selected according to observations previously done. After confirming by OD readings and microscopic observation of the existence of cells firmly adhered to the coupons (i.e., cells attached to the supports after vigorous washing with distilled water), the cells present at the top surface of each coupon were manually removed using a cell scraper (VWR), concentrated in 1 mL deionized water, and transferred to Eppendorf tubes. After vortex homogenization (VWR, maximum speed/10 s), cells were counted in a Neubauer chamber. When needed, samples were fixed with standard Lugol solution, concentrated by centrifugation (3 min at 10000 rpm) (Griffiths et al. 2011) using a ScanFuge Mini centrifuge, and counted immediately or after storage at 4 °C.
Biofilm development in fed-batch conditions
Next, the development of the microalgal biofilm was encouraged and assessed. The experimental set-up was the one described in the “Substrate materials” section, but biofilm growth was promoted in the coupons remaining from the adhesion tests by renovating twice the media of each Petri dish (at the 6th and 10th day of culture) using full-strength BBM. Sampling was done at 240 and 312 h and biofilm development was assessed by cell counting using a Neubauer chamber and OD readings at 680 nm.
Complementary tests were done using triplicate Petri dishes with PVC and PP coupons to confirm observed trends. In addition, it was evaluated in triplicate the effect of diminishing the light intensity (e.g., due to shading) on cell adhesion using PVC coupons in closed Petri dishes placed under 40 (dishes with extra plastic cover) or 60 μmol photons m−2 s−1 (dishes without plastic cover), on the rocking platform. Sampling was done after 72 and 144 h of experiment for evaluation of OD680nm and counting cells firmly attached to the coupons.
Later, a higher scale evaluation of biofilm formation was tested using PVC plates with 10.6 cm × 21 cm × 0.4 cm, placed under the same environmental conditions as before in the rocking platform (although only at 15 rpm, to avoid media splashing). The protocol developed for small-scale tests was used: initial inoculation of BBM/10 with C. vulgaris SAG 211-12 culture at OD680 = 0.05 of exponential phase cells, followed by change to full BBM, cell scraping at the end of the experiment, and biomass drying overnight at 80 °C for gravimetric evaluation of the production. Several runs were done in order to get useful information for the development of the new microalgal biofilm reactor.
Development of a laboratory-scale rotating flat plate photobioreactor
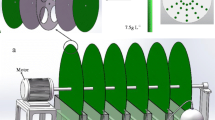
In order to implement the production of C. vulgaris SAG 211-12 as a biofilm, a new bioreactor was developed, taking the concept of the RBC to another level. In fact, differently from the Algadisk, the rotating algal biofilm reactor, RABR (Christenson and Sims 2012) or PRBC reactors, in our work disks were not used, but four flat PVC plates (43 cm × 8.3 cm × 0.5 cm, L × W × H), arranged horizontally at 90° angles in a shaft rotating at 2.8 rpm. The developed rotating flat plate photobioreactor, RFPPB, was provided with a special plate fixation system at the two lateral plate-supporting disks, which enabled easy removal of each plate without disassembling the whole unit. This way, maximum light exposition was guaranteed for both sides of each plate (shadow minimization) and the plate removal for harvest and replacement was facilitated. The whole assemblage had also a small footprint. Two types of plates were tested in duplicate: smooth PVC and rough PVC (after polishing with n° 120 sand paper). The whole system was placed in an 8 L maximum volume trough.
A photoautotrophic growth mode was chosen using BBM medium, at 22 ± 2 °C, under the same illumination conditions used for the adhesion and biofilm growth tests, being fine bubble aeration provided by 0.2 μm filtered air. Since the reactor trough was dark gray, suspended growth was discouraged, and only when emerging from the trough did the plates receive light at each rotation. The biofilm development protocol included initial disinfection of the whole system (washing with water, followed by washing with ethanol at 70% v/v and rinsing with sterilized water), filling the reactor with BBM/10, inoculation with C. vulgaris at OD680 = 0.05, change to BBM/2 after cell adhesion, and compensation for evaporation with sterile distilled water. After 18 days, the plates were removed, scraped, and the biomass dried at 80 °C for 24 h for initial biofilm dry weight determination. Next, the reactor trough was cleaned, disinfected, and refilled with growth medium and the microalgal colonies remaining attached to the plates served as inoculum for regrowth tests (in duplicate, at harvest intervals of 8 days). Biofilm formed was assessed visually by photographic recording and by dry weight determination. The biomass concentration in suspension in the photobioreactor trough was also assessed.
Calculations and statistical analysis
Photobioreactor performance was evaluated as surface biomass yield (Y, g m−2), surface productivity (P S , g m−2 day−1, Eq. 1), which is based on the area of the substrate, or as footprint productivity (P F , g m−2 day−1, Eq. 2), which is based on the footprint area of the reactor, according to Zhang et al. (2015). The gravimetric method (dry weight-based) was used after harvesting the biomass from each plate surface by scraping, subsequently to a growth-harvest cycle.
where DW X is total harvested biomass dry weight, in g, A P is the wet area of both faces of the four plates (0.23 m2), and n the cultivation period in days;
where A F is the footprint area of the bioreactor (0.14 m2).
