Abstract
Thiamine release during synthetic mutualism between Chlorella sorokiniana co-immobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense was measured under stress conditions of pH, light intensity, and nitrogen starvation in short-term experiments. Thiamine release in the co-immobilized treatment was significantly higher at acidic pH compared to thiamine released by either microorganism alone. Under slightly alkaline pH, C. sorokiniana released the highest amount of thiamine. At stressful pH 6, the co-immobilized treatment released a higher quantity of thiamine than the sum of thiamine released by either microorganisms when immobilized separately. Release of thiamine by C. sorokiniana alone or co-immobilized was light intensity dependent; with higher the light intensity, more thiamine was released. Extreme light intensity negatively affected growth of the microalgae and release of thiamine. Nitrogen starvation during the first 24 h of culturing negatively affected release of thiamine by both microorganisms, where C. sorokiniana was more severely affected. Partial or continuous nitrogen starvation had similar negative effects on C. sorokiniana, but co-immobilization improved thiamine release. These results indicate that thiamine is released during synthetic mutualism between C. sorokiniana and A. brasilense, and this happens specifically during the alleviation of pH stress in the microalgae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mutualistic interactions, either naturally occurring or man-made (synthetic mutualism—Imase et al. 2008; Momeni et al. 2011), among different species of either microorganisms or macro-organisms can improve the performance of the partners and are based on the exchange of resources and services (Bronstein 1994; Doebeli and Knowlton 1998; Kessler and Heil 2011). The composition of exudates produced by an organism can indicate the type of microorganisms that are interacting in a specific environment (Kamilova et al. 2006). Exudates from plant are a key determinant of the microbial community structure in the rhizosphere (Barea et al. 2013; Cesco et al. 2012; Nannipieri et al. 2008; Hartmann et al. 2009).
Vitamins are one of the lesser-studied compounds in plants and microalgae exudates, and most studies are decades old (Aaronson et al. 1977; Nishijima et al. 1979; Schönwitz and Ziegler 1994). Green unicellular microalgae of the genus Chlorella produce and exude several vitamins, such as thiamine, riboflavin, biotin, cobalamin, and pantothenic acid (Aaronson et al. 1977; Nishijima et al. 1979; Pratt and Johnson 1965). These microalgae are intensively studied for many biotechnological applications (Lebeau and Robert 2006; de-Bashan and Bashan 2010; Perez-Garcia and Bashan 2015).
Diverse plant growth-promoting bacteria (PGPB) can produce vitamins in artificial culture media, as well. At least 11 vitamins are produced by PGPB in vitro and, so far, seven of these occur in relation to interactions with plants. Growth parameters of in vitro culturing play the major role determining which vitamin is produced and in what quantity (Palacios et al. 2014). Specifically, this study used the PGPB Azospirillum brasilense. The general characteristics of this PGPB is a highly motile bacterium (Bashan and Holguin 1994; Bashan and Levanony 1987), having high affinity for colonizing roots (Bashan et al. 1986; Levanony et al. 1989; Puente et al. 1999), but poor survival in soils without plants (Bashan et al. 1995; Bashan 1999). It affects plant growth, including microalgae, by many small-scale mechanisms working together or in cascade, a theory called “multiple mechanism theory” (Bashan and de-Bashan 2010). This PGPB can produce several vitamins in vitro, including thiamine, riboflavin, biotin, pantothenic acid, and niacin (Dahm et al. 1993; Rodelas et al. 1993). Production mainly depends on the kind of carbon source in the culture media and on environmental factors, such as temperature and pH (Dahm et al. 1993; Rodelas et al. 1993).
Functions and requirements for vitamins in plants and bacteria have been extensively studied (Smith et al. 2007 and references therein). Specifically, thiamine serves as a co-factor in diverse metabolic pathways in plants (Schyns et al. 2005). During plant–microbe interactions, vitamins influence proliferation of PGPB in and around the root system, thereby enhancing their competitiveness (Palacios et al. 2014). Because of the common occurrence of microbial vitamin production by PGPB and assimilation of microbe-synthesized vitamins by plants, vitamins have a role in plant development and rhizosphere interactions (Baya et al. 1981).
The main factors affecting production of vitamins in microalgae and bacteria are environmental conditions, especially temperature, pH, nutrient deficiencies, and light intensity. Some changes from optimal conditions produce stress in microorganism. The pH of the culture medium is one of the most important factors, which affects optimal growth of algal cultures (Khalil et al. 2010). Intense light has a strong negative effect on pigment concentration (Kana et al. 1997). Limited available nitrogen or nitrogen starvation affects microalgae in different ways, particularly carbohydrate and lipid metabolism in general and specifically in Chlorella spp. (Choix et al. 2012a, b; Khozin-Goldberg and Cohen 2011; Přibyl et al. 2012; Widjaja et al. 2009).
Many PGPB and especially Azospirillum spp. can mitigate diverse stressors, including salt, osmotic, and oxidative stress in plants (Bacilio et al. 2004; Bashan and de-Bashan 2010; Cassán et al. 2009; Rodrigues et al. 2013; Rojas-Tapias et al. 2012). In the microalgae Chlorella sorokiniana UTEX 2714 (formerly C. vulgaris), when co-immobilized with the PGPB A. brasilense, this can ameliorate pH and tryptophan stress (de-Bashan et al. 2005), promote cellular and population growth (Gonzalez and Bashan 2000), and enhance production of carbohydrates, lipids, fatty acids, and photosynthetic pigments (Choix et al. 2012a, b; de-Bashan et al. 2002; Leyva et al. 2014). Consequently, Chlorella spp. and A. brasilense were proposed as a model to study plant–bacteria interactions (de-Bashan and Bashan 2008). This created a synthetic mutualism where numerous positive effects were recorded for both partners. So far, the microalgae have received more attention (de-Bashan et al. 2015).
