Abstract
Microalgae are ideal candidates for bioremediation and biotechnological applications. However, salinity and nutrient resource availability vary seasonally and between cultivation sites, potentially impacting on biomass productivity. The aim of this study was to screen pollutant-tolerant freshwater microalgae (Desmodesmus armatus, Mesotaenium sp., Scenedesmus quadricauda and Tetraedron sp.), isolated from Tarong power station ash-dam water, for their tolerance to cultivation at a range of salinities. To determine if biochemical composition could be manipulated, the effects of 4-day nutrient limitation were also determined. Microalgae were cultured at 2, 8, 11 and 18 ppt salinity, and nutrient uptake was monitored daily. Growth, total lipid, fatty acid (FA), and amino acid contents were quantified in biomass harvested while nutrient-replete and, after 4 days, nutrient-deplete. D. armatus showed the highest salinity tolerance actively growing in up to 18 ppt while Mesotaenium sp. was the least halotolerant with decreasing growth rates from 11 ppt. However, Mesotaenium sp. at 2 and 8 ppt had the highest biomass productivity and nutrient requirements of the four species, making it ideal for nutrient remediation of eutrophic freshwater effluents. Salinity and nutrient status had minimal influence on total lipid and FA contents in D. armatus and Mesotaenium sp., while nutrient depletion induced an increase of total lipid and FAs in S. quadricauda and Tetraedron sp., which was further increased with increasing salinity. As none of the growth conditions affected amino acid profiles of the species, these findings provide a basis for species selection based on site-specific salinity conditions and nutrient resource availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cultivation of microalgae has the potential to provide critical ecosystem services through bioremediation of atmospheric industrial pollution (e.g. CO2 and NO) (Brune et al. 2009; Ho et al. 2011) and nutrient-rich waters from agriculture, aquaculture or urban sewage (Chan et al. 2014). In parallel, the resulting biomass can be used for production of commodities and high-value compounds such as protein or fatty acids (Mata et al. 2010; Pulz and Gross 2004; Stephens et al. 2010).
An important consideration for the feasibility of large-scale microalgae cultivation is water availability and salinity (Borowitzka and Moheimani 2013). Industrial sites, such as coal-fired power stations or sewage plants, may provide low-salinity wastewaters; however, in most cases, groundwater is predominantly available, which, in many parts of Australia, is often saline (<5 ppt) (Hart et al. 1991; Peck and Hatton 2003). This is of particular concern in tropical areas, where high evaporation rates year-round may lead to problems of increased salinity. For example, in a 100,000-L culture pond with a 5 ppt starting salinity and a ~5 % day−1 evaporation rate (North East Queensland, Australia, December) (BOM 2006), salinity would increase to ~7.5 ppt in 10 days and ~10 ppt in 20 days despite daily replacement. Consequently, when screening microalgae for biotechnological applications, it is important to determine species-specific halotolerance to identify species with broader salinity tolerance ranges providing greater flexibility in water requirements and applicability across different cultivation sites (Borowitzka and Moheimani 2013).
Many microalgae have the ability to tolerate fluctuations in salinity (Brown 1982; Kirst 1989; von Alvensleben et al. 2013) through the K+/Na+ pump as well as osmolyte production (e.g. glycine betaine, proline, sucrose, glycerol), which contributes to the osmotic potential for cell turgor and volume control and the latter protecting and restoring damaged proteins, nucleic acids and membrane lipids (Erdmann and Hagemann 2001). Despite this, salinity stress often leads to decreased biomass productivity due to the high energy cost of osmoregulation (Oren 1999) and is often associated with an over-production of reactive oxygen species (ROS) (Erdmann and Hagemann 2001; Mahajan and Tuteja 2005; Sudhir and Murthy 2004). Importantly, this natural response to salinity stress can also be exploited in order to manipulate the biochemical composition of microalgae, as evidenced in, e.g. increased fatty acid content with increasing salinity in the marine microalgae Isochrysis sp. and Nannochloropsis oculata (Renaud and Parry 1994) and in the freshwater microalga Chlamydomonas mexicana (Salama et al. 2014).