The assays were performed in duplicate or triplicate and the results are represented graphically. For experiments using duplicates, results of independent data sets are expressed as data points or as means of data points, for simplicity of the graphical representation when only showing OD trends. For assays using triplicates, results are expressed as mean ± SD, and statistical analysis of data was done using the GraphPad Prism 6 software (GraphPad Software, USA). Normality of data was checked by Shapiro-Wilk test, non-parametric Mann-Whitney test was performed for the comparison between two independent data sets, and Kruskal-Wallis test followed by Dunn’s post hoc test was used for multiple comparisons, at a significance level of p < 0.05.
Lipid extraction and quantification
Lipids were extracted for quantification using a modified Bligh and Dyer (1959) method (Mata et al. 2016): (1) the biomass sample obtained by scraping the reactor plates was frozen and then lyophilized. A certain amount of dried material was weighed in a pre-weighed centrifuge glass tube. (2) Chloroform (Riedel de Haën, p.a.), methanol (Riedel de Haën, p.a.) and distilled water were added in ratios of 1:2:0.8 (v/v), respectively. (3) The centrifuge tube containing the C. vulgaris sample with chloroform, methanol, and water was sonicated for 30 min (Bandelin Sonorex TK30). (4) A second extraction step was then performed by adding the co-solvents at ratios of 2:2:1.8 (v/v) of chloroform, methanol, and distilled water, respectively. (5) The sample was sonicated for another 30 min and then centrifuged at 3000 rpm, for 15 min (ECCO Tvp 25 No. 8601 centrifuge). (6) After centrifugation, three layers became visible: an upper layer containing water and methanol, a central layer consisting of the extracted microalgae cake, and a lower layer which contained the lipids and chloroform. The upper layer was discarded and the lower layer was carefully recovered with a syringe to a previously weighed glass tube. (7) Chloroform was evaporated to dryness in a laboratorial hood at room temperature (about 25 °C), and the purified lipids extract remained in the glass tube. (8) The tube containing the lipids was weighed again and the microalgae lipid content was estimated by the difference of the weight of the tube with and without the lipids extracted.
Lipid composition
Transesterification of microalgal lipids
The lipids extracted from the Chlorella vulgaris biofilm were transesterified to obtain fatty acid methyl esters (FAME), using the Lepage and Roy (1984) method, with slight modifications as described by Mata et al. (2013): 10 mg of the crude lipids were dissolved in 2 mL of a freshly prepared mixture of chloroform-methanol (2:1, v/v) in a 10 mL Pyrex tube sealed with a Teflon screw cap; 1 mL of methanol (Riedel de Haën, p.a.) as reagent and 0.3 mL of sulfuric acid (Scharlau Chemie, reagent grade, 95–97%) as catalyst were added; the sealed tube containing the mixture was weighed; the tube was vigorously shaken for 5 min; finally, the mixture was reacted in the tube at 100 °C, for 10 min, in a digestor (ECO 16 Thermoreactor Velp Scientifica) after which it was cooled down to room temperature by immersion in a water bath; 1 mL of distilled water was added for phase separation (two distinct phases are formed, the upper layer rich in water, methanol, glycerol, and sulfuric acid, and the lower layer rich in chloroform and esters) and the upper phase was removed; 1 mL of distilled water was added again to the tube for a gentle water washing of esters with chloroform layer (denser than the water layer) followed by discard of the water rich upper layer (less dense)—this step was repeated two more times; the esters rich layer was filtered using a disposable Nylon syringe filter (13 mm diameter, 0.2 μm pore, Cronus, UK); the chloroform was evaporated from the esters in a laboratorial hood, at room temperature (of about 25 °C).
Analysis of FAME by gas chromatography
The FAME obtained from the C. vulgaris lipids were analyzed by gas chromatography (GC) according to the EN 14103:2010 standard, using as internal standard methyl heptadecanoate (99.5% purity, Fluka) with a concentration of 10.256 mg mL−1. This analysis was performed using a gas chromatograph (DANI GC 1000 DPC) equipped with a TRB-WAX (Capillary Column, Teknokroma) for FAME’s (30 m, 0.32 mm internal diameter, and 0.25 μm film thickness). The injector, flame ionization detector (FID), and oven temperatures were set to 250, 250, and to 195 °C, respectively. The carrier gas used was helium, at a flow rate of 1 mL min−1. Injection was made in a split mode, using a split ratio of 1:80, and the injected volume was 0.1 μL. Analyses were done in duplicate.
Results
Algal performance in BBM
Microalgae were successfully acclimated to BBM media with different strengths. In standard BBM and volumes of 1000 mL, exponential phase was reached after 2–3 days from inoculation and maximum cell densities were attained at the 6th day with 4.64 × 107 cells mL−1. Maximum growth rates (μ) were observed at the 2nd day of cultivation (μ = 0.68 day−1). It must be noted that BBM is usually regarded as only a maintenance medium and pH was not controlled. Standard curves (data not shown) were drawn relating number of cells (cells mL−1) with suspension absorbance (OD680) and cell dry weight (g L−1) with suspension absorbance OD680.
