Abstract
Neurodegenerative disorders, which affect memory, cognition, and social functions, can be treated using neurotrophic agents to support neuronal development and protect mature neurons from atrophy. We screened 34 tropical seaweed species collected from Indonesian coastal areas for their neurite-outgrowth-promoting activity (NOPA) in fetal rat hippocampal neurons in vitro. Based on the number and total length of primary neurites, red seaweeds had greater NOPA than green and brown seaweeds. The red seaweed Kappaphycus alvarezii showed the highest NOPA. Addition of the ethanol extract to the culture (1 μg mL−1) significantly accelerated initial neuronal maturation from stage I to stage II (70 %; P < 0.05) within 24 h and increased the number of neurites that developed multipolar characteristics (48 %; P < 0.05). These results indicate that the aquaculturable carrageenan producer K. alvarezii might be a promising source of neurotrophic compounds to enhance memory and learning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common age-associated dementia requires neurotrophins to prevent neuronal death or produce neuronal cells. Neurotrophins promote neurite outgrowth processes and survival. Neuronal death can be prevented by endogenous neurotrophic factors (NFs), such as nerve growth factor and brain-derived neurotrophic factors (Akagi et al. 2015). Neurotrophic factors play roles in both the development and the maintenance of the nervous system, including neuronal differentiation, maturation, and survival. Their ability to elicit pro-survival and pro-functional responses in neurons make them candidate drugs for treating neurodegenerative disorders (Weissmiller and Wu 2012).
Seaweeds are an abundant marine resource that provide important compounds to marine animals and may be used for the treatment of various human diseases. The use of seaweeds has increased due to their various advantages and ease of cultivation. In addition to their use as foodstuffs, several seaweeds have also been used as herbal medicines (Smit 2004; Kolanjinathan et al. 2014). Several studies have reported neurotrophic activities of Sargassum fulvellum (Hannan et al. 2012), Gelidium amansii (Hannan et al. 2013, 2014), Undaria pinnatifida (Bhuiyan et al. 2015), Gracilariopsis chorda (Mohibbullah et al. 2015a), and Porphyra yezoensis (Mohibbullah et al. 2015b) in primary cultures of fetal rat hippocampal neurons. Kamei and Sagara (2002) screened 300 seaweed species for their neurite-outgrowth-promoting activity (NOPA) in a rat adrenal medulla pheochromocytoma cell line (PC12D). Although most samples showed no activity, the brown alga Sargassum macrocarpum showed prominent NOPA. Sargaquinoic acid (Kamei and Tsang 2003) and sargachromenol (Tsang et al. 2005) isolated from S. macrocarpum promoted neurite outgrowth via signaling pathways in PC12D cells.
In the present study, we screened 34 tropical seaweed species for NOPA in a primary culture of rat hippocampal neurons. The hippocampus is especially vulnerable to damage during the early stages of Alzheimer’s disease (Mu and Gage 2011). In primary cultures, hippocampal neurons readily polarize, develop extensive axonal and dendritic arbors, and form numerous functional synaptic connections with one another, which are useful for determining many aspects of neuronal development (Kaech and Banker 2006; Seibenhener and Wooten 2012). Using these hippocampal neuron cells, NOPAs of tropical seaweed extracts were compared based on the number and total length of primary neurites, and the dose dependence of the effect on neuronal maturation by the seaweed with the greatest potential, Kappaphycus alvarezii, was investigated.
Materials and methods
Collection of seaweed samples
Thirty-four species of wild seaweed were collected from the coast of Sayang Heulang (7° 40′ 08.1″ S, 107° 41′ 42.9″ E) and Karimunjawa (5° 48′ 27.6″ S, 110° 22′ 01.3″ E), Java, Indonesia, in 2014 and 2015. Tissues were thoroughly rinsed with freshwater to remove all extraneous matter, such as epiphytes, sand, salts, and shells. The seaweed samples were identified and dried at room temperature for 3–5 days. Dried tissues were cut into small pieces, pulverized to fine powder in a grinder (HMF-340, Hanil Co., Seoul, South Korea), and stored in zipper plastic bags under dark conditions at 20 °C for further use. Voucher specimens were deposited in the author’s laboratory (Y.K. Hong).
