Abstract
The in vitro digestibility of lipids from Chaetoceros muelleri that were cultured in Guillard medium (F/2) and agricultural fertilizer medium (AF/2) and performance index was examined in enzymatic extracts of Litopenaeus vannamei larvae. The highest enzymatic activity (EA) of lipases was observed in Zoea I (1.53 ± 0.14 EA/10 EU) and Mysis I (1.26 ± 0.01 EA/10 EU (EU are units of enzymatic activity)) when C. muelleri was grown in the F/2 medium. The highest concentration of the unsaturated fatty acids (UFAs) in C. muelleri was observed with F/2 medium (9.96 μg FA/106 cells), and the lowest was obtained with (AF/2) medium (6.68 μg FA/106 cells). The percentage of the total highly unsaturated fatty acids (HUFAs) in Zoea (18.23 %) and Mysis I (F/2 = 19.32 %, AF/2 = 20.25 %) larvae was similar between culture media. The performance indices (PI) of shrimp larvae (Zoea I to Mysis I) differed between culture media, with higher values of PI recorded with the F/2 medium-cultured algae (14.3 ± 1.1) with respect to the values with the AF/2 medium-cultured algae (8.0 ± 1.8). We concluded that due to the effect of the nitrogen source in AF/2 medium, C. muelleri cells have a lower HUFA content and L. vannamei larvae experience decreased lipid digestibility and performance index.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The white shrimp Litopenaeus vannamei is a native species of the western Pacific Coast of Latin America, from Perú to México (Liao and Chien 2011). Penaeid shrimp progress through several larval stages, the first of which is a naupliar stage, when nutrients are obtained from the yolk sac. From the Zoea I to Mysis III stages, they feed on microalgae, and at the Mysis stage, they usually begin to feed on brine shrimp nauplii until metamorphosis (González-Félix et al. 2002).
More data on lipid digestibility in shrimp is needed to improve commercial larval production. To this end, many techniques have been developed, such as in vivo digestibility (apparent digestibility coefficients and markers), digestibility in vitro (Versaw et al. 1989), the o-phthaldialdehyde (OPA) method, and the pH-shift method. For shrimp, a method has been designed to evaluate in vitro digestibility by measuring lipids and lipases (Nolasco et al. 2006).
The biochemical composition of the culture medium can influence microalgal growth and quality. The main factors that alter the biochemical composition in microalgal cultures are temperature, pH, culture age, chemical forms, availability of nutrients, and quantity and spectral composition of light (Sánchez-Saavedra and Voltolina 2006).
Agricultural fertilizers have been used as nutrient sources (nitrates, ammonium, and urea) in substitution of nitrates of F/2 medium (Guillard 1975) for microalgal culture medium with excellent results. Additionally, agricultural fertilizers that are used as nutrient sources can modify the biochemical composition of Chaetoceros muelleri (Pacheco-Vega and Sánchez-Saavedra 2009). When various nitrogen sources are used for microalgae production, it is necessary to determine the biochemical composition of the cells that are supplied as food and evaluate lipid digestibility in vitro. Regarding the lipid content in microalgae used as feed, its effects on growth, metabolism, assimilation, and survival of the marine organisms that cannot synthesize them must be determined (Brown et al. 1989).
The aim of the present study was to examine the performance index and lipid digestibility at various larval stages of L. vannamei that were fed on C. muelleri, in two culture media.
Materials and methods
Diatom cultures
Non-axenic batch cultures of Chaetoceros muelleri (strain Ch m, UABCS culture collection, México) were maintained in F/2 medium (Guillard 1975) used as a control, and the experimental medium, agricultural fertilizer (AF/2), was prepared with liquid agricultural fertilizers. AF/2 (Table 1) was prepared with 72 % phosphoric acid as the phosphorus source and with a liquid fertilizer, with 32 % of the total nitrogen as urea (16.4 %) and ammonium nitrate (15.6 %), which were added to achieve the same P and N concentrations as in F/2 medium. The concentration of the nitrogen and phosphorus in the experimental media was measured spectrophotometrically on a DR/4000 Hach. Nitrates were measured by the cadmium reduction method, nitrite was measured by diazotization method, ammonia was measured by salicylate method, and orthophosphates were measured by acid method (Hach 1997).