C. vulgaris adhesion and biofilm formation tests
Preliminary adhesion tests
In these tests, substrates PVC, glass, and polyurethane were placed in glass trays with BBM/10 and inoculated with C. vulgaris. Adhesion was evaluated by OD680 readings in the suspension at the beginning and at selected times of the experience. It was expected that, in case of adhesion, OD values would decrease when compared with the initial ones due to the microalgae change from suspended to adhered form. Figure 1 shows an initial OD rise in all tested conditions. This can be explained by an initial multiplication of suspended cells, as the environmental conditions favored growth (especially in the control vessel where there was no shadow effect caused by the presence of the adhesion substrates) and by the absence or negligible adhesion. Nevertheless, after 2.5 h, different behaviors were observed: a constant drop in OD in the control (possibly caused by sedimentation but mostly by visible wall growth) and OD stabilization in the substrate vessels, which was faster in glass and polyurethane and slower in PVC substrates. After this period, OD decreased, probably due to adhesion phenomena, simultaneously in glass and polyurethane substrates, but not in PVC. Possibly, the assay had insufficient duration for the adhesion of C. vulgaris to this substrate.
After initial colonization was seen to occur with C. vulgaris SAG 211-12, the next step was promotion of biofilm development and its maintenance. Substrates were kept for 6 days under the same conditions, but new medium was added (BBM/10) and evaporation compensated when needed. OD readings showed the same trend as before, and substrates were visually inspected and image scans taken for better visualization, being biofilm formation and maintenance confirmed.
Microalgal immobilization tests with coupons
The materials chosen as substrates for tests with coupons were PVC, Inox, PP, and polyethylene (PE). Glass and polyurethane were excluded due to difficulties to cut them in coupons and because they were not adequate for building a bioreactor. PE, PP, and Inox were considered because there are reports on their use in the literature. In these tests, the quantification of the cells effectively adhered to the coupons was done by counting with a Neubauer hemocytometer.
Adhesion evaluation in BBM/10 (batch mode)
After inoculation, the number of cells showing effective adhesion to the substrates was tentatively evaluated after 48 h of inoculation for the substrate PE, but it was too low for quantification using a Neubauer chamber. So, adhesion quantification was assayed after 72 h of inoculation for the other substrates and for all of them at 144 h.
The results in Fig. 2a show that at 72 h of testing a higher cell adhesion was observed in PVC coupons than in PP and Inox. The maximum number of cells adhered to PVC was the double of the cells attached to the other substrates, attaining 2.5 × 105 cells mL−1 or 5.0 × 104 cells cm−2 of substrate.
The results obtained at 144 h are depicted in Fig. 2b and show that for all the substrates assayed, the number of adhered cells increased when compared to 72 h, denoting biofilm growth. PVC continues to be the material attaining the highest number of attached cells (1.2 × 106 cells mL−1). It was also observed that the material with the lowest number of cells adhered was Inox (1.0 × 105 cells mL−1).
Biofilm development evaluation in BBM (fed-batch mode)
In the following tests, the culture medium was changed to BBM full strength to foster biofilm development, and after 240 h of incubation, the cell counting showed a global increase in attached cells compared to batch mode. The substrate material with the highest capacity to form and maintain a biofilm continued to be PVC, with a maximum of 2 × 106 cells mL−1 attained as illustrated in Fig. 2c. Nevertheless, at 312 h (Fig. 2d), it was observed that the capacity to form biofilm tends to be similar among substrates and with the same behavior as in batch mode. This behavior can be explained hypothesizing that, once the biofilm is formed, specificities of the adhesion surface like hydrophobicity, and surface topography, might have changed.
Another important aspect resulting from this experiment is related with the quantification of the amount of biomass formed on the different substrates used and their evolution along time. This evaluation is shown in Fig. 3a where it can be observed that the maximum cellular density was obtained with PVC (1.6 × 106 cells mL−1) up to 240 h of biofilm growth, but it decayed strongly for longer times. For PP, biofilm growth tended to be slower but prolonged in time, attaining a cellular concentration of 1.1 × 106 cells mL−1 after 312 h. For PE and Inox, the behavior observed shows an initial lag phase, a short growth phase (with a lower maximum cellular density, between 8.2 × 105 and 6.3 × 105 cells mL−1, respectively) and reaching a stationary phase of development after 240 h of incubation time.
Evolution of the density of Chlorella vulgaris SAG 211-12 cells adhered to each substrate along the duration of the experiment (a). Evolution of the absorbance of the cells in suspension along the duration of the experiment for each substrate assayed in duplicate (b). Vertical lines in a show changes of culture medium (BBM full strength)
Similar to the preliminary tests, where OD was used as a proxy to evaluate the existence of cellular adhesion, in these tests, the OD evolution was also followed for the cells in suspension in the medium (non-adhered cells and/or cells eventually detached from the substrates), for each material and for the control, along the incubation time. The biofilm growth peak at 240 h, especially for PVC, can be observed in Fig. 3b as a sharp drop in the OD of suspended cells. Additionally, comparing both graphics of Fig. 3, an inverse relationship can be seen between adhered cells counting and the concentration (absorbance) of cells in suspension, up to 240 h of testing.
In summary, from the work performed, and after analyzing the concentration of cells adhered to the different substrates for all time points, it can be said that PVC is globally the most appropriate substrate material for C. vulgaris SAG 211-12 adhesion and biofilm development. Moreover, we can conclude that Inox is the substrate that showed lower adhesion properties.
Adhesion and biofilm development complementary tests
In order to confirm the results obtained with PP and PVC in the first adhesion and biofilm development assays, the experiments were repeated in triplicate. Results showed again that the maximum number of adhered cells was found in PVC, with 1.4 × 106 cells mL−1 at 72 h and 1.0 × 106 cells mL−1 at 144 h (Online Resource 1).