Preparation of extracts
Fine powder samples were sieved through a 36-mesh filter to obtain a small particle size. Two gram of each sample was extracted with 100 mL of 95 % ethanol in a 250-mL flask. The mixture was incubated on an orbital shaker at 200 rpm at room temperature. After 24 h, the extract was separated from the residue using a filtration set with a vacuum pump through Advantec No. 2 filter paper. The filtrate was concentrated in vacuo, and the salts contained in the extract were eliminated by repeated extractions with 100 % ethanol until no salt remained visible. After drying under a stream of nitrogen gas, the extracts were dissolved in dimethyl sulfoxide (DMSO) to 8 mg mL−1 and stored at −20 °C in airtight vials until required.
Culture and treatment of primary hippocampal neurons
Primary hippocampal neurons were cultured in 24-well polystyrene plates. All cell culture reagents were from Invitrogen (USA) unless otherwise stated. All animal care and use were in accordance with the institutional guidelines and approved by the Institutional Animal Care and Use Committee of the College of Medicine, Dongguk University, South Korea. Primary hippocampal neurons were cultured as described previously (Goslin et al. 1998; Hannan et al. 2012). Briefly, rats (Sprague–Dawley) at day 19 of pregnancy were euthanized with isoflurane, and the fetuses were collected. The hippocampi dissected from the brain were collected in Hank’s balanced salt solution (HBSS) and incubated in 0.25 % trypsin in HBSS for 12 min at 37 °C to dissociate tissues. The cells were counted with a hemocytometer and plated at a density of ∼1–2 × 104 cells cm−2 onto poly-DL-lysine-coated glass coverslips in 24-well plates. Cultures were maintained in serum-free neurobasal medium supplemented with B27 and incubated at 37 °C under 5 % CO2 and 95 % air. Extract or vehicle control (DMSO, final concentration ≤0.5 %) was added to the culture medium prior to plating.
Image acquisition
Images (1388 × 1039 pixels) were acquired using a Leica Microscope DM IRE2 equipped with I3 S, N2.1S, and Y5 filter systems (Leica Microsystems AG, Germany) and a high-resolution CoolSNAP CCD camera (Photometrics Inc., Germany) under the control of a computer using Leica FW4000 software. The microscope was used for phase-contrast and epifluorescence microscopy. The digital images were processed using Adobe Photoshop 7.0 for Windows.
Image analysis and quantification
Morphometric analyses and quantification were performed using ImageJ version 1.48 software (http://imagej.nih.gov/ij) with the simple neurite tracer plug-in (National Institute of Health, Bethesda, MA, USA) and Sholl plug-in (http://biology.ucsd.edu/labs/ghosh/software). Morphometric parameters, such as the number of primary neurites (NPN; neurites that originated directly from the soma), total length of primary neurites (TLPN; the sum of the length of primary neurites), and length of the longest neurite (LLN), were measured. Neurons that were not intermingled with the processes of adjacent neurons were selected for analysis. Neuronal cell populations at different developmental stages and cells that exhibited unipolar, bipolar, and multipolar characteristics were also counted (Dotti et al. 1988). A vehicle control (medium with DMSO) was compared with those treated with seaweed extracts.
Statistical analysis
Data are expressed as means ± standard error. All data were checked for the normality of the distribution using a Kolmogorov–Smirnov test. Normally distributed data were analyzed by Student’s t test and one-way analysis of variance followed by the least-significant-difference test with significance set at P < 0.05. All calculations were carried out using SPSS 17.0 software for Windows (SPSS Inc., USA).
Results
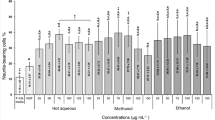
The NOPA of seaweed ethanolic extracts was evaluated based on the NPN and TLPN of embryonic hippocampal neurons. Those morphometric analyses were performed in vitro at day 2 (DIV 2). Among the 34 seaweeds tested, 14 showed neurotrophic activity based on the NPN, and 18 species showed such activity based on the TLPN (Tables 1, 2, and 3). Most of the seaweeds showed dose-dependent neurotrophic activities. Activity increased in a dose-dependent manner at 1–10 μg mL−1, but it decreased or was inhibited at 20 μg mL−1. In our screening experiment, extracts of Gelidiella acerosa (EGA) and K. alvarezii (EKA) showed highly significant positive effects (P < 0.001) on NOPA in developing rat hippocampal neurons at the lowest dose, 1 μg mL−1 (Table 3). Upon addition of EGA, NOPAs based on NPN and TLPN were 1.31- and 1.92-fold, respectively, higher than those of the vehicle control. The addition of EKA resulted in 1.22- and 1.71-fold increases in NOPAs based on NPN and TLPN, respectively, compared with the vehicle control. Thus, we selected EKA for further evaluation because K. alvarezii is one of the most abundant aquaculturable carrageenophytes. Four of 10 chlorophytes (Caulerpa racemosa, Codium decorticatum, Ulva reticulata, and Valoniopsis pachynema, Table 1), 1 of 8 phaeophytes (Padina australis, Table 2), and 11 of 16 rhodophytes (Acanthophora muscoides, Acanthophora spicifera, Amphiroa fragilissima, Eucheuma spinosum, Gelidium latifolium, Gracilaria coronopifolia, Gracilaria heteroclada, Gracilaria salicornia, Gracilaria tenuistipitata, Gracilaria verrucosa, and Palmaria palmata, Table 3) were other seaweed species that exhibited activity. Most rhodophytes (81 %) exhibited greater NOPA compared with chlorophytes (40 %) and phaeophytes (13 %). Furthermore, Acanthophora spicifera, E. spinosum, Gracilaria tenuistipitata, and K. alvarezii showed marked NPN activity, and Acanthophora muscoides, Acanthophora spicifera, and Gelidiella acerosa showed considerable TLPN activity. Additionally, Halimeda renschii and Sargassum polycystum exhibited marked inhibition of NPN and TLPN.