Thiamine is required by all cells because of its role as a co-factor in the formation of thiamine pyrophosphate for enzymes essential to carbon and amino acid metabolism (Jurgenson et al. 2009). The specific role of thiamine during plant–bacteria interaction, as a co-factor in different metabolic pathways, activates defense reactions in plants and promotes plant growth (for review see Palacios et al. 2014). To start to explain the possible roles of thiamine during mutuality interaction of the microorganisms, it is essential to explore the conditions that are conducive for this vitamin under mutual culturing. To this end, we studied the release of thiamine during artificial mutualism between C. sorokiniana and A. brasilense. We specifically tested the hypothesis that cultivating A. brasilense with C. sorokiniana in very close proximity in an alginate bead affects the release of thiamine by the bacteria–microalgae association under stressors of low and high light intensities, low and high pH, and presence or absence of a nitrogen source, compared to ideal culturing. Microorganisms immobilized alone served as controls.
Materials and methods
Microorganisms and initial growth conditions prior experiments
The unicellular microalgae Chlorella sorokiniana (UTEX 2714, University of Texas, Austin, TX) (formerly Chlorella vulgaris Beijerink; Bashan et al. 2015) and the bacterium Azospirillum brasilense Cd (DSM 1843; Leibniz-Institut DMSZ, Braunschweig, Germany) were used. To produce cultures of the microalgae, 10 mL of axenic culture of microalgae was inoculated into 90 mL sterile mineral medium (C30), as described in Gonzalez et al. (1997). The medium contains (in g L−1) KNO3 (25), MgSO4·7H2O (10), KH2PO4 (4), K2HPO4 (1), and FeSO4·7H2O (1) and contains (in μg L−1) H3BO3 (2.86), MnCl2·4H2O (1.81), ZnSO4·7H2O (0.11), CuSO4·5H2O (0.09), and NaMoO4 (0.021). The inoculated medium was incubated at 27 ± 2 °C at 140 rpm under light intensity of 60 μmol photons m−2 s−1 for 7 days, then harvested by centrifugation at 6000×g for 5 min. To eliminate traces of growth medium, the pellets were rinsed three times in saline solution (0.85 % NaCl w/v). The bacterium was cultivated in BTB-2 medium (Bashan et al. 2011), which contains (in g L−1) NaCl (1.2), MgSO4·7H2O (0.25), K2HPO4 (0.13), CaCl2 (0.22), K2SO4 (0.17), NH4Cl (1), Na2SO4 (2.4), NaHCO3 (0.5), Na2CO3 (0.09), Fe-EDTA (0.07), tryptone (5), and yeast extract (5), with 8 mL glycerol. The pH was adjusted to 7 with 1 M KOH, incubated at 32 ± 2 °C at 120 rpm for 16 h, and then harvested by centrifugation at 6000×g for 5 min. Elimination of traces of growth medium was done as described earlier.
Immobilization of microorganisms
The microorganisms were immobilized, using the method described by de-Bashan et al. (2004), where 40 mL C. sorokiniana culture (6.0 × 106 cells mL−1) were mixed with 160 mL sterile, 6000 cP 2 % alginate solution (alginate mixed at 14,000 and 3500 cP), and stirred for 15 min. Using an automatic bead maker, this mixture was dropped into a 2 % CaCl2 solution under slow stirring (de-Bashan and Bashan 2010). The beads were stabilized for 1 h at 28 ± 1 °C and washed in sterile saline solution (0.85 % NaCl). Azospirillum brasilense (approximately 1.0 × 109 CFU mL−1) was immobilized similarly. Immobilization normally reduces the number of A. brasilense but not C. sorokiniana in the beads; therefore, a second incubation step for recovering the lost population of the bacteria (secondary growth) was necessary (nutrient broth, 10 %, w/v, #N7519 Fluka-Sigma-Aldrich) (Bashan 1986). Under starvation conditions for secondary multiplication, we used N-free OAB medium containing (in g L−1) KOH (4.80), malic acid (5), NaCl (1.20), MgSO4·7H2O (0.25), K2HPO4 (0.13), CaCl2 (0.22), K2SO4 (0.17), Na2SO4 (2.40), NaHCO3 (0.50), Na2CO3 (0.09), and FeIIIEDTA (0.07), and (in μg L−1) H3BO3 (0.2), MnCl2·4H2O (0.2), ZnCl2 (0.15), CuCl2·2H2O (0.2), and NaMoO4·2H2O (20) (Bashan and de-Bashan 2015). Light intensity, temperature, and pH in the secondary multiplication of the bacterium were similar to ideal conditions of growth under experiments described earlier. For co-immobilizing the two microorganisms in the same bead, after washing the cultures, each culture was re-suspended three times in 10 mL sterile saline solution (0.85 % NaCl v/v) and then mixed in the alginate before forming the beads. It is well established that immobilization of these microorganisms in alginate beads prevents their release from the beads (Covarrubias et al. 2012). Under close confinement, they are forced to interact with each other (de-Bashan and Bashan 2008; de-Bashan et al. 2015).
Experimental culture conditions
After secondary incubation, the beads were washed three times in sterile saline solution to remove remnants of the medium. For each experiment, 20 g of beads with microorganisms, either immobilized alone or co-immobilized, was inoculated in 150 mL synthetic growth medium (SGM), described in de-Bashan et al. (2011), containing (in mg L−1) NaCl (7), CaCl2 (4), MgSO4·7H2O (2), K2HPO4 (217), KH2PO4 (8.5), Na2HPO4 (33.4), and NH4Cl (191). The inoculated medium was incubated under autotrophic conditions under different light intensities, pH, and nitrogen conditions: (1) four different light intensities (30, 60, 300, and 500 μmol photons m−2 s−1) at pH 7; (2) pH 6 (adjusted with 1 N HCl) and pH 7 and pH 8 (adjusted with 0.3 N NaOH) at 60 μmol photons m−2 s−1. The N starvation experiments were conducted in synthetic N-free-medium at pH 7, 60 μmol photons m−2 s−1. (3) Two regimens of nitrogen starvation were tested. The first was done during secondary growth (24 h after immobilizing the microorganisms) and a second regimen during the entire experiment. The diazotroph A. brasilense does not fix atmospheric nitrogen during highly oxygenated culturing conditions with C. sorokiniana, maintaining the N-free medium. All experiments were stirred at 140 rpm at 27 ± 2 °C for 144 h.