Illumination is a very important parameter for microalgal development, so tests were performed with PVC for two different light intensities. The results obtained (Online Resource 1) showed no differences in the concentration of cells adhered to the substrate under the conditions assayed.
Once again, it assessed the possibility of using simple indicators of cell adhesion and biofilm formation, such as the variation of cells in suspension measured as absorbance. It was observed that, for each type of substrate tested, the higher the adhered cell concentration value, the lower the concentration of suspended cells in the culture medium (Online Resource 2).
These results allowed us to choose PVC as the best material for bioreactor development. Tests done to assess adhesion and biofilm formation in large PVC plates, using the protocols developed for coupons, were also successful (data not shown).
Laboratory-scale rotating flat plate photobioreactor
RFPPB performance
The proposed RFPPB was subjected to preliminary tests of performance, with biomass harvest at fixed intervals of 8 days and regrowth cycles without inoculation. The colony distribution was uniform and the biofilm formed was evenly distributed (Online Resource 3). Removal of the individual plates from the system was easy and the scraping process fast.
Nevertheless, an effect was observed in one of the tests when comparing harvested biomass characteristics from roughed PVC plates versus smooth ones and from the front and backsides of each plate (Online Resource 4). Independent from the plate side considered, rough PVC showed higher fresh and dry biomass weights (around 20% more). Regarding plate sides (A or P, being A the upward side regarding the rotational movement), effects varied depending whether fresh or dry biomass results were analyzed. For fresh weight, and independent from the PVC type used, the posterior side biomass was 30% higher than the anterior side values. As to dry weight, the anterior side (A) resulted in 10% more biomass than the posterior side (P), irrespective of the material used. These findings can be attributed to higher hydration of the biofilm scraped from the posterior plate sides, which is translated in higher dry to wet biomass ratio values for anterior compared to posterior faces.
Suspended growth in the bioreactor trough was very small (maximum below 0.13 OD and pH 7.2, or 0.50 OD at the last harvest, with pH of 8.4), a fact probably related to suboptimal conditions for the cell growth under these conditions. Some flocs of cells were observed, probably resulting from detached material from the plates, but were not quantified.
Although preliminary, data on bioreactor performance can be found in Table 1. Biomass yields increased steadily with successive harvest and regrowth cycles, reaching 3.35 g DW per adhesion surface.
Regarding productivity, and using a harvest frequency of 8 days, values doubled at each harvest time (Table 1), although started to decelerate at the second harvest, considering either areal or footprint productivity. This might indicate exhaustion of nutrients and/or CO2 in the culture medium.
Lipid content and profile of the C. vulgaris biofilm
The C. vulgaris biofilm collected by scraping was frozen and then lyophilized for total removal of water. Upon lipid extraction as described previously, it was found that the C. vulgaris SAG 211-12 biofilm contained 10.3 ± 0.1% (w/w dry basis) of lipids. The fatty acid profile of the lipids produced by C. vulgaris SAG 211-12 attached to the plates of the RFPPB is presented in Table 2.
The fatty acids found in the highest percentages (% of total fatty acids) were the γ-linolenic acid [C18:3 n-3] (24.32%), followed by the palmitic acid [C16:0] (20.81%) and the heptadecenoic acid [C17:1] (12.65%), in a slightly lower amount, while linoleic acid (C18:2) composition was ∼ 10%. Noteworthy, these results show a clear predominance of fatty acids composed of 16 and 18 carbons in the carbon chain, as well as a much lower ratio of saturated to unsaturated ones. The fraction of saturated FAME was ∼ 23.36%, whereas the fraction of mono unsaturated FAME was about 1/3 the total (∼ 34.33%) and the fraction of polyunsaturated FAME was the highest one (∼ 42.31%).
Discussion
The economical production of microalgal biomass, namely of Chlorella strains, is an important subject for the industry and so methods to reduce the impact of factors like harvest, while increasing productivity, are of utmost importance. In this line of thought, microalgae cultivation as biofilms offers many advantages, but still needs developments. In the present work, the biofilm formation capacity of C. vulgaris SAG 211-12 on several substrates was assessed, and a new photobiorector using the best attachment material tested (PVC) was developed and preliminarily tested.