Microalgal nutrient uptake (e.g. nitrogen and phosphate) varies widely between species (Aravantinou et al. 2013; Dortch et al. 1984) which has multiple implications for large-scale production depending on whether these nutrients need to be purchased or whether they are freely available in nutrient-rich wastewater, requiring remediation. If nutrient-rich wastewater is available, species selection should identify species with high nutrient consumption and tolerance to eutrophic conditions for timely wastewater remediation (Mata et al. 2010). In contrast, if nutrient-rich wastewater is not available, nutrient provision will incur substantial costs, and species with lower nutrient consumption would be advantageous for biomass production. This is particularly important with the observed global peak of phosphate production and fertilizers, in general, becoming increasingly expensive if effective recycling methods are not adopted (Cordell et al. 2009; Dawson and Hilton 2011). As microalgal growth is positively correlated with nutrient availability (MacIntyre and Cullen 2005), nutrient provision at a cost will particularly affect economics of large-volume bio-products. Similarly to salinity, nutrient condition manipulations are commonly used to favourably alter the biochemical composition of microalgal biomass, for example, to induce the rapid accumulation of triacylglycerols (TAG) (Gao et al. 2013; Olofsson et al. 2014; Rodolfi et al. 2009) or pigments (Imamoglu et al. 2009) in a number of algal species in commercial production. Whilst nutrient limitation leads to cessation of active biomass production, benefits are incurred if it leads to substantially higher accumulation of target compounds (e.g. β-carotene in Dunaliella salina, astaxanthin in Haematococcus pluvialis and lipids in Nannochloropsis spp. (Richardson 2011)) through diverting carbon usage for growth to carbon storage in biomolecules.
Considering the enormous diversity of algal species (Guiry 2012) and the common stress response to up-regulate the content of cellular components that are desirable in commercial production of algae, bioprospecting for new microalgal species amenable to cultivation and environmental tolerance trials connected to biochemical plasticity remain important tasks. Research to date for Desmodesmus armatus, Mesotaenium sp. and Tetraedron sp. is limited, in particular, concerning their potential biotechnological applications (Pulz and Gross 2004). Scenedesmus spp., however, have been extensively investigated with established potential for wastewater remediation and in biotechnological applications, e.g. pigment and biofuel production (Martı́nez et al. 2000; Muller et al. 2005; Guedes et al. 2011; Garcia-Moscoso et al. 2013). Therefore, this study investigated nutrient requirements and responses of four freshwater microalgal species (D. armatus, Mesotaenium sp., Tetraedron sp. and Scenedesmus quadricauda) isolated from ash dam water of a Queensland power station to changes in salinity and flow-on effects on biochemical compositions. The second aim was to identify if nutrient limitation could be used to favourably alter the biochemical profiles and productivity of the same species and if this effect was linked to the level of salinity stress.
Materials and methods
Freshwater microalgae were isolated from Tarong power station (Stanwell Corp.) in Queensland, Australia, and maintained at the North Queensland Algal Identification/Culturing Facility (NQAIF) culture collection (James Cook University, Townsville, Australia). Of the 13 successfully isolated species, Desmodesmus armatus (Trebouxiophyceae) (culture accession # NQAIF301), Mesotaenium sp. (Conjugatophyceae) (NQAIF303), Scenedesmus quadricauda (Chlorophyceae) (NQAIF304) and Tetraedron sp. (Chlorophyceae) (NQAIF295) were chosen for the experiments, not only because they were the dominant and most abundant species but also because of their cosmopolitan distribution which minimizes biosecurity concerns. The fact that they were isolated from ash dam waters from a coal-fired power plant points to their potential resilience in demanding environments and likely suitability for remediation purposes. Individually aerated (0.45 μm filtered air; Durapore; Millipore) batch cultures were maintained at 24 °C, with a 12:12-h photoperiod and light intensity of 42 μmol photons m−2 s−1. Monoclonal cultures with low bacterial numbers (<1 mL−1) were established in a total culture volume of 2 L in modified L1 culture medium (Andersen et al. 2005), with 5 mg L−1 instead of 3 mg L−1phosphate. Preliminary studies identified high phosphate uptake rates by Mesotaenium sp. and S. quadricauda; consequently, medium phosphate concentrations were increased to reduce phosphate replenishment requirements and avoid medium phosphate depletion. To account for differences in cell size, all species were inoculated at 100 mg dry weight L−1. To maintain nutrient-replete biomass, cultures were re-fertilized with nitrate and phosphate when near depletion, until there was sufficient N replete biomass for harvest for biochemical analyses. Modified L1 culture medium was prepared at four salinities: 2, 8, 11 and 18 parts per thousand (ppt) (0.03, 0.14, 0.19 and 0.31 M, respectively) NaCl in filtered seawater (FSW) (pre-filtration Whatman GF/C, followed by 0.45 μm Durapore, Millipore). All materials were sterilized by autoclaving, and cultures were handled and inoculated aseptically in a laminar flow cabinet. Replicate cultures (2 L, n = 3) of all species were inoculated at a dry weight (DW) of ~100 mg L−1 for each salinity. Inoculation was carried out from salinity-specific, 6-month-acclimated mother cultures.