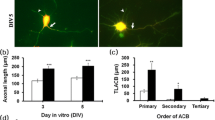
Because K. alvarezii had the greatest NOPA and biomass, we further investigated the extract of this seaweed. First, to evaluate the concentration dependency of the effect, 0.1 to 60 μg mL−1 EKA was added to hippocampal neuron cultures. Typical micrographs of a culture grown for 2 days are shown in Fig. 1a. Growth parameters—the NPN, TLPN, and LLN—peaked at 1 μg mL−1 (P < 0.05) and decreased gradually at higher doses (Fig. 1b). Therefore, EKA was added to neuron cultures at 1 μg mL−1 in subsequent experiments.
Optimal EKA concentration for neurite-outgrowth-promoting activity. a Representative phase-contrast photomicrographs of EKA (1 μg mL−1) and vehicle. b EKA concentration optimization for NPN (triangle), TLPN (square), and LLN (circle) of hippocampal neurons after 2 days of incubation. V indicates vehicle. Different letters (a–d) indicate significant differences among the means (P < 0.05). Data represent means ± SE (n = 60–80 neurons for each concentration)
EKA influenced the initial development of neurons using the optimal dose, 1 μg mL−1. Neurites formed during initial neuronal differentiation function as precursors for dendrites and axons in mature neurons. Neurons are categorized into the following developmental stages (Fig. 2a): lamellipodia (stage I), transition (fan-shaped process; stage 1.5), and minor processes (stage II). The number of neurons at each maturation stage differed significantly after 24-h incubation (P < 0.05). In cultures to which EKA was added, 70 % of the cells were in developmental stage II, and only 13 % were in stage I; however, ∼46 % of the cells in the vehicle group were in stage II, and 27 % were in stage I (Fig. 2b, (1)). At 48 h, the number of neurons in stage II increased significantly (P < 0.05), to 85 % (vs. to only 64 % in the vehicle control), upon the addition of EKA to the medium (Fig. 2b, (2)). Thus, the addition of EKA to the fetal hippocampal neuron culture medium significantly accelerated the maturation of neuronal cells (P < 0.05). Furthermore, we counted the number of processes to determine the neuron type and categorized minor process (stage II) neurons into unipolar (at least one process), bipolar (two processes), and multipolar (more than two processes, Fig. 3a). Of the neuronal cells cultured in EKA-containing medium for 24 h, 48 % developed multipolar characteristics compared with 21 % in the control culture (Fig. 3b, (1)). After 48 h, only 9 % of the total cells in EKA medium did not exhibit bipolar characteristics, whereas 28 % of the cells in the vehicle medium remained unipolar (Fig. 3b, (2)). Thus, the number of processes in EKA-treated cultures was significantly increased compared with that in the vehicle control at both 24 and 48 h (P < 0.05). These results clearly indicate that EKA not only enhances neuronal maturation but also accelerates their initial differentiation.