Adhesion evaluation and biofilm formation in C. vulgaris SAG-211-12
In order to cultivate microalgae as biofilms, an adequate immobilization substrate must be chosen, and this process can be based, for instance, in an estimation of the attached cells. However, there are very few studies in the literature on microalgal adhesion evaluation, and normally elaborated methods of study are reported, such as direct counting of microalgal cells adhered to supports using epifluorescence microscopy (Sekar et al. 2004; Irving and Allen 2011). Nevertheless, such methods are time consuming and expensive and difficult to implement for quick screenings of adhesion in large-scale biomass production systems, and thus, alternative procedures should be available. Application of simple methodologies for attachment and biofilm formation assessment, like spectrophotometry, can be found in studies of bacterial adhesion to medical devices (An and Friedman, 1997). For bacteria, either the substrate is observed directly with a spectrophotometer after staining, or the cells are washed off the surface, stained, and the optical density of the solution examined. In this field, adhesion and biofilm formation studies can be performed not only in batch but also in fed-batch systems, which enable more realistic conditions, by changing the culture medium at defined intervals. This way more biofilm can be produced (Cerca et al. 2004). In the present work, as the microalga used had green pigments, it was tested the possibility of directly using optical density changes of a suspension, measured at 680 nm, as a rapid method of assessing the adhesion of C. vulgaris SAG 211-12 to different materials. It was hypothesized that the OD of the microalgal suspension would show a decrease when adhesion occurs, which could be corroborated by visual observations and by the concomitant increase of the number of cells attached to the substrate. This was in fact observed for all the substrates tested, especially for the most performing one (PVC, Fig. 3). In view of the results obtained in the present work, the use of the optical density of the suspended culture as a quick and simple adhesion indicator can be envisaged as a screening method for surface attachment studies with microalgae, although more research should be done. Regarding the biofilm production on different substrates, results show that this microalga has adhesion and biofilm formation capacities, especially on PVC. High maximum number of cells (over 1.0 × 106 mL−1) was obtained, but the results of the adhesion to different substrates in batch mode showed variation in biomass values for the duplicate and triplicate tests performed (Fig. 2 and Online Resource 1). This is an aspect already observed in other studies (Irving and Allen 2011; Blanken et al. 2014) and may be due to an instability in the initial biofilm development under the experimental conditions assayed, along with problems in the collection and treatment of the material adhered, which increase as the mass of cells retrieved became bigger. Thus, in this case, substrate analysis based on adhesion trends, more than in biomass absolute values, could be a more adequate methodology. Regarding the types of substrates tested, the results of our work show a tendency for C. vulgaris SAG 211-12 preference for PVC up to 144 h of batch testing, with low to moderate adhesion to stainless steel, PE, and PP. Although it is difficult to compare exhaustively the present work with others in the literature, not only because of the difference in strains (and even species) used but also due to the different methodologies employed to assess adhesion, some examples can be given to illustrate the multiplicity of possible experimental outcomes. In Sekar et al. (2004), it is referred the significant variation of the behavior of an autochthonous C. vulgaris strain isolated from a cooling system with the materials used, being the maximum colonization observed in Inox, followed by Perspex and glass after 48 h of testing. However, in the work of Irving and Allen (2011), the best results were obtained with PU and the worse with PE and glass for C. vulgaris strain CPCC no. 90. On the other hand, Orandi et al. (2012) showed that PVC enabled the formation of a mature biofilm of a microbial consortium dominated by Ulothrix sp., and Blanken et al. (2014) tested rough and smooth stainless steel woven meshes and a coated, sanded polycarbonate disk to produce Chlorella sorokiniana biofilms, with results favoring the rough mesh. Explanations for microalgal substrate preference are diverse and difficult to generalize, and so it is difficult to predict algal adhesion to surfaces. Some authors say that surface characteristics like hydrophobicity are important factors, although reports of no correlation of adhesion with this parameter exist (e.g., Sekar et al. 2004; Irving and Allen 2011), and others point that surface topography deserves consideration, with more structured surfaces promoting attachment (e.g., Irving and Allen 2011; Blanken et al. 2014). Other reasons for adhesion preferences or avoidances can also be evoked, such as substrate toxicity (e.g., Sekar et al. 2004). In our work, all the surfaces tested were flat, non-patterned (smooth), non-toxic, and hydrophobic according to the referred literature. Therefore, the preference of C. vulgaris SAG 211-12 for PVC is difficult to justify and can be an attribute of the strain, substrate, and culture conditions used.
Biofilms tend to grow with the age of the culture, and a study of their behavior for prolonged periods is important from a practical point of view. The use of fed-batch cultivation, where the culture medium is changed at pre-defined periods, can foster biofilm development (Cerca et al. 2004). This approach was followed in our work, and biomass increased for all materials at 240 h, but it resulted in a leveling of attached cell numbers for all materials after 312 h of cultivation. This behavior was also reported by authors like Irving and Allen (2011) after 7 days of cultivation and can be explained hypothesizing that, once the biofilm is formed, specificities of the adhesion surface like hydrophobicity, and surface topography might have changed, and so no substrate preferences are observed.
The novel rotating flat plate photobioreactor
One of the most important aims of this work was the development of a new photobioreactor. Therefore, it is important to discuss the rationale behind its development, the advantages facing other models, and the potential for improvement.
Among the (few) types of existing biofilm-based algal cultivation systems, the rotating designs based on the RBC principle are appealing. These systems are of common use in biological wastewater treatment and some of their characteristics, like small footprint and large surface area, are important for more efficient microalgal bioreactor development. Moreover, it is widely accepted that attached growth biomass systems provide easy harvest when compared to suspended ones (Gross et al. 2015a).