Algal growth
Algal growth was determined by measuring turbidity (OD750 nm), correlated to cells L−1and dry weights (DW; g L−1) following previously described methods (von Alvensleben et al. 2013). Turbidity calibration curves were medium-blanked for each salinity, while DW samples were corrected for salt content using salinity-specific blanks. Results were correlated to generate linear equations (R 2 > 0.95) used to determine cell numbers and respective DWs of each species at each salinity from turbidity measurements. Growth of all species was then determined every second day using turbidity for 20 days, and obtained data were transformed to cell numbers and DWs as described in von Alvensleben et al. (2013). Volumetric biomass productivities (Eq. 1) were determined using a method modified from Su et al. (2011).
where DW 1 and DW 2 = initial and final DW (g L−1), respectively, t 1 and t 2 = initial and final culture time points (days) per identified growth period, respectively.
Nutrient analyses
Medium nitrate (NO3 −), nitrite (NO2 −) and phosphate (PO4 3−) concentrations were determined every second day. For this, 5 mL culture samples were centrifuged at 3000 × g at 20 °C for 20 min, followed by 0.45-μm filtration (Durapore; Millipore Kilsyth) of the supernatant to remove any suspended material. From this, 1.25 mL was used for nitrate (NO3 −), 1.25 mL for nitrite (NO2 −) and 200 μL for phosphate (PO4 3−) determination. The phosphate assay required dilution with deionized water (Elix 5, Millipore) when PO4 3− concentrations exceeded 2.5 mg L−1 to ensure accuracy of the assay.
Nitrate and nitrite were determined spectrophotometrically in triplicate using a method adapted from Carvalho et al. (1998) to suit a 96-well plate format as described in detail in the supplementary material. Nitrite (λ = 540 nm) and nitrate + nitrite (λ = 220 nm) concentrations were calculated by comparison of absorbences with serial dilution linear regression calibration curves (R 2 > 0.95), generated previously from known nitrate and nitrite concentrations (0–32.21 and 0–43.38 μM sodium nitrate and sodium nitrite, respectively). Nitrate concentrations were calculated by subtracting the nitrite concentration from the total nitrate + nitrite concentration.
Similarly, phosphate was determined spectrophotometrically (λ = 610 nm) in triplicate following standard methods adapted from Van Veldhoven and Mannaerts (1987) and R&D Systems, Malachite Green Phosphate Detection Kit (Cat. Number: DY996, www.RnDSystems.com) to suit a 96-well plate format, as described in detail in the supplementary material. Phosphate concentrations were then calculated from serial dilution linear regression calibration curves (R 2 > 0.95), generated previously from known phosphate concentrations (0–50 μM).
Biochemical analyses
Biomass samples for biochemical analyses were harvested from 500 mL samples by centrifugation as described previously (von Alvensleben et al. 2013) when nitrate-replete during late logarithmic growth (day 14 for D. armatus, Mesotaenium sp. and Tetraedron sp. and day 12 for S. quadricauda) and 4 days after total nitrogen (N) depletion during the initial stationary phase (days 22–28 in D. armatus, days 20–24 in Mesotaenium sp., day 24 in S. quadricauda and days 24–30 in Tetraedron sp.). Cultures were classified as nutrient-replete and nutrient-deplete based on increasing and decreasing (NO2 −) secretion patterns, and nutrient depletion was assured by harvesting 4 days after medium nutrient depletion (Malerba et al. 2012). Biomass pellets were freeze-dried and stored in air-tight vials under nitrogen at 4 °C until further analysis.
Total lipids were determined gravimetrically following a direct extraction and transesterification method as described in detail in von Alvensleben et al. (2013).
Fatty acid extraction, transesterification and analysis
Fatty acids were extracted from freeze-dried samples in a single-step extraction and transesterification procedure modified from Gosch et al. (2012) followed by GC-MS analysis. Briefly, 2 mL acetylchloride,/acetylchloride, 95:5 (v/v)) and 300 μL internal standard (nonadecanoic acid, cat# 72332‐1G‐F, Sigma-Aldrich, Australia), 0.2 mg L−1 in methanol) were added to approximately 30 mg (±0.1 mg) dry biomass in Teflon-capped glass vials. Samples were heated at 100 °C for 1 h and allowed to cool, after which 1 mL hexane was added. To ensure complete partitioning of the formed fatty acid methyl esters (FAMEs) into the hexane layer, the samples were heated again at 100 °C for 1 min to form a single methanol/hexane phase. One-millilitre de-ionized water was then added to the cooled sample to facilitate phase separation. The hexane phase containing the FAMEs was collected and filtered through a 0.2 μm PTFE syringe filter prior to injection on the GC column. All solvents were of HPLC grade. Butylated hydroxy-toluene (BHT) (0.01 %) was added as an antioxidant during the extraction.