Cell counting of microorganisms
For each treatment, three beads from a 250-mL flask were sampled. Each bead was solubilized by immersion for ~30 min (~28 °C) in 1 mL of citrate buffer containing (in mM) sodium citrate (55), EDTA anhydride (30), and NaCl2 (150), in a final volume of 1 L and adjusted to pH 8 with NaOH. Azospirillum brasilense cells were counted by the fluorescein diacetate method described by Chrzanowski et al. (1984) under a fluorescent microscope (BX41, Olympus, Japan). Chlorella sorokiniana was counted under a light microscope using a Neubauer hemocytometer connected to an image analyzer (Image ProPlus 4.5; Media Cybernetics, USA) (Gonzalez and Bashan 2000).
High-performance liquid chromatography (HPLC) analysis
HPLC (1100, Agilent Technologies, USA) was used for all analyses. Chromatograms were analyzed and recorded using HPCHEM integrating software (version G2170BA; Agilent Technologies). A modification of a HPLC method by Sánchez-Machado et al. (2004) was used. The HPLC system was equipped with a reversed phase column (LiChrosorb-RP 18, 10 μm particle size, 250 × 4 mm; Merck, Germany) and was run isocratically (all analyses use the same concentration of solvents) using 0.1 M phosphate buffer (pH 7)/acetonitrile (72:28 v/v) serving as the mobile phase. The injection volume was 30 μL and the flow rate was 0.65 mL min−1. Thiamine and its derivatives, thiamine monophosphate and thiamine diphosphate, were measured indirectly by fluorescence detection after post-oxidation to thiochrome, thiochrome monophosphate, and thiochrome diphosphate, respectively. The post-oxidation procedure was 500 μL 1 % potassium ferricyanide (dissolved in 15 % aqueous NaOH) added to 1 mL standard solution or sample; the solution was shaken for 10 s and then mixed with 100 μL concentrated H3PO4. The settings of the fluorescence detector were 370 nm for excitation and 435 nm for emission. For vitamin B1 and its two derivatives, the standards were thiamine hydrochloride (T-4625; Sigma), thiamine monophosphate chloride dehydrate (T-8637; Sigma), and thiamine diphosphate (C-8754; Sigma).
Determination of thiamine in exudates
One gram of beads (approximately 25 beads per replicate and per treatment) was dissolved in 15 mL citrate buffer as described above. Once dissolved, sample was centrifuged at 6000×g for 5 min. The pellet was discarded and the supernatant was added to 10 mL of the culture medium to obtain at the end a mixed sample (25 mL) of exudates representing both inside and outside beads. The solutions were centrifuged at 6000×g for 10 min at room temperature, filtered through a 0.22-μm membrane filter (GSWP02500; EMD Millipore), and analyzed by HPLC. Net release of thiamine was measured by subtracting the thiamine released by each microorganism immobilized alone from the amount of thiamine released by the co-immobilized treatment.
Experimental designs and data analysis
The setup of all experiments was in batch cultures. Each experiment was performed in triplicate, where each 250-mL Erlenmeyer flask served as a replicate. Each setup contained three treatments: beads containing C. sorokiniana, beads containing A. brasilense (these two serving as controls), and beads containing the two co-immobilized microorganisms (n = 9). Each experiment was repeated twice. The quantity of thiamine made of the sum of thiamine monophosphate and thiamine diphosphate are expressed as total thiamine per milliliter of the solution. The linearity of the calibration curves was tested by a correlation analysis, followed by a regression analysis. Data of counted cells and vitamin production were analyzed first by one-way ANOVA and then by LSD (least statistical difference) post hoc analysis, with significance set at P <0.05, using statistical software (Statistica 6.0; StatSoft, USA). The comparison between thiamine production by co-immobilized treatment versus thiamine production by C. sorokiniana and A. brasilense (immobilized separately) were analyzed by orthogonal contrast analysis with significance set at P <0.05, using analytical software (SAS 9.00; SAS Institute, USA).
Results
Effect of pH of the growth medium on thiamine release
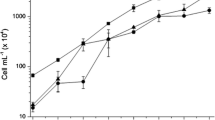
Thiamine released during co-immobilization of C. sorokiniana with A. brasilense was significantly higher at pH 6 after 96 h compared to thiamine released by each microorganism immobilized alone (Fig. 1a). The amount of thiamine released by co-immobilized microorganisms (116 ± 45.3 ng mL−1) was higher than the sum of the thiamine concentration released by either microorganism immobilized alone (28.5 ± 8.2 ng mL−1 for C. sorokiniana; 37.3 ± 4 ng mL−1 for A. brasilense; P < 0.0045). A similar result was obtained at pH 7, where exudation of thiamine in the co-immobilized treatment (64.5 ± 4.02 ng mL−1 after 96 h) was also higher than the amount released by either of the microorganisms immobilized alone (Fig. 1b). At pH 8, C. sorokiniana released the highest amount of thiamine after 96 h (241.7 ± 144.7 ng mL−1) (Fig. 1c). At other sampling times, the co-immobilized treatment released amounts of thiamine that were similar to the sum of thiamine released by each microorganism immobilized alone. These quantities were lower than the quantities released at pH 6. Analyzing the same data and comparing the effects of different pH treatments revealed that pH had no significant effect on release of thiamine by either microorganism (except at pH 8 for C. sorokiniana and pH 6 and 7 for co-immobilized microorganisms after 96 h) (Fig. S1a–c). At pH 6 and 7, a gain in thiamine was detected, with a net release of thiamine by the co-immobilized microorganisms of 50.19 ± 20.97 ng mL−1 at pH 6 and 9.2856 ± 1.0125 ng mL−1 at pH 7. At pH 8, the yield of thiamine in the co-immobilized treatment was similar to the sum of the thiamine released by C. sorokiniana and A. brasilense immobilized alone, indicating no gain of thiamine (Fig. 1c).