The construction of the new RFPPB was based on the premise of using a rotating system but innovating in design, with a durable substrate favorable for adhesion of a microalga like C. vulgaris SAG 211-12 (as is PVC), maximizing light radiation incidence and area available for microalgal attachment in a small footprint, while facilitating harvest. The prototype designed (Fig. 4 (I)) is also inexpensive, efficient, and scalable. Moreover, the new design chosen, using rectangular flat plates placed in a horizontal shaft (a paddle-wheel-like reactor), is also more favorable to operation under continuous regime, as the liquid flow direction (in-out) will be parallel to the longest dimension of the plates of the reactor, thus maximizing the contact between the attached microalgae, nutrients, and CO2 present in the flow. This contrasts favorably with existent models based on the classical RBC concept, like the PRBC (photorotating biological contactor, Orandi et al. 2012), constructed using 16 roughened PVC disks mounted in an horizontal shaft; the RABR (rotating algae biofilm reactor, Christenson and Sims 2012), which has PVC cylinders covered with fabrics or aluminum wheels placed in a shaft, covered with cotton cord for microalgal attachment; and the Algadisk reactor (Blanken et al. 2014), which uses four disks with different materials, horizontally placed in a medium trough. In these systems, the disks, placed sequentially in the horizontal shaft, enter the medium trough vertically, with the first disk facing the flow inlet point. Proper spacing of the disks enables adequate aeration and flow distribution. Nevertheless, mixing problems can occur in the medium trough, as denoted by the need of a mixing paddle in the PRBC to prevent short-circuiting.
(I) Rotating plate photobioreactor (RFPPB) projected and tested in this work. (II) Schematic representation of solar light incidence over the disks of a RBC-type photobioreactor during part of a day (a); variation of solar position over an RBC along 1 day (b); schematic representation of the new RFPPB photobioreactor and solar radiation incidence over the plates of the RFPPB at noon (c); solar incidence variation over the RFPPB during 1 day (d)
Light is a critical parameter for microalgal production. Existing biofilm photobioreactor designs favor light penetration using, for example, flat support materials instead of textured ones, which should be placed vertically to reduce footprint. However, this creates shadow effects (Gross et al. 2015a). A better understanding of the optimization of light incidence achieved with the new photobioreactor design can be obtained comparing the RFPPB reactor with a RBC-type reactor with vertically placed disks (Fig. 4 (II, a, b)) and assuming daily solar illumination. In the RBC, the angle of light incidence over one disk increases as the number of disks decreases, with a maximum of 90° in case of only one disk, with the light source perpendicular to the surface of the disk. Moreover, a maximum angle of 45° is obtained when the distance between disks is equal to their radius. In the RFPPB, contrary to the RBC, exposure to light is not a limiting factor for microalgal growth because it is possible, at a given time, that a light incidence is at 90°, as it can be seen in Fig. 4 (II, c, d)). In addition, the constructed bioreactor enables the maximization of the biofilm area exposed to light, as all the area of one plate receives light radiation at a certain point of its rotation, avoiding shading, as occurs in the RBC due to the disks being placed in series in a shaft.
Moreover, in case of excessive (photoinhibitory) solar radiation being achieved, the proposed photobioreactor can minimize this problem as the number of plates can be changed easily, thus controlling the radiation per unit of growth surface, i.e., dissipating the total radiation by an increased surface area used for biofilm growth (spatial dilution of light referred by Tredici and Zittelli 1998).
Regarding the controlled illumination used indoors, the proposed model has also some practical use advantages compared, for instance, with the Algadisk photobioreactor proposal (Blanken et al. 2014), where the lateral disk illumination used complicates the design and the harvest, which increases costs.
In normal operation, RBC-type bioreactors need periodic cleaning or flushing. Another clear advantage of the proposed RFPPB, when compared with other RBC-based models, is the fact that it is possible to remove one plate at a time for microalgal harvest and/or cleaning, without the need to alter the position of the others, or to remove all the other plates from the shaft. Thus, harvest time and effort are reduced, resulting in lower processing costs.
As to the novel photobioreactor operation, it is similar to other types of biofilm-based reactors, with initial microalgal inoculation in a suitable medium and several cycles of production and harvest. As reported also for other photobioreactors (Johnson and Wen 2010; Christenson and Sims 2012; Gross et al. 2013), the colonies remaining on the plates after harvest by scraping served as inoculum for further microalgae growth, thus diminishing work and costs with reinoculation.
As the RFPPB was designed to produce microalgae as a biofilm, it was not expected the production of important suspended biomass. In fact, very low suspended growth was observed (maximum OD680 of 0.50), as observed also in other rotating biofilm photobioreactors (e.g., Blanken et al. 2014, with OD below 1.0). These results can be attributed to insufficient light for photosynthesis in the bioreactor trough (which was opaque and was almost entirely covered by the biofilm plates, thus impeding light penetration from above).
Bioreactor performance and potential improvements
An evaluation of the RFPPB performance showed maximum biomass yield values that are 300% higher than those obtained by Gross et al. (2013) using Chlorella vulgaris (UTEX #265), continuous light, optimized BBM for 15 days and non-optimal cotton surfaces (1.2 to 1.35 g m−2), but are 500% lower than the one obtained using the best surface material (16.20 g m−2). Data from their work also shows that yields doubled with increasing rotational speed of the RAB system to 4 rpm. Rotational speed is, in fact, an important factor affecting performance in rotating biological contactors as it affects nutrient and gas mass transfer to and from the biofilm (Cortez et al. 2008). The speed of rotation also affects microalgal growth by the alternating exposition to the air and to the culture medium and biofilm formation by the shear stress applied (Gross et al. 2013). Thus, as we have used only 2.8 rpm rotation speed (and BBM/2 as medium), there is room for improvements of our bioreactor performance, which will lead to higher yields of biomass.