Effects of EKA on neuronal development. a Phase-contrast photomicrographs showing initial differentiation of developing neurons: stage I (lamellipodia, 1), stage 1.5 (transition stage, fan-shaped process, 2), and stage II (minor process, 3). b Relative ratio of neurons (%) in the various developmental stages of stage 1 (black bar), stage 1.5 (white bar), and stage 2 (gray bar) after 24 h (1) and 48 h (2) of incubation. Values are percentages of 1000–1200 neurons
Effects of EKA on the processes of neuronal development. a Typical phase-contrast images of neurons with unipolar (1), bipolar (2), and multipolar (3) characteristics. b Relative ratios of neurons (%) that exhibited unipolar (black bar), bipolar (white bar), and multipolar (gray bar) characteristics after 24 h (1) and 48 h (2) of incubation. Values are percentages of 500–800 neurons
Discussion
We compared the effects of common tropical seaweeds on the neurite outgrowth of primary hippocampal neurons. Among the 34 seaweed species tested, most rhodophytes enhanced neurite outgrowth based on morphological parameters. Most agar or carrageenan producers also demonstrated NOPAs. Few phaeophytes possessed neurotrophic factors, and many inhibited neurite outgrowth, likely due to their phenolic or tannin components. It will be also important to identify NOPA inhibitors to warn humans about consuming food that contains these substances. Our data suggest that K. alvarezii (also commercially known as cottonii) exhibited the greatest enhancement of neuronal differentiation. Approximately 1.5 million tonnes of dry cottonii was harvested from aquaculture in Indonesia in 2009 (Pambudi et al. 2010). The effect of EKA on initial neuronal development was evidenced by the early development of multipolar characteristics. Neuron cells that develop neurites earlier are thought to develop more extensive arborization. In our previous study (Hannan et al. 2013), the red seaweed Gelidium amansii exhibited the greatest NOPA. At DIV 3, its ethanol extract showed increased NPN (133 %), TLPN (186 %), and LLN (211 %) values; however, K. alvarezii at DIV 2 showed increased NPN (137 %), TLPN (159 %), and LLN (144 %) values. The optimal dose of EKA (1 μg mL−1) was markedly lower than that of Gelidium amansii (15 μg mL−1) for NOPA. Thus, K. alvarezii was assumed to have potent neurotrophic compounds. This carrageenan producer K. alvarezii also exhibited antioxidant (Kumar et al. 2008), cardiovascular protective (Matanjun et al. 2010), antimicrobial (Prabha et al. 2013), and anti-inflammatory (Ranganayaki et al. 2014) activities.
NF levels in the brain affect neuronal development and survival; thus, NFs are required to prevent neuronal aging and neurodegenerative symptoms, such as Alzheimer’s disease (Heese et al. 2006). The Chinese medicinal herb Achyranthes bidentata has been reported to prevent the apoptosis and enhance the survival and growth of rat hippocampal neurons in primary cultures (Tang et al. 2008). Ginkgolide B from Ginkgo biloba increased brain-derived NFs and protected hippocampal neurons from apoptosis (Xiao et al. 2010). The brown seaweed S. macrocarpum exhibits NOPA and supports the survival of PC12D cells due to production of sargaquinoic acid (Kamei and Tsang 2003) and sargachromenol (Tsang et al. 2005). Sargassum fulvellum also promoted maturation, synaptogenesis, and survival of hippocampal neuron cells (Hannan et al. 2012). The active compound from S. fulvellum responsible for the differentiation of PC12D cells was pheophytin a (Ina et al. 2007). Phlorotannins from Eisenia bicyclis have been shown to inhibit β-amyloid cleavage enzyme (Jung et al. 2010), which initiates the formation of β-amyloid in Alzheimer’s disease (Tang et al. 2006). The ethanol extract of K. alvarezii contains alkaloids, carbohydrates, glycosides, proteins, amino acids, phenolic compounds, flavonoids, and terpenoids (Prabha et al. 2013). The aqueous extract had considerable quantities of alkaloids, saponin, phenols, proteins, phytosterols, amino acids, sugars, reducing sugars, flavonoids, steroids, and tannins (Ranganayaki et al. 2014). The major active compound for NOPAs from EKA has not been determined. Our data suggest that the abundant, aquaculturable, and edible K. alvarezii may exert beneficial effects on the health of the human brain. Efforts to isolate the active compounds and determine the underlying molecular mechanism(s) are in process.