Thiamine in exudates at pH 6 (a), 7 (b), and 8 (c) produced and released by Chlorella sorokiniana and Azospirillum brasilense immobilized separately or immobilized together in alginate beads and growth of these microorganisms in these cultures (d, e, f, g, h, i). Values along curves denoted by different capital letters differ significantly, using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE
Regardless of the pH, C. sorokiniana had larger populations when co-immobilized with A. brasilense (Fig. 1d–f, Fig. S1d, f). When immobilized alone, cultures of C. sorokiniana grown at pH 6 had significantly smaller populations (Fig. 1d). However, when co-immobilized, the population recovered and no significant difference was detected between populations growing at different pH levels (Fig. S1f); this indicates alleviation of stress. At all pH levels of the cultures of A. brasilense, populations were always smaller in the co-immobilized treatment (Fig. 1g–i, Fig. S1e).
Effect of light intensity on thiamine release
Under all light conditions, both microorganisms released thiamine into the growth medium (Fig. 2), and this release was light intensity dependent in the range of 30–300 μmol photons m−2 s−1. As light intensity increased, thiamine release increased (Fig. 2a–c, Fig. S2a), reaching the highest level at 300 μmol photons m−2 s−1. At 500 μmol photons m−2 s−1, release of thiamine dropped to the lowest level of all treatments (Fig. 2d, Fig. S2a–c).
Comparison of thiamine content in exudates under light intensities of 30, 60, 300, and 500 μmol photon m−2 s−1 released by Chlorella sorokiniana and Azospirillum brasilense immobilized separately or immobilized together in alginate beads (a, b, c, d) and growth of Chlorella sorokiniana immobilized alone and co-immobilized with Azospirillum brasilense in these cultures (e, f, g, h). Values along curves denoted by different capital letters differ significantly using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE
At 30 μmol photons m−2 s−1, there is no difference in the amount of thiamine released by either treatment. At 60 and 500 μmol photons m−2 s−1 at 96 h, more thiamine was released in the co-immobilized treatments; however, only at 60 μmol photons m−2 s−1 was more thiamine released by the co-immobilized than the sum of thiamine released by each microorganism immobilized alone (Fig. 2b). At this time duration and light intensity, the net release of thiamine in the co-immobilized treatment was 6.22 ± 3.01 ng mL−1. At 300 μmol photons m−2 s−1, the highest release of thiamine occurred in C. sorokiniana alone and co-immobilized; only after 144 h is there a difference between the two (Fig. 2c, Fig. S2a, c). At this time duration and light intensity, net release of thiamine in the co-immobilized treatment was 6.22 ± 3.01 ng mL−1.
Except under 30 μmol photons m−2 s−1, A. brasilense enhanced growth of C. sorokiniana in all cases (Fig. 2e–h). Although there was no growth of C. sorokiniana at 500 μmol photons m−2 s−1, in co-immobilization with A. brasilense after incubation for 144 h was there a slight, but significant, enhanced growth (Fig. 2h), indicating a reduction of stress after an adaptation period. Growth of A. brasilense was similar at all the tested light intensities (Fig. S2e).
Effect of nitrogen starvation on release of thiamine
Release of thiamine by C. sorokiniana, when immobilized alone, ceased after 48 h of incubation (8.81 ± 3.23 ng mL−1). Even when nitrogen was restored after 24 h of incubation, release of thiamine by C. sorokiniana did not recover (Fig. 3a, b). Nitrogen starvation during the first 24 h of the secondary growth phase negatively affected thiamine release by the microalga and the bacterium, but C. sorokiniana was more affected (Fig. 3b).
Comparison of thiamine content in exudates released by Chlorella sorokiniana and Azospirillum brasilense immobilized separately or immobilized together in alginate beads under nitrogen starvation for the full experiment or partial nitrogen starvation (a, b) and growth of C. sorokiniana (c, d) and A. brasilense (e, f) under these conditions. Values along curves denoted by different capital letters differ significantly using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE
Release of thiamine under the co-immobilized treatment and in A. brasilense immobilized alone was similar under both conditions, whether the nitrogen was restored or not (Fig. 3a, b). The quantities of released thiamine in these treatments were at all sampling times higher than when C. sorokiniana was cultured alone (Fig. 3a, b). The sole difference was that during the co-immobilized treatment, release of thiamine slowly continued over the entire 144 h of the experiment (Fig. 3a, b). There was no significant difference in the growth of C. sorokiniana, whether immobilized alone or with A. brasilense in both nitrogen-absent regimes (Fig. 3c, d). However, under partial nitrogen starvation, C. sorokiniana, when immobilized alone or co-immobilized with A. brasilense and A. brasilense immobilized alone, growth was greater than under full starvation regimes (Fig. 3c–f).