In biofilm reactors, an improvement in substrate roughness can increase attachment and so biomass production (Sekar et al. 2004; Gross et al. 2015a). In our case, there was no observable effect on final biomass of the improvement of the PVC plate adhesion surface characteristics (roughness), which was also referred in other works with C. vulgaris (Irving and Allen 2011). This might be explained by the fact that, after initial colonization, and during regrowth, the effect of surface characteristics is mitigated. Nevertheless, this type of tests should be repeated for confirmation of our results.
Some detached material was observed at the bottom of the RFPPB reactor. This material is produced as the biofilm thickens and becomes more susceptible to shear forces (Gross et al. 2015a), which can be important in a rotating bioreactor. As also commented by other authors (Blanken et al. 2014), the productivity of the RFPPB could be increased if this biomass was harvested and quantified.
Another factor that could affect productivity is the harvest frequency. Nevertheless, the harvest frequency used (8 days) is close to the 7 days used by other authors (Gross et al. 2013; Blanken et al. 2014).
As referred above, our results are within the range of values found by Gross et al. (2013) for their preliminary tests on adhesion surfaces and therefore are promising considering the fact that the bioreactor operation was not yet optimized. In the referred work, areal productivity increased also with rotational speed, up to 4 rpm. Hence, by improving this factor, as well as the culture medium (composition, pH), CO2 input and the illumination characteristics (intensity, photoperiod), we expect that significantly higher productivities can be achieved.
Lipid profile of microalgae produced in RFPPB
The biofilm produced in the novel RFPPB contained 10.3% lipids. This result is in accordance with the one found by Griffiths et al. (2014) when cultivating C. vulgaris under N-replete conditions and within the range determined by Matos et al. (2014) for suspended cultures supplemented with desalination wastewater. However, the lipid content of the C. vulgaris cells attached to the RFPPB plates just reached the lowest value achieved by Münkel et al. (2013) while using a flat panel airlift reactor.
Regarding the fatty acid profile, the C. vulgaris SAG 211-12 cultivated as a biofilm were rich in polyunsaturated fatty acids (PUFAs) like linoleic (omega 6 fatty acid, a widely recognized food supplement) and linolenic (omega 3 fatty acid), also known as essential fatty acids, which were found to be high in C. vulgaris. The importance of these acids for the cell is evident from the sum of linoleic and γ-linolenic acids, which was approximately 34.3% of the total fatty acid content. These essential fatty acids are an obligatory dietary requirement for humans and animals (Batista et al. 2013). Polyunsaturated fatty acids are typical components of membrane lipids.
Not only lipid productivity but also the composition of the fatty acid profile plays an important role for future applications of microalgae lipids. High amounts of saturated and monounsaturated fatty acids are the basis for an economically feasible production of biofuels from microalgae. The importance of such acids in accordance with the biodiesel standard EN 14214 is described in Griffiths et al. (2011). Among others, Chlorella vulgaris is one of the most promising microalgae for the accumulation of triglycerides and therefore interesting for biodiesel production.
In conclusion, C. vulgaris SAG 211-12 showed good adhesion capacity to Inox, PP, PE, and PVC and growth as a biofilm. Initial behavior differences of firmly adhered cells towards substrate materials were attenuated with full biofilm development. A new rotating flat plate concept photobioreactor, the FRPPB, was built using PVC, the best immobilization substrate. The bioreactor is robust, is inexpensive, promotes good biofilm development, has higher light radiation incidence than conventional rotating disks, has small footprint, has easy maintenance, and has easy biomass harvest, thus providing a new cost-effective microalgae production system. The first tests were promising, yielding 3.35 g DW m−2 of biomass and a lipid content of 10.3% (w/w dry basis), rich in PUFA.
References
An YH, Friedman RJ (1997) Laboratory methods for studies of bacterial adhesion. J Microbiol Methods 30:141–152
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2:164–173
Blanken W, Janssen M, Cuaresma M, Libor Z, Bhaiji T, Wijffels R (2014) Biofilm growth of Chlorella sorokiniana in a rotating biological contactor based photobioreactor. Biotechnol Bioeng 111:2436–2445
Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J (2004) Influence of batch or fed-batch growth on Staphylococcus epidermidis biofilm formation. Lett Appl Microbiol 39:420–424
Chini Zittelli G, Rodolfi L, Bassi N, Biondi N, Tredici M (2013) Photobioreactors for microalgae biofuel production. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 115–131
Christenson LB, Sims RC (2012) Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol Bioeng 109:1674–1684
Cortez S, Teixeira P, Oliveira R, Mota M (2008) Rotating biological contactors: a review on main factors affecting performance. Rev Environ Sci Biotechnol 7:155–172
Craggs RJ, Adey WH, Jenson KR, St John MS, Green FB, Oswald WJ (1996) Phosphorus removal from wastewater using an algal turf scrubber. Water Sci Technol 33:191–198
Feng P, Deng Z, Hua Z, Fan L (2011) Lipid accumulation and growth of Chlorella zofingiensis in flat plate photobioreactors outdoors. Bioresour Technol 102:10577–10584