Fatty acid analysis was carried out in scan mode on an Agilent 7890 GC (DB-23 capillary column with a 0.15 μm cyanopropyl stationary phase, 60 m × 0.25 mm inner diameter) equipped with a flame ionization detector (FID) and connected to an Agilent 5975C electron ionization (EI) turbo mass spectrometer (Agilent Technologies, Australia), for identification of FAMEs as previously described (Gosch et al. 2012; von Alvensleben et al. 2013).
Amino acid analyses
Amino acid profiling of biomass from nutrient-replete and nutrient-deplete cultures at 2 and 11 ppt salinity was carried out by the Instrument Analysis Center of Shanghai Jiao Tong University using a Hitachi L8900 Amino Acid Analyzer (Tokyo, Japan) in accordance with the Chinese national standard protocol for amino acid determination (GB/T 5009.124-2003). Briefly, 10 mg samples were hydrolyzed for 22 h at 110 °C in a vacuum glass tube, using 6 M HCl. Samples were dried in a vacuum, and the residue was diluted with 0.02 M HCl prior to analysis on the amino acid analyzer, together with amino acid standards. Alanine, arginine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine and valine were quantified. The sum of these amino acids is considered total protein throughout this study.
Carbohydrate determination
Total carbohydrate content was determined by difference, containing also dietary fibre and some organic acids, following the method by Sims (1978) using Eq. 2. For this, ash (dry inorganic) content (mg g−1 DW) was determined by combustion at 500 °C for 24 h in a muffle furnace (Yokogawa-UP 150, AS1044).
Total lipid and FA productivities
Total fatty acid (TFA) productivities were determined using Eq. 3, where TFA1 was determined in nutrient-replete conditions and TFA 2 in nutrient-deplete conditions, and t 1 and t 2 represent the replete and deplete harvest time points for FA determination, respectively.
Statistical analyses
All statistical analyses were carried out in Statistica 12 (StatSoft Pty Ltd.). Repeated measures ANOVAs were used to determine the effects of salinity on culture growth and biomass productivity and the effects of both salinity and nutrient status on biochemical profiles within and between species. One-way ANOVAs were used to clarify single variable effects generally within species across salinities. Tukey’s post hoc tests were used to determine significant differences assigned at P < 0.05. Homogeneity of variances and normality assumptions were verified using Levene’s tests. Non-metric multidimensional scaling (nMDS, Primer 6) (Clarke 1993) was used to evaluate patterns in fatty acid and amino acid quality (% of total FA or amino acid (AA)) relative to salinity and nutrient status. Increasing distances between points in the ordination plot represent decreasing similarity between those points. The Bray-Curtis similarity coefficient was used as a distance measure, derived from square-root-transformed data of individual fatty acid proportions (mean of replicates, n = 3). Pearson product correlations for the relative proportions of the individual FAs or groups of FAs (>0.8) are shown in the vector loading plot. The length and direction of the vectors indicate the strength of the correlation and direction of change between the two axes, i.e. the relative differences in specific fatty acids between the demarcated groups.
Results
Effect of salinity on growth
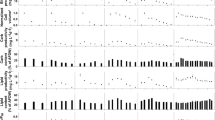
Lower salinities (2, 8 and 11 ppt) had no effect on growth of D. armatus, S. quadricauda or Tetraedron sp.; however, 18 ppt significantly reduced or completely inhibited growth in all three species (Fig. 1a, c, d) (repeated measures ANOVA: D. armatus; F (1,3) = 95.5, P < 0.05, S. quadricauda; F (1,3) = 179.5, P < 0.05 Tetraedron sp. F (1,3) = 44.3, P < 0.05). In Mesotaenium sp., cultures at 2 and 8 ppt reached similar final biomass content (~1.1 g L−1), but there was a significant decrease in biomass production with increasing salinity at 11 (0.8 g L−1) and 18 ppt (0.2 g L−1) (repeated measures ANOVA, F (1,3) = 1549.4, P < 0.05) (Fig. 1b).