Discussion
Synthetic mutualism between C. sorokiniana and A. brasilense is man-made and so far not found in nature. As is, it does not have ecological importance. This created mutualism has two purposes: (1) to study the metabolism of plant–bacterial interaction because it highly resembles, in several metabolic pathways, the interaction between PGPB and higher plants (de-Bashan and Bashan 2008; de-Bashan et al. 2015); and (2) to explore potential usefulness of this mutualism for biotechnological gains (see “Introduction” for references). Specifically, this study of thiamine extrusion, known to participate in PGPB–plant interactions (Palacios et al. 2014), and as a co-factor in carbon and amino acid metabolism in all organisms (Jurgenson et al. 2009), is aimed to describe new functional interactions and to increase our understanding of eukaryote–prokaryote cell interactions. At the same time, this research aims to provide a tool for biotechnological development. These ideas are supported by two examples of thiamine contribution to auxotrophic–thiamine marine microalgae: (1) co-culturing of the phytoplankton microalgae Ostreococcus lucimarinus with a Pseudoalteromonas sp., strain TW7. This strain, which synthesizes thiamine, showed the bacterial influence on thiamine availability, allowing the microalgae to grow. This bacterium can increase thiamine availability beyond de novo synthesis (Paerl et al. 2015). (2) The marine bacterium Dinoroseobacter shibae, a symbiont of cosmopolitan marine microalgae, can provide thiamine to auxotroph–thiamine microalgae (Wagner-Döbler et al. 2010). However, the novel contribution of this study is to show changes in available thiamine between two interacting microorganisms that can produce and consume thiamine and both are benefited from the interaction.
During synthetic mutualism, the PGPB A. brasilense enhanced production of diverse compounds, such as carbohydrates, lipids, and photosynthetic pigments in C. sorokiniana during their interaction within alginate beads (Choix et al. 2012a, b; Gonzalez and Bashan 2000; de-Bashan et al. 2002; Leyva et al. 2014). This study specifically extends the effects when both microorganisms interact under optimal or stressful growth conditions by releasing thiamine. This study demonstrated that, during synthetic mutualism, C. sorokiniana, co-immobilized with A. brasilense, enhances the release of thiamine into the growth medium under stress conditions of pH.
By measuring the relative effect of each stressor, only at pH 6 (stressful condition for growth of microalgae) more thiamine was released in the co-immobilized treatment than the sum of thiamine released by C. sorokiniana and A. brasilense when immobilized separately. This may happen because production of thiamine by microalgae is related to cell multiplication (Nishijima et al. 1979) and A. brasilense alleviates the effect of pH stress in C. sorokiniana (de-Bashan et al. 2005), leading to recovering and enhancement of growth in the microalga. Under non-stressed growth conditions at pH 7 and light intensity of 60 μmol photon m−2 s−1 (serving as controls in our study), more thiamine was released under the co-immobilized treatment. Under these optimal growth conditions, several metabolic effects have been reported for the microalgae C. sorokiniana, when co-immobilized with the bacteria A. brasilense (Gonzalez and Bashan 2000; Choix et al. 2012a, b, 2014; de-Bashan et al. 2002; Leyva et al. 2014, 2015). The well-established role of thiamine as a cofactor in several metabolic pathways (Schyns et al. 2005) can explain the enhancement of thiamine released to the media during optimal synthetic mutualism of these microorganisms, allowing more available thiamine for both species.
Release of thiamine and cell growth of C. sorokiniana was light dependent when immobilized alone or co-immobilized with A. brasilense. Lv et al. (2010) report that cell growth of C. sorokiniana increases with the increase of light intensity. Thiamine diphosphate acts as a co-factor of an important number of enzymes in carbohydrate metabolism (Schyns et al. 2005). Therefore, we theorized that the increase in carbon metabolism influenced by light intensity could induce greater production of thiamine (which also is able to be released to the medium) by C. sorokiniana that, in turn, facilitates carbon assimilation. Yet, light may have limits. We found that under a high light intensity, release of thiamine and population growth of C. sorokiniana, when immobilized alone, were negatively affected. It is plausible that the effect of the highest light intensity on growth of C. sorokiniana is due to a photo-inhibition, a light-induced depression of photosynthesis caused by excessive light (Smith and Underwood 2000). Similar effects have been reported for other microalgae (Kana et al. 1997; Pal et al. 2011). When C. sorokiniana was co-immobilized with A. brasilense, growth recovered after 144 h, indicating alleviation of stress by A. brasilense. Under intense irradiation, the photosynthetic apparatus does not use light energy properly; the excess energy leads to the formation of highly active oxygen molecules (Bar et al. 1995). The interaction between C. sorokiniana and A. brasilense enhances production of photosynthetic pigments in C. sorokiniana, which may improve photosynthesis (de-Bashan et al. 2002). An increase in auxiliary photo-protective photosynthetic pigments in wheat seedlings by A. brasilense was reported in wheat plants (Bashan et al. 2006), but not in microalgae. The decrease of concentration of thiamine in exudates may be a consequence of two metabolic processes. Since vitamin excretion is related to cell multiplication (Nishijima et al. 1979), at very high light intensity (for C. sorokiniana), the light inhibits growth of C. sorokiniana cells as it inhibits production and release of thiamine. Also, thiamine is sensitive to strong light and is degraded (Dănet and Calatayud 1994; Hagen et al. 1991).
Under nitrogen starvation, release of thiamine and growth of C. sorokiniana cells were negatively affected. This happened because nitrogen starvation mainly affects photosynthetic efficiency, causing inactivation of the photosystem II in unicellular microalgae (Berges et al. 1996). In our study, the negative effect of nitrogen starvation in the first 24 h was enough to completely stop the growth of C. sorokiniana. Even when nitrogen was added 24 h later, the microalgae did not recover. Floreto et al. (1996) reported that the green macroalgae Olva pertusa, when starved of nitrogen, was negatively affected, mainly reducing biomass and growth rate. This major stress event probably inhibited the release of thiamine.
Under nitrogen starvation, we did not find thiamine when C. sorokiniana was immobilized alone. This occurs because the thiazole ring of thiamine is produced from the amino acid glycine, from which one atom of carbon and one atom of nitrogen are taken (Nosaka 2006). Under nitrogen starvation in the diatom Thalassiosira pseudonana, N-containing osmolytes such as proline, homarine, and glycine betaine are replaced by accumulation of dimethylsulfonium propionate, which does not contain nitrogen (Bucciarelli and Sunda 2003). Therefore, we assume that the low release of thiamine during nitrogen starvation in our study results from the absence of precursors of thiamine, such as glycine. Although Bertrand and Allen (2012) propose that thiamine may enhance the ability of effectively responding to and recovering from nitrogen starvation in phytoplankton, this did not happen in our study. Even when thiamine was present in the exudates of microorganisms in the co-immobilized treatment, growth of C. sorokiniana did not recover. Taken together, this shows that nitrogen availability is a primary factor controlling cellular responses in C. sorokiniana, surpassing thiamine production and release.