Gao F, Yang Z-H, Li C, Zeng G-M, Ma D-H, Zhou L (2015) A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour Technol 179:8–12
Griffiths MJ, Garcin C, van Hille RP, Harrison ST (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Methods 85:119–123
Griffiths MJ, van Hille RP, Harrison ST (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98:2345–2356
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201
Gross M, Jarboe D, Wen Z (2015a) Biofilm-based algal cultivation systems. Appl Microbiol Biotechnol 99:5781–5789
Gross M, Mascarenhas V, Wen Z (2015b) Evaluating algal growth performance and water use efficiency of pilot-scale revolving algal biofilm (RAB) culture systems. Biotechnol Bioeng 112:2040–2050
Irving TE, Allen DG (2011) Species and material considerations in the formation and development of microalgal biofilms. Appl Microbiol Biotechnol 92:283–294
Johnson MB, Wen Z (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85:525–534
Kesaano M, Sims RC (2014) Algal biofilm based technology for wastewater treatment. Algal Res 5:231–240
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Li T, Piltz B, Podola B, Dron A, de Beer D, Melkonian M (2016) Microscale profiling of photosynthesis-related variables in a highly productive biofilm photobioreactor. Biotechnol Bioeng 113:1046–1055
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications. Renew Sust Energ Rev 14:217–232
Mata TM, Melo AC, Meireles S, Mendes AM, Martins AA, Caetano NS (2013) Potential of microalgae Scenedesmus obliquus grown in brewery wastewater for biodiesel production. Chem Eng Trans 32:901–906
Mata TM, Martins AA, Oliveira O, Oliveira S, Mendes AM, Caetano N (2016) Lipid content and productivity of Arthrospira platensis and Chlorella vulgaris grown under mixotrophic conditions with salinity stress. Chem Eng Trans 49:187–192
Matos AP, Torres RCO, Morioka LRI, Moecke EHS, França KB, Sant’Anna ES (2014) Growing Chlorella vulgaris in photobioreactor by continuous process using concentrated desalination: effect of dilution rate on biochemical composition. Int J Chem Eng. https://doi.org/10.1155/2014/310285
Münkel R, Schmid-Staiger U, Werner A, Hirth T (2013) Optimization of outdoor cultivation in flat panel airlift reactors for lipid production by Chlorella vulgaris. Biotechnol Bioeng 110:2882–2893
Orandi S, Lewis DM (2013) Biosorption of heavy metals in a photo-rotating biological contactor—a batch process study. Appl Microbiol Biotechnol 97:5113–5123
Orandi S, Lewis DM, Moheimani NR (2012) Biofilm establishment and heavy metal removal capacity of an indigenous mining algal-microbial consortium in a photo-rotating biological contactor. J Indust Microbiol Biotechnol 39(9):1321–1331. https://doi.org/10.1007/s10295-012-1142-9
Ozkan A, Kinney K, Katz L, Berberoglu H (2012) Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour Technol 114:542–548
Percival SL, Knapp JS, Edyvean RGJ, Wales DS (1998) Biofilms, mains water and stainless steel. Water Res 32:2187–2201
Sekar R, Venugopalan V, Satpathy K, Nair K, Rao V (2004) Laboratory studies on adhesion of microalgae to hard substrates. Hydrobiologia 512:109–116
Seth JR, Wangikar PP (2015) Challenges and opportunities for microalgae mediated CO2 capture and biorefinery. Biotechnol Bioeng 112:1281–1296
Shen Y, Zhang H, Xu X, Lin X (2015) Biofilm formation and lipid accumulation of attached culture of Botryococcus braunii. Bioprocess Biosyst Eng 38:481–488
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Stein JR (1973) Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge
Travieso L, Pellón A, Benıtez F, Sánchez E, Borja R, O’Farrill N, Weiland P (2002) BIOALGA reactor: preliminary studies for heavy metals removal. Biochem Eng J 12:87–91
Tredici MR, Zittelli GC (1998) Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotechnol Bioeng 57:187–197
Wang SK, Stiles AR, Guo C, Liu CZ (2014) Microalgae cultivation in photobioreactors: an overview of light characteristics. Eng Life Sci 14:550–559
Yin S, Wang J, Chen L, Liu T (2015) The water footprint of biofilm cultivation of Haematococcus pluvialis is greatly decreased by using sealed narrow chambers combined with slow aeration rate. Biotechnol Lett 37:1819–1827
Zhang L, Chen L, Wang J, Chen Y, Gao X, Zhang Z, Liu T (2015) Attached cultivation for improving the biomass productivity of Spirulina platensis. Bioresour Technol 181:136–142
Acknowledgements
This work was financially supported by the Project FCT UID/EQU/00305/2013 and Project POCI-01-0145-FEDER-006939 (Laboratory for Process Engineering, Environment, Biotechnology and Energy – LEPABE funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI)—and by national funds through FCT—Fundação para a Ciência e a Tecnologia—and partially supported also by the Strategic Funding UID/Multi/04423/2013 through national funds provided to CIIMAR (Interdisciplinary Centre of Marine and Environmental Research) by FCT and European Regional Development Fund (ERDF), in the framework of the program PT2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Online Resource 1
(DOCX 38 kb).
Online Resource 2
(DOCX 58 kb).
Online Resource 3
(DOCX 1995 kb).
Online Resource 4
(DOCX 17 kb).
Rights and permissions
About this article
Cite this article
Melo, M., Fernandes, S., Caetano, N. et al. Chlorella vulgaris (SAG 211-12) biofilm formation capacity and proposal of a rotating flat plate photobioreactor for more sustainable biomass production. J Appl Phycol 30, 887–899 (2018). https://doi.org/10.1007/s10811-017-1290-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1290-4