Both media were prepared with 1-μm filtered seawater that was enriched with 0.107 mol L−1 sodium silicate (Pacheco-Vega and Sánchez-Saavedra 2009), and a magnetic stirrer was used to mix the solution. The semi-continuous cultures were maintained in triplicate in 9-L polyethylene bags (28 × 66 cm) for each culture medium at 19 °C, with continuous light at 150 μmol photons m−2 s−1 and 20 % daily dilutions to allow periodic harvest of cultured cells. To determine the content of lipids and fatty acids in microalgae, 600 mL of the microalgae culture was harvested and centrifuged at 1076×g for 40 min at 10 °C. The cell pellet was washed with 5 % ammonium formate solution, frozen at −40 °C, and lyophilized.
Extraction and measurement of fatty acids
Triplicate samples from each experimental culture were dried with a Labconco freeze dry system/freezone 4.5. Fatty acids were extracted by direct transesterification (Carrapiso and García 2000) and analyzed (1 μL) on a Hewlett Packard G1800B automatic injection gas chromatograph with an Omegawax 250 capillary column (30 m, 25 mm diameter) and 0.25 μM of liquid phase. High-purity (99 %) helium was used as the carrier gas at 1.2 mL min−1. Fatty acids were identified by comparing retention times with individual fatty acid standards (SIGMA Fatty Acids kit). Each fatty acid was quantified by measuring the proportion of its peak area to the percentage of total fatty acid peak area. The concentration of fatty acids in microalgae was measured using the lipid content per Bligh and Dyer (1959).
Nauplii maintenance
Shrimp nauplii of L. vannamei (Boone 1931) were supplied by AQUALAB, Bahía de La Paz, Baja California Sur, México, and transported to the Pichilingue Aquaculture Laboratory, Baja California Sur State University (UABCS), La Paz, Baja California Sur, México. Seven hundred thousand nauplii were transported in an oxygen-saturated polyethylene bag and acclimated gradually for 2 h at 29.5 ± 0.5 °C. Each experimental unit contained 1500 larvae at the start of the experiment. The buckets contained 15 L of seawater at a daily exchange rate of 20 %. The nauplii were maintained on a 12:12 light/dark cycle that was provided by natural ambient conditions of the laboratory and with an air supply. The developmental stages of the shrimp were analyzed by random sampling of five shrimp larvae per treatment every 2 h on ×100 Olympus II-CHK microscope.
Each treatment of shrimp nauplii that were fed C. muelleri cultivated in the two culture media (AF/2 and F/2) was administered in quadruplicate. At the beginning of the experiment, each experimental container was supplied with 7 × 104 cells mL−1. The cell density was increased daily by adding new cells every day, to reach a concentration of 15 × 104 cell mL−1 on the third day. The cell density of the microalgae was measured every 2 h by cell counting using hemocytometer, and when necessary, algal cells were added to attain the required concentration. The shrimp larvae were weighed in different live stages in each treatment. The survival of shrimp larvae was calculated by differences between the numbers of organisms at the initial time related with the evaluated at the end of the experiment.
Enzymatic extract preparations
Samples at the Zoea I stage (2 × 105 larvae) were homogenized in cold 50 mM Tris–HCl buffer, pH 7.5. Enzyme homogenates from Mysis I were also processed at the end of the experiment. The samples were frozen and kept at −30 °C for 1 day until analysis. Homogenates for lipase activity were prepared in 1.5-mL vials using glass rods to macerate the samples, which were centrifuged at 16,000×g for 15 min at 5 °C. The supernatants were recovered, adjusted to pH 8, aliquoted into 1-mL samples, and frozen at −20 °C until analysis.