References
Akagi M, Matsui N, Akae H, Hirashima N, Fukuishi N, Fukuyama Y, Akagi R (2015) Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as Alzheimer’s disease. J Pharmacol Sci 127:155–163
Bhuiyan MMH, Mohibbullah M, Hannan MA, Hong YK, Choi JS, Choi IS, Moon IS (2015) Undaria pinnatifida promotes spinogenesis and synaptogenesis and potentiates functional presynaptic plasticity in hippocampal neurons. Am J Chin Med 43:529–542
Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8:1454–1468
Goslin K, Asmussen H, Banker G (1998) Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K (eds) Culturing nerve cells, 2nd edn. MIT Press, Massachusetts, pp 339–370
Hannan MA, Kang JY, Hong YK, Lee HS, Chowdhury MTH, Choi JS, Choi IS, Moon IS (2012) A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis. In Vitro Cell Dev Biol Anim 48:535–544
Hannan MA, Kang JY, Hong YK, Lee HS, Choi JS, Choi IS, Moon IS (2013) The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother Res 27:21–29
Hannan MA, Kang JY, Mohibbullah M, Hong YK, Lee HS, Choi JS, Choi IS, Moon IS (2014) Gelidium amansii promotes dendritic spine morphology and synaptogenesis, and modulates NMDA receptor-mediated postsynaptic current. In Vitro Cell Dev Biol Anim 50:445–452
Heese K, Low JW, Inoue N (2006) Nerve growth factor, neural stem cells and Alzheimer’s disease. Neurosignals 15:1–12
Ina A, Hayashi KI, Nozaki H, Kamei Y (2007) Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int J Dev Neurosci 25:63–68
Jung H, Oh S, Choi J (2010) Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg Med Chem Lett 20:3211–3215
Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415
Kamei Y, Sagara A (2002) Neurite outgrowth promoting activity of marine algae from Japan against rat adrenal medulla pheochromocytoma cell line, PC12D. Cytotechnology 40:99–106
Kamei Y, Tsang CK (2003) Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int J Dev Neurosci 21:255–262
Kolanjinathan K, Ganesh P, Saranraj P (2014) Pharmacological importance of seaweeds. World J Fish Mar Sci 6:1–15
Kumar KS, Ganesan K, Rao PVS (2008) Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty—an edible seaweed. Food Chem 107:289–295
Matanjun P, Mohamed S, Muhammad K, Mustapha NM (2010) Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J Med Food 13:792–800
Mohibbullah M, Bhuiyan MMH, Hannan MA, Getachew P, Hong YK, Choi JS, Choi IS, Moon IS (2015a) The edible red alga Porphyra yezoensis promotes neuronal survival and cytoarchitecture in primary hippocampal neurons. Cell Mol Neurobiol. doi:10.1007/s10571-015-0247-x
Mohibbullah M, Hannan MA, Choi JY, Bhuiyan MMH, Hong YK, Choi JS, Choi IS, Moon IS (2015b) The edible marine alga Gracilariopsis chorda alleviates hypoxia/reoxygenation-induced oxidative stress in cultured hippocampal neurons. J Med Food 18:960–971
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85
Pambudi L, Meinita MDN, Ariyati RW (2010) Seaweed cultivation in Indonesia: recent status. Mar Biosci Biotechnol 4:6–10
Prabha V, Prakash DJ, Sudha PN (2013) Analysis of bioactive compounds and antimicrobial activity of marine algae Kappaphycus alvarezii using three solvent extracts. Int J Pharm Sci Res 4:306–310
Ranganayaki P, Susmitha S, Vijayaraghavan R (2014) Study on metabolic compounds of Kappaphycus alvarezii and in vitro analysis of anti-inflammatory activity. Int J Curr Res Acad Rev 2:157–166
Seibenhener ML, Wooten MW (2012) Isolation and culture of hippocampal neurons from prenatal mice. J Vis Exp 65:3634
Smit AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16:245–262
Tang K, Hynan L, Baskin F, Rosenberg R (2006) Platelet amyloid precursor protein processing: a bio-marker for Alzheimer’s disease. J Neurol Sci 240:53–58
Tang X, Chen Y, Gu X, Ding F (2008) Achyranthes bidentata blume extract promotes neuronal growth in cultured embryonic rat hippocampal neurons. Prog Nat Sci 19:549–555
Tsang CK, Ina A, Goto T, Kamei Y (2005) Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 132:633–643
Weissmiller AM, Wu C (2012) Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl Neurodegener 1:14
Xiao Q, Wang C, Li J, Hou Q, Li J, Ma J, Wang W, Wang Z (2010) Ginkgolide B protects hippocampal neurons from apoptosis induced by beta-amyloid 25–35 partly via up-regulation of brain-derived neurotrophic factor. Eur J Pharmacol 647:48–54
Acknowledgments
This work was supported by a research grant from Pukyong National University (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal care and use were in accordance with the institutional guidelines and approved by the Institutional Animal Care and Use Committee of the College of Medicine, Dongguk University, South Korea
Rights and permissions
About this article
Cite this article
Tirtawijaya, G., Mohibbullah, M., Meinita, M.D.N. et al. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. J Appl Phycol 28, 2515–2522 (2016). https://doi.org/10.1007/s10811-016-0795-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0795-6