At 2 and 8 ppt, highest final biomass density was achieved by Mesotaenium sp., >1000 mg L−1, whereas all other species reached between 650 and 950 mg L−1 (Fig. 1). At 11 ppt, biomass density was highest for D. armatus and Mesotaenium sp. (~930 and 850 mg L−1, respectively). With similar growth patterns from 2 to 11 ppt, S. quadricauda and Tetraedron sp. reached the lowest biomass density (730–770 and 710–760 mg L−1, respectively) (Fig. 1). At 18 ppt, D. armatus exhibited the highest growth reaching ~700 mg L−1 (Fig. 1), whereas Mesotaenium sp. and Tetraedron sp. cultures showed minimal growth (260–300 mg L−1) and S. quadricauda growth was completely inhibited, producing insufficient biomass for biochemical composition analysis at this salinity.
With the exception of Mesotaenium sp., salinity had no significant effect on biomass productivity (mg L−1 day−1) from 2 to 11 ppt for each species (repeated measures ANOVA, F (1,2) = 0.4, P = 0.6) (Fig. 2) but was significantly lower at 18 ppt in all species (repeated measures ANOVA, F (1,3) = 90.2, P < 0.05) (Fig. 2). Mesotaenium sp. showed similar productivities at 2 and 8 ppt, but a significant decrease (one-way ANOVA, F = (1,3) = 88.8, P < 0.05) at both 11 and 18 ppt. Between species, Mesotaenium sp. cultured at 2 and 8 ppt had the highest biomass productivity (52–54 mg L−1 day−1) (repeated measures ANOVA, F (1,9) = 9, P < 0.05) (Fig. 2). Productivities were similar (31–43 mg L−1 day−1) in D. armatus and S. quadricauda cultures from 2 to 11 ppt, as well as Mesotaenium sp. at 11 ppt and Tetraedron sp. at 2 ppt. Overall, Tetraedron sp. had the lowest biomass productivity at salinities above 2 ppt (10–31 mg L−1 day−1). At 18 ppt, D. armatus showed the highest productivity (17 mg L−1 day−1) followed by Tetraedron sp. and Mesotaenium sp. with minimal productivity (9–10 mg L−1 day−1) (Fig. 2).
Nutrient dynamics
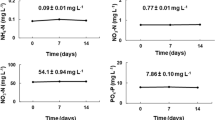
Within species, the highest total N-uptake was always at 2 ppt (1.5–1.9 mg L−1 day−1) (Table 1). Total N-uptake was lower but not significantly different in cultures at 8 and 11 ppt in D. armatus, Mesotaenium sp. and S. quadricauda (1.4–1.5 mg L−1 day−1) (repeated measures ANOVA, F (1,2) = 2.7, P = 0.1) (Table 1). Tetraedron sp. showed a significantly lower intermediate uptake at 8 and 11 ppt (one-way ANOVA, F (1,3) = 27.3, P < 0.05) (~1.1 mg L−1 day−1). Between species, the highest total N uptake was observed in S. quadricauda and Tetraedron sp. cultures at 2 ppt (~1.9 mg L−1 day−1), followed by Mesotaenium sp. (1.6 mg L−1 day−1), then D. armatus (1.5 mg L−1 day−1) (Table 1).
Salinity had no significant effect on phosphate uptake in D. armatus (0.59–0.64 mg L−1 day−1) (one-way ANOVA, F (1,3) = 0.02, P = 0.9), S. quadricauda (1.5–2.1 mg L−1 day−1) (one-way ANOVA, F (1,2) = 1.7, P = 0.2) or Tetraedron sp. (0.36–0.57 mg L−1 day−1 ) (one-way ANOVA F (1,3) = 1.8, P = 0.2). In Mesotaenium sp., phosphate uptake was significantly higher at 2 ppt (~2.3 mg L−1 day−1) (one-way ANOVA, F (1,3) = 49.1, P < 0.05), but not significantly different between cultures from 8 to 18 ppt (0.34–0.75 mg L−1 day−1) (main-effects ANOVA, F (1,2) = 3.4, P = 0.06) (Table 1). Between species, phosphate uptake was highest in Mesotaenium sp. at 2 ppt (2.2 mg L−1 day−1) and S. quadricauda (1.5–2.1 mg L−1 day−1) at all salinities (repeated measures ANOVA, F (1,3) = 69.8, P < 0.05), requiring re-fertilization every second day.
Correlating biomass productivity (mg L−1 day−1) and total N and P uptake rates (mg L−1 day−1) (Table 2) showed that, despite differences in uptake rates, these were closely correlated with biomass productivity resulting in similar N-uptake per mg g−1 DW across all salinities within species. The highest total N-uptake per unit biomass was observed in S. quadricauda and Tetraedron sp. at 2 ppt (~0.06 mg N mg−1 DW L−1 day−1) (Table 2). Phosphate uptake per unit biomass within species was generally lower at 2 ppt (except in Mesotaenium sp. and S. quadricauda) and increased at higher salinities. The highest phosphate uptake per unit biomass was observed in S. quadricauda at 2 ppt (~0.06 mg N mg−1 DW L−1 day−1) (Table 2).
Biochemical composition
Total lipid and TFA content. Total lipid content was 50 % higher than TFA content, which is indicative of a large contribution of other lipid-soluble compounds, particularly pigments, to the total lipid fraction (Lim et al. 2012; von Alvensleben et al. 2013). Salinity had minor effects on total lipid content in nutrient-replete conditions, with Mesotaenium sp. showing the highest total lipid content (20–25 % of DW) and Tetraedron sp. the lowest (14–18 % of DW) (Fig. 3). Nutrient depletion induced an increase of total lipid with increasing salinity in S. quadricauda (repeated measures ANOVA, F (1,2) = 14.3, P = 0.03) and Tetraedron sp. (~5–10 % increase) (repeated measures ANOVA, F (1,3) = 0.161, P = 0.9) whereas nutrient depletion had no effect on total lipid content in D. armatus (repeated measures ANOVA, F (1,3) = 1.1, P = 0.4) and Mesotaenium sp. cultures (F (1,3) = 0.9, P = 0.5) (Fig. 3).
Salinity did, however, have a significant effect on TFA contents in all species in both nutrient-replete and nutrient-deplete conditions (species- and nutrient-specific one-way ANOVAs, P < 0.05), except for S. quadricauda in replete conditions (one-way ANOVAs, F (1,2) = 2.2, P = 0.2). These significant effects were mainly driven by the small variance between replicates. In nutrient-replete conditions, TFA content decreased with increasing salinity in D. armatus from 2 (9 %) to 18 ppt (7 %) (one-way ANOVA, F (1,3) = 71.6, P < 0.05), Mesotaenium sp. (7–8 %) (one-way ANOVA, F (1,3) = 204.9, P < 0.05) and S. quadricauda (6.7–7.2 %) (one-way ANOVA, F (1,2) = 2.2, P = 0.2) from 2 to 11 ppt. In nutrient-replete Tetraedron sp. cultures, TFA content increased at 8 and 11 ppt (~6.5 %). In all species, nutrient-replete cultures at 18 ppt contained the lowest TFA, except for Mesotaenium sp. which contained its highest TFA content (12–13 % of DW) at this salinity.
Nutrient depletion had a small but significant effect on TFA driving an increase (1–6 % increase) in TFA content in all species (repeated measures ANOVAs, D. armatus: F (1,1) = 289, P < 0.05, Mesotaenium sp.: F (1,1) = 15, P < 0.05, S. quadricauda: F (1,1) = 58.8, P < 0.05, Tetraedron sp.: F (1,3) = 323.8, P < 0.05) at all salinities, except in Mesotaenium sp. at 18 ppt where a decrease (1 %) was observed. Between species, TFA content was highest in D. armatus and Mesotaenium sp. (7–13 % of DW) and lowest in S. quadricauda and Tetraedron sp. (5–7 % of DW) (Fig. 3).
Fatty acid profiles
The most abundant (>5 mg g−1 DW) fatty acids present in all species were C16:0, C18:1, C16:4, C18:2 and C18:3 (α-linolenic acid), except in Mesotaenium sp., which did not contain C16:4 but had high amounts of C16:2 (S. Table 2). Excluding cultures at 18 ppt, Mesotaenium sp. contained the highest C16:0 (15–35 mg g−1 DW) and C18:2 concentrations (10–35 mg g−1 DW) and D. armatus the highest C16:4 (9–15 mg g−1 DW) and C18:3 (α) (20–29 mg g−1 DW) concentrations. MDS analyses (Fig. 4) showed distinct groupings of biomass samples based on their fatty acid profiles. In D. armatus (Fig. 4a, stress 0.01), nutrient-replete cultures from 2 to 11 ppt and nutrient-deplete cultures at 2 ppt grouped together and were characterized by high proportions of omega-3 (ω-3) (52–56 %) and polyunsaturated