In vitro digestibility
The in vitro digestibility of lipids (enzymatic activity) is a new method of correlating data from in vivo digestibility experiments (Nolasco et al. 2006). We used this technique to measure lipase activity in Zoea I and Mysis I larvae using emulsified lipids according to Nolasco et al. (2006), as described in Table 2. Lipids from C. muelleri were extracted according to Bligh and Dyer (1959) and dried with nitrogen gas. Samples were immediately stored at −20 °C for analysis. The emulsion was prepared by homogenizing the mixture of a solution distilled water/gum arabic/lipids (91.3:4.8:3.9). Lipase activity was measured by continuous monitoring pH-STAT (718 Stat Tiotrino, Metrohm) method. To calculate the enzymatic activity (EA), the reaction was run for 4800 s at 25 °C by mixing and NaOH consumption was measured every 300 s. The EA was calculated according to the following equation:
where ∆V = consumed volume of 0.1 N NaOH (mL) to keep the reaction at pH 8.0 and ∆T = slope of the equation for the linear function between NaOH consumed (Y-axis) versus elapsed time (X-axis). Distilled water was used as blank, NaOH = normality of NaOH solution, and Vol. enzyme = volume of enzymatic extract in microliter.
Determination of enzymatic activity
Lipase activity was calculated as 1 μg naphthol released per minute using a coefficient of molar extinction of 0.02 at 540 nm (Versaw et al. 1989) and was related to the soluble protein content in the homogenates. Each unit of lipase represented the amount of enzyme that was required to increase the optical density by 0.01 unit min−1 at 540 nm. The soluble protein concentration in the homogenates was measured spectrophotometrically at 595 nm according to Bradford (1976) method using bovine serum albumin as the standard. For the pH-Sat test 10, enzymatic units were used.
The effect of urea on lipid digestibility was measured according to Versaw et al. (1989). The urea concentrations in the reaction were 6, 12.5, 25, 50, and 100 μmol L−1. Digestive gland homogenates of shrimp larvae were prepared by removing the insoluble material by centrifugation at 70,970×g for 10 min at 4 °C immediately. The digestibility assay was performed by mixing enzymatic extract with lipid substrate. This reaction was incubated at 25 °C for 90 min and stopped with trichloroacetic acid (TCA) and ethanol/ethyl acetate (1:1).
Performance index
Performance index (PI) was calculated using the following equation (Díaz-Iglesias et al. 1991):
where T = time elapsed in days, n = number of survivors, P fin = average weight at the end, and P ini = average initial weight.
For the fatty acid analysis, samples of Zoea I L. vannamei (2 × 105 organisms) were collected and frozen at −40 °C. Fatty acids were extracted as described above.
Statistical analysis
Before the statistical analysis, the data were tested for homoscedasticity and normality. Differences between enzymatic activities were analyzed by a two-way analysis of variance (ANOVA). The effects of urea concentration included in the media and lipid digestibility were analyzed by one-way ANOVA. Differences in performance index were analyzed by one-way ANOVA. The percentages that were obtained from the fatty acid profiles of shrimp larvae were arcsine square root-transformed before analysis and evaluated by one-way ANOVA. When significant differences were noted Tukey a posteriori test was used. Statistical analyses were performed using STATISTIC 7.0 for Windows (Statsoft, USA) and α = 0.05.
Results
The concentration of total soluble protein from L. vannamei enzymatic extracts was higher at stages Zoea I and Mysis I in larval that were fed C. muelleri that was raised in (F/2) versus (FA/2) (Table 3). However, lipase-specific activity was significantly higher (51 %) in larvae that were fed FA/2-cultured algae than in Zoea I to Mysis I larvae that were fed F/2-cultured algae (Table 3).
The amounts of saturated fatty acids (SFAs) and monounsaturated acids (MUFAs) in C. muelleri cultured in F/2 and AF/2 media showed insignificant difference did not differ (P > 0.05). However, the concentrations of HUFAs, such as arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), differed between media (Table 4).
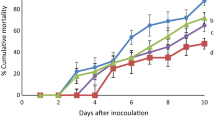
Mean in vitro enzymatic activity (EA) was significantly higher (P < 0.05) in Zoea I (1.53 ± 0.14) and Mysis I (1.26 ± 0.01) larvae when the C. muelleri was grown in F/2 medium compared with the AF/2 medium (Table 5). Mean survival differed significantly for Mysis I larvae between treatments, with higher values in F/2 (52.01 ± 5.69) versus AF/2 (25.27 ± 4.32). The dry weight was higher from Zoea I to Mysis I in F/2 (0.268 ± 0.152 mg) compared with AF/2 (0.152 ± 0.054 mg). Lipid digestibility by shrimp lipase was significantly affected by the culture medium of microalgae. This was confirmed with the result shown in Fig. 1.