In our study, A. brasilense released thiamine under all growth conditions that we tested. The production of B vitamins by this bacterium depends on a carbon source (Rodelas et al. 1993) and pH (Dahm et al. 1993). In our study, A. brasilense had the ability to release thiamine in a mineral medium without any external carbon source; however, the release of thiamine was enhanced under acidic pH, but only during the first 48 h. This can be explained since the bacterium can use stored energy compounds such as poly-β-hydroxybutyrate to regulate their metabolism, and this favors establishment and survival in competitive environments under suboptimal conditions (Kadouri et al. 2003). We propose that, because thiamine is an important co-factor in diverse metabolic pathways, the stored carbon ensures production of thiamine when a carbon source is limited.
The exact function of thiamine in this synthetic mutualism is still an open question, currently under investigation. On one hand, both microorganisms are self-producers of vitamin B1 and can sustain life without external thiamine. Yet, the interaction released thiamine. There are at least two plausible explanations that thiamine has a yet-to-be-discovered function in other metabolic activities that promote microalgal growth and metabolism: (1) enhancing production of indole-3-acetic acid (IAA; Meza et al. 2015a, b), in which thiamine is a known co-factor for IAA production. (2) Thiamine may participate in enhanced carbohydrate metabolism in microalgae. Thiamine is an important co-factor in carbohydrate metabolism in all organisms. Recently, Choix et al. (2012a, b, 2014) show that during this synthetic mutualism, total content of carbohydrates and especially starch and its specific metabolism are significantly enhanced. So far, it is impossible to separate the metabolic effect of each of the partners in this synthetic mutualism experiment. Therefore, our experiments can only show that applying pH stress conditions to the microalgae enhanced release of thiamine. However, we cannot show the direct effect of stressors on thiamine biosynthesis of each microorganism. An effort is currently underway to develop a method to separate the effect of each partner (J.P. Hernandez, personal communication).
One of the main findings of this study is that during the interaction of the two microorganisms, thiamine is always available for both microorganisms, as evident by the release of this vitamin in the exudates. Also, this indirectly indicates a constant production of thiamine. When alleviation of stress in C. sorokiniana by A. brasilense occurs, this indicates that release of thiamine has recovered. This suggests a yet-to-explore link between stress mitigation in C. sorokiniana by A. brasilense and thiamine.
Conclusions
These results show that
-
1.
Thiamine is released during synthetic mutualism between C. sorokiniana and A. brasilense.
-
2.
Larger amounts of thiamine are released specifically during alleviation of a pH stress in the microalgae.
References
Aaronson S, Dhawale SW, Patni NJ (1977) The cell content and secretion of water-soluble vitamins by several freshwater algae. Arch Microbiol 112:57–59
Bacilio M, Rodriguez H, Moreno M, Hernandez J-P, Bashan Y (2004) Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol Fert Soils 40:188–193
Bar E, Rise M, Vishkautsan M, Arad SM (1995) Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J Plant Physiol 146:527–534
Barea J-M, Pozo M-J, Azcón R, Azcón-Aguilar C (2013) Microbial interactions in the rhizosphere. In: de Brujin FJ (ed) Molecular microbial ecology of the rhizosphere. John Wiley & Sons, New York, pp 29–44
Bashan Y (1986) Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl Environ Microbiol 51:1089–1098
Bashan Y (1999) Interactions of Azospirillum spp. in soils: a review. Biol Fert Soils 29:246–256
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 108:77–136
Bashan Y, de-Bashan LE (2015) Inoculants for Azospirillum. In: Cassán F, Okon Y, Creus C (eds) Handbook for Azospirillum. Springer, Berlin, pp 469–485
Bashan Y, Holguin G (1994) Root-to-root travel of the beneficial bacterium Azospirillum brasilense. Appl Environ Microbiol 60:2120–2131
Bashan Y, Levanony H (1987) Horizontal and vertical movement of Azospirillum brasilense Cd in the soil and along the rhizosphere of wheat and weeds in controlled and field environments. J Gen Microbiol 133:3473–3480
Bashan Y, Levanony H, Klein E (1986) Evidence for a weak active external adsorption of Azospirillum brasilense Cd to wheat roots. J Gen Microbiol 132:3069–3073
Bashan Y, Puente ME, Rodriguez-Mendoza MN, Toledo G, Holguin G, Ferrera-Cerrato R, Pedrin S (1995) Survival of Azospirillum brasilense in the bulk soil and rhizosphere of 23 soil types. Appl Environ Microbiol 61:1938–1945
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577
Bashan Y, Bustillos JJ, Leyva LA, Hernandez J-P, Bacilio M (2006) Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol Fert Soils 42:279–285
Bashan Y, Trejo A, de-Bashan LE (2011) Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol Fert Soils 47:963–969
Bashan Y, Lopez BR, Huss VAR, Amavizca E, de-Bashan LE (2015) Chlorella sorokiniana (formerly C. vulgaris) UTEX 2714, a non-thermotolerant microalga useful for biotechnological applications and as a reference strain. J Appl Phycol. doi:10.1007/s10811-015-0571-z
Baya AM, Boethling RS, Ramos-Cormenzana A (1981) Vitamin production in relation to phosphate solubilization by soil bacteria. Soil Biol Biochem 13:527–531
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. J Plant Physiol 110:689–696
Bertrand EM, Allen AE (2012) Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Front Microbiol 3:1–16. doi:10.3389/fmicb.2012.00375
Bronstein JL (1994) Our current understanding of mutualism. Q Rev Biol 69:31–51