The fatty acid composition of the shrimp larvae reflected the composition of the microalgal diets cultured in F/2 and AF/2 media (Table 6). Zoea I shrimp larvae had a higher percentage of saturated fatty acids (SFAs) than Mysis I larvae fed with C. muelleri that was cultured in F/2 and AF/2 media (Table 6). Zoea I larvae had a lower total monounsaturated fatty acid content (ΣMUFA) than Mysis larvae fed with C. muelleri cultured with AF/2 and F/2 media. No significant differences were observed with regard to polyunsaturated fatty acids (ΣPUFAs), highly unsaturated fatty acids (ΣHUFAs), and eicosapentaenoic acid (EPA) (P > 0.05) between samples. Arachidonic acid (ARA) and docosahexaenoic acid (DHA) content differed between treatments (Table 6).
Discussion
The differences in polyunsaturated fatty acids are attributed to the differences in the chemical nitrogen sources in C. muelleri and other species as described by several groups (Fidalgo et al. 1995; Lourenço et al. 2002; Liang et al. 2006). Changes in highly polyunsaturated fatty acids in algal cells depend not only on the nitrogen source and the characteristics of a species inter alia. Our results show that C. muelleri grown with urea, nitrates, and ammonium (AF/2 medium) as nitrogen sources produced lower amounts of polyunsaturated fatty acids.
The differences detected in lipid digestibility by Zoea I and Mysis I L. vannamei were also observed by González et al. (1994) during the ontogenesis of Litopenaeus schmitti, noting increased enzymatic activity from Nauplii to Zoea I, as in L. vannamei. Our results in shrimp larvae demonstrate that the enzymatic activities were constant until Mysis I, following a similar pattern as in L. vannamei in Rivera-Pérez et al. (2010) in L. vannamei. The present results suggest that the digestive gland, which is the principal organ in the synthesis and secretion of enzymes, is maturing and the midgut caeca is degenerating or experiencing a slowdown in enzyme production. Further, the activities of various enzymes are considered hallmarks of the mature hepatopancreas during early development (Lovett and Fólder 1990). The resulting enzymatic activity followed a similar pattern as in Ziaei-Nejad et al. (2006) with an induced strong enzymatic inhibition in early larvae. The variations in digestibility that we observed might be attributed to ontogenetic changes in the digestive system that caused variations in enzymatic activity in L. vannamei larvae. In addition, the differences in enzymatic activity correlated with the changes in lipid composition in C. muelleri; higher concentrations of n–3 HUFA increased the efficiency and absorption of lipids (by approximately five to ten times) (Geurden et al. 1997).
The presence of urea in the experimental medium (AF/2) also affected digestibility. Urea denatures proteins, which affects an increase in the ionic strength of a solution (Madigan et al. 2003). Urea concentrations from 0.5 to 8 M affect lipase activity (Abuin et al. 2005) by the incorporation of urea to the micellar interface producing a decrease of the association of 2-NA with the micelles on lipid digestibility. The concentration of urea in the experimental medium (AF/2) was 35 μM, which altered the lipase activity in shrimp larvae, seen in Fig. 1; the digestibility decreased if any quantity of urea was added.
In the feed of shrimp cultures, the proportion of saturated and unsaturated fatty acids can affect the digestibility of lipids with a diet that contained a higher proportion of SFAs is lower than one with a higher proportion of the HUFAs (Merican and Shim 1995). Glencross et al. (2002) reported high levels of digestibility in Penaeus monodon for all fatty acids with unsaturated bonds. Longer-chain saturated fatty acids were the least digestible; lower digestibility values were obtained as fatty acid chain length increases. The results of this study demonstrate that the amount of lipids in the diet influences their digestibility and that the fatty acid composition of the total lipids in a diet affects their digestibility in total and individually. Thus, differences in the fatty acid composition of C. muelleri were affected by the chemical composition of the culture medium (F/2 or AF/2).
The fatty acid composition at various stages of L. vannamei was altered by the nitrogen source that was used to grow C. muelleri in F/2 and AF/2 media; changes in the SFAs occurred at the Zoea I and Mysis I stages. At the Zoea I stage, the fatty acid composition depends primarily on the contribution of the yolk sac, not on exogenous feeding (Fraser 1989). Nauplii shrimp are physiologically prepared to metabolize endogenous yolk reserves, which are the chief metabolic substrate for energy production, allowing larvae to become independent of external feeding before the opening of the mouth (Rivera-Pérez et al. 2010).
However, low EPA content in shrimp eggs has been associated with a low survival to Zoea III (Palacios et al. 2002). Therefore, the difference in performance index from the Zoea I to the Mysis I might be attributed to high levels of SFAs, MUFAs, ARA, and DHA in the C. muelleri that was added as feed in the treatments. The various fatty acid contents in C. muelleri feed were used for the biosynthesis of new tissue and maintaining cell tissue during the early developmental stages of shrimp larvae. The results of this study showed equal HUFA percentages in Zoea I and Mysis I. An increase in the proportion of HUFAs from Zoea I to Mysis I was observed by D’Souza and Loneragan (1999) and González-Félix et al. (2002) in various crustacean species (L. vannamei, L. japonicus, Penaeus semisulcatus, and P. monodon).
Our results show that regardless of the HUFA content in C. muelleri and Mysis I of L. vannamei, a constant level of HUFAs is maintained. Glencross et al. (2002) concluded that the fatty acid composition in the diet influences the digestibility of lipids and the fatty acid composition in shrimp larvae. Furthermore, the differences in the PI in shrimp larvae show that lipolytic digestion has a direct effect on the development of Zoea L. vannamei. The differences in digestibility reflect the ability of Mysis I L. vannamei to maintain constant HUFA proportion. Moreover, the presence of urea in the culture medium decreases the lipolytic digestibility in L. vannamei shrimp, affecting PI in the AF/2 treatment.
Due the nutritional requirements during the development of L. vannamei larvae, we recommend feeding shrimp larvae with C. muelleri that is grown in F/2 medium to obtain better results with regard to performance index.
References
Abuin E, Lissi E, Solar C (2005) Effect of urea on the enzymatic activity of a lipase entrapped in AOT-heptane-water reverse micellar solutions. J Colloid Interface Sci 283:87–93
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown MR, Jeffrey SW, Garland CD (1989) Nutritional aspects of microalgae used in mariculture, a literature review. In: Marine laboratories report 205. Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australia, p 44
Carrapiso A, García C (2000) Development in lipid analysis: some new extraction techniques and in situ transesterification. Lipids 35:1167–1177
Díaz-Iglesias E, Brito-Pérez R, Báez-Hidalgo M (1991) Cría de postlarvas de langosta Panulirus argus en condiciones de laboratorio. Memorias del Taller Internacional sobre Ecología y Pesquerías de Langosta. Rev Invest Mar Cuba 12:323–331
D’Souza FM, Loneragan NR (1999) Effects of monospecific and mixed-algae diets on survival, development and fatty acid composition of penaeid prawn (Penaeus spp.) larvae. Mar Biol 133:621–633
Fidalgo JP, Gid A, Abalde J, Herrero C (1995) Culture of the marine diatom Phaeodactylum tricornutum with different nitrogen sources: growth, nutrient conversion and biochemical composition. Cah Biol Mar 36:165–173
Fraser AJ (1989) Triacylglycerol content as a condition index for fish, bivalve, and crustacean larvae. Can J Fish Aquat Sci 46:1868–1873
Geurden I, Coutteau P, Sorgeloos P (1997) Effect of a dietary phospholipid supplementation on growth and fatty acid composition of European sea bass (Dicentrarchus labrax L.) and turbot (Scophthalmus maximus L.) juveniles from weaning onwards. Fish Physiol Biochem 16:259–272
Glencross BD, Smith DM, Thomas MR, Williams KC (2002) The effect of dietary n-3 and n-6 fatty acid balance on the growth of the prawn Penaeus monodon. Aquaculture 205:157–169