Bucciarelli E, Sunda WG (2003) Influence of CO2, nitrate, phosphate, and silicate limitation on intracellular dimethylsulfoniopropionate in batch cultures of the coastal diatom Thalassiosira pseudonana. Limnol Oceanogr 48:2256–2265
Cassán F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L, Renella G, Landi L, Nannipieri P (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol Fert Soils 48:123–149
Choix FJ, de-Bashan LE, Bashan Y (2012a) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzyme Microb Technol 51:294–299
Choix FJ, de-Bashan LE, Bashan Y (2012b) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense. II. Heterotrophic conditions. Enzyme Microb Technol 51:300–309
Choix FJ, Bashan Y, Mendoza A, de-Bashan LE (2014) Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J Biotechnol 177:22–34
Chrzanowski TH, Crotty RD, Hubbard JG, Welch RP (1984) Applicability of fluorescein diacetate method of detecting active bacteria in freshwater. Microb Ecol 10:179–185
Covarrubias SA, de-Bashan LE, Moreno M, Bashan Y (2012) Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl Microbiol Biotechnol 93:2669–2680
Dahm H, Rózycki H, Strzelczyk E, Li CY (1993) Production of B-group vitamins by Azospirillum spp. grown in media of different pH at different temperatures. Zbl Mikrobiol 148:195–203
Dănet AF, Calatayud JM (1994) FIA-spectrophotometric determination of thiamine after UV-irradiation. Talanta 41:2147–2151
de-Bashan LE, Bashan Y (2008) Joint immobilization of plant growth-promoting bacteria and green microalgae in alginate beads as an experimental model for studying plant-bacterium interactions. Appl Environ Microbiol 74:6797–6802
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627
de-Bashan LE, Bashan Y, Moreno M, Lebsky VK, Bustillos JJ (2002) Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Can J Microbiol 48:514–521
de-Bashan LE, Antoun H, Bashan Y (2005) Cultivation factors and population size control uptake of nitrogen by the microalgae Chlorella vulgaris when interacting with the microalgae growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol Ecol 54:197–203
de-Bashan LE, Schmid M, Rothballer M, Hartmann A, Bashan Y (2011) Cell-cell interaction in the eukaryote-prokaryote model using the microalgae Chlorella vulgaris and the bacterium Azospirillum brasilense immobilized in polymer beads. J Phycol 47:1350–1359
de-Bashan LE, Hernandez J-P, Bashan Y (2015) Interaction of Azospirillum spp. with microalgae; a basic eukaryotic–prokaryotic model and its biotechnological applications. In: Cassán F, Okon Y, Creus C (eds) Handbook for Azospirillum. Springer, Berlin, pp 367–388
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. Proc Natl Acad Sci U S A 95:8676–8680
Floreto EAT, Teshima S, Ishikawa M (1996) Effects of nitrogen and phosphorus on the growth and fatty acid composition of Ulva pertusa Kjellman (Chlorophyta). Bot Mar 39:69–74
Gonzalez LE, Bashan Y (2000) Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl Environ Microbiol 66:1527–1531
Gonzalez LE, Cañizares RO, Baena S (1997) Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour Technol 60:259–262
Hagen SR, Muneta P, Augustin J, LeTourneau D (1991) Stability and utilization of picloram, vitamins, and sucrose in a tissue culture medium. Plant Cell Tiss Org 25:45–48
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Imase M, Watanabe K, Aoyagi H, Tanaka H (2008) Construction of an artificial symbiotic community using a Chlorella-symbiont association as a model. FEMS Microbiol Ecol 63:273–282
Jurgenson CT, Begley TP, Ealick E (2009) The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78:569–603
Kadouri D, Jurkevitch E, Okon Y (2003) Involvement of the reserve material poly-β-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl Environ Microbiol 69:3244–3250
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant-Microbe Interact 19:250–256
Kana TM, Geider RJ, Critchley C (1997) Regulation of photosynthetic pigments in micro-algae by multiple environmental factors: a dynamic balance hypothesis. New Phytol 137:629–638
Kessler A, Heil M (2011) The multiple faces of indirect defenses and their agents of natural selection. Funct Ecol 25:348–357
Khalil ZI, Asker MMS, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotechnol 26:1225–1231
Khozin-Goldberg I, Cohen Z (2011) Unraveling algal lipid metabolism: recent advances in gene identification. Biochimie 93:91–100
Lebeau T, Robert JM (2006) Biotechnology of immobilized micro algae: a culture technique for the future? In: Rao S (ed) Algal cultures, analogues of blooms and applications. Science Publishers, Enfield, pp 801–837
Levanony H, Bashan Y, Romano B, Klein E (1989) Ultrastructural localization and identification of Azospirillum brasilense Cd on and within wheat root by immuno-gold labeling. Plant Soil 117:207–218
Leyva LA, Bashan Y, Mendoza A, de-Bashan LE (2014) Accumulation of fatty acids in Chlorella vulgaris under heterotrophic conditions in relation to activity of acetyl-CoA carboxylase, temperature, and co-immobilization with Azospirillum brasilense. Naturwissenschaften 101:819–830
Leyva LA, Bashan Y, de-Bashan LE (2015) Activity of acetyl-CoA carboxylase is not directly linked to accumulation of lipids when Chlorella vulgaris is co-immobilised with Azospirillum brasilense in alginate under autotrophic and heterotrophic conditions. Ann Microbiol 65:339–349
Lv J-M, Cheng L-H, Xu X-H, Zhang L, Chen H-L (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Meza B, de-Bashan LE, Bashan Y (2015a) Involvement of indole-3-acetic acid produced by Azospirillum brasilense in accumulating intracellular ammonium in Chlorella vulgaris. Res Microbiol 166:72–83
Meza B, de-Bashan LE, Hernandez J-P, Bashan Y (2015b) Accumulation of intra-cellular polyphosphate in Chlorella vulgaris cells is related to indole-3-acetic acid produced by Azospirillum brasilense. Res Microbiol 166:399–407