González R, Fraga V, Carrillo O (1994) Cambios ontogénicos en la actividad de las principales enzimas digestivas de Penaeus schmitti. Rev Invest Mar Cuba 15:262–268
González-Félix ML, Gatlin DM, Lawrence A, Pérez-Velázquez M (2002) Effect of various dietary lipids on quantitative essential fatty acid requirements of juvenile Pacific white shrimp Litopenaeus vannamei. J World Aquacult Soc 33:330–340
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chantey MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 29–60
Hach (1997) Water analysis handbook. Hach Company, Loveland, p 1309
Liang Y, Beardall J, Heraud P (2006) Effects of nitrogen source and UV radiation on the growth, chlorophyll fluorescence and fatty acid composition of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). J Photochem Photobiol 82(B):161–172
Liao IC, Chien Y-H (2011) The Pacific white shrimp, Litopenaeus vannamei, in Asia: the world’s most widely cultured alien crustacean. In: Galin BS, Clark PF, Carlton JT (eds) In the wrong place-alien marine crustaceans: distribution, biology and impacts. Springer, Netherlands, pp 489–519
Lourenço SO, Barbarino E, Mancini-Filho J, Schinke KP, Aidar E (2002) Effects of different nitrogen sources on the growth and biochemical profile of 10 marine microalgae in batch culture: an evaluation for aquaculture. Phycologia 41:158–168
Lovett D, Fólder DL (1990) Ontogenic change in digestive enzyme activity of larval and postlarval white shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol Bull 178:144–159
Madigan M, Martinko J, Parker J (2003) Biología de los microorganismos. In: Macromolecules, Capítulo 3, 10th edn. Editorial Person, Madrid, p 1011
Merican ZO, Shim KF (1995) Apparent digestibility of lipid and fatty acid in residual lipids of meal by adult Penaeus vannamei. Aquaculture 133:275–286
Nolasco H, Del Monte-Martínez A, Hinojosa P, Civera-Cerecedo R, Vega-Villasante F (2006) Digestibilidad in vitro de lípidos alimentarios para el camarón. In: Cruz-Suárez E, Ricque DM, Tapia-Salazar M, Nieto-López M, Villareal-Cavazos DA, Puello Cruz A, García-Ortega A (eds) Avances en nutrición Acuícola, VIII Simposium Internacional de Nutrición Acuícola. Monterrey, Universidad Autónoma de Nuevo León, pp 15–17
Pacheco-Vega JM, Sánchez-Saavedra MP (2009) The biochemical composition of Chaetoceros muelleri Lemmermann grown with an agricultural fertlizer. J World Aquac Soc 40(4):556–560
Palacios E, Racotta LS, Heras H, Marty Y, Moal J, Jean-Francois S (2002) Relation between lipid and fatty acid composition of eggs and larval survival in white pacific shrimp (Penaeus vannamei, Boone, 1931). Aquac Int 9:531–543
Rivera-Pérez C, Navarrete Del Toro MA, García-Carreño FL (2010) Digestive lipase activity through development and after fasting and re-feeding in the white leg shrimp Penaeus vannamei. Aquaculture 300:163–168
Sánchez-Saavedra MP, Voltolina D (2006) The growth rate, biomass production and composition of Chaetoceros sp. grown with different light sources. Aquac Eng 35:161–165
Versaw KW, Cuppet LS, Winters DD, Williams EL (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54:1557–1568
Ziaei-Nejad S, Habibi Rezaei M, Azari Takami G, Lovett DL, Ali-Reza M, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252:516–524
Acknowledgments
This study was founded by the CICESE (623108) and SEP-CONACyT (130074) and to UABCS (AICM-CTM-04-11-25-11-05-24) projects. Thanks to Patricia Hinojosa for her technical support at the Comparative Physiology Laboratory of the Centro de Investigaciones Biológicas del Noroeste (CIBNOR). We would like to thank “AQUALAB” for supplying the shrimp larvae.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacheco-Vega, J.M., Sánchez-Saavedra, M.d.P., Cadena-Roa, M.A. et al. Lipid digestibility and performance index of Litopenaeus vannamei fed with Chaetoceros muelleri cultured in two different enriched media. J Appl Phycol 28, 2379–2385 (2016). https://doi.org/10.1007/s10811-015-0750-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0750-y