Momeni B, Chen C-C, Hillesland KL, Waite A, Shou W (2011) Using artificial systems to explore the ecology and evolution of symbioses. Cell Mol Life Sci 68:1353–1368
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G, Valori F (2008) Effects of root exudates in microbial diversity and activity in rhizosphere soils. In: Nautiyal CS, Dion P (eds) Molecular mechanisms of plant and microbe coexistence. Springer, Heidelberg, pp 339–365
Nishijima T, Shiozaki R, Hata Y (1979) Production of vitamin B12, thiamine, and biotin by freshwater phytoplankton. Bull Jpn Soc Sci Fish 45:199–204
Nosaka K (2006) Recent progress in understanding thiamin biosynthesis and its genetic regulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 72:30–40
Paerl RW, Bertrand EM, Allen AE, Palenik B, Azam F (2015) Vitamin B1 ecophysiology of marine picoeukaryotic algae: strain-specific differences and a new role for bacteria in vitamin cycling. Limnol Oceanogr 60:215–228
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Palacios OA, Bashan Y, de-Bashan LE (2014) Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—an overview. Biol Fert Soils 50:415–432
Perez-Garcia O, Bashan Y (2015) Microalgal heterotrophic and mixotrophic culturing for bio-refining: From metabolic routes to techno-economics. In: Prokop A, Bajpai R, Zappi M (eds) Algal biorefineries II: products and biorefinery design. Springer International, Switzerland, (In Press). doi:10.1007/978-3-319-20200-6_3
Pratt R, Johnson E (1965) Production of thiamine, riboflavin, folic acid and biotin by Chlorella vulgaris and Chlorella pyrenoidosa. J Pharm Sci 54:871–874
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Puente ME, Holguin G, Glick BR, Bashan Y (1999) Root surface colonization of black mangrove seedlings by Azospirillum halofraeference and Azospirillum brasilense in seawater. FEMS Microbiol Ecol 29:283–292
Rodelas B, Salmerón V, Martinez-Toledo MV, González-López J (1993) Production of vitamins by Azospirillum brasilense in chemically-defined media. Plant Soil 153:97–101
Rodrigues AC, Bonifacio A, Antunes JEL, da Silveira JAG, Figueiredo MVB (2013) Minimization of oxidative stress in cowpea nodules by the interrelationship between Bradyrhizobium sp. and plant growth-promoting bacteria. Appl Soil Ecol 64:245–251
Rojas-Tapias DF, Bonilla RR, Dussán J (2012) Effect of inoculation with plant growth-promoting bacteria on growth and copper uptake by sunflowers. Water Air Soil Pollut 223:643–654
Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P (2004) Simultaneous determination of thiamine and riboflavin in edible marine seaweeds by high-performance liquid chromatography. J Chromatogr Sci 42:117–120
Schönwitz R, Ziegler H (1994) Exudation of water-soluble vitamins and of some carbohydrates by intact roots of maize seedlings (Zea mays L.) into a mineral nutrient solution. J Plant Physiol 107:7–14
Schyns G, Potot S, Geng Y, Barbosa TM, Henriques A, Perkins JB (2005) Isolation and characterization of new thiamine-deregulated mutants of Bacillus subtilis. J Bacteriol 187:8127–8136
Smith DJ, Underwood GJC (2000) The production of extracellular carbohydrates by estuarine benthic diatoms: the effect of growth phase and light and dark treatment. J Phycol 36:321–333
Smith AG, Croft MT, Moulin M, Webb ME (2007) Plants need their vitamins too. Curr Opin Plant Biol 10:266–275
Wagner-Döbler I, Ballhausen B, Berger M et al (2010) The complete genome sequence of the algal symbiont Dinoroseobacter shibae: a hitchhiker’s guide to life in the sea. ISME J 4:61–77
Widjaja A, Chien C-C, Ju Y-H (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem E 40:13–20
Acknowledgments
At CIBNOR, we thank Manuel Moreno, Francisco Hernandez, and Juan-Pablo Hernandez for technical support and Ira Fogel for English and editorial suggestions. At Auburn University, we thank John Mcinroy for final English editing. Alejandro Palacios of the Autonomous University of Baja California Sur, La Paz, Mexico provided advice in statistical analysis. This study was supported by Consejo Nacional de Ciencia y Tecnologia of Mexico (CONACYT-Basic Science-2009, contract 164548) and time for writing by The Bashan Foundation, USA. O.A.P. was a recipient of a graduate fellowship (CONACYT 226169) and small periodic grants from the Bashan Foundation. This is contribution 2015-004 from the Bashan Institute of Science, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is dedicated to the memory of the German/Spanish mycorrhizae researcher Dr. Horst Vierheilig (1964–2011) of CSIC, Spain
The term “release” stands for “extrusion”, “excretion”, and “produce”. A specific term is used when the exact mechanism is known.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Comparison of thiamine release by Chlorella sorokiniana and Azospirillum brasilense at different pH of the medium when immobilized separately or immobilized together in alginate beads (a, b, c) and growth of these microorganisms in these cultures (d, e, f). Values along curves denoted by different capital letters differ significantly using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE. (PDF 262 kb)
Fig. S2
Comparison of thiamine content in exudates under different light intensities released by Chlorella sorokiniana (a), Azospirillum brasilense (b) immobilized alone and co-immobilized (c) and comparison of growth of these microorganisms in these cultures (d, e, f). Values along curves denoted by different capital letters differ significantly using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE. (PDF 270 kb)
Rights and permissions
About this article
Cite this article
Palacios, O.A., Bashan, Y., Schmid, M. et al. Enhancement of thiamine release during synthetic mutualism between Chlorella sorokiniana and Azospirillum brasilense growing under stress conditions. J Appl Phycol 28, 1521–1531 (2016). https://doi.org/10.1007/s10811-015-0697-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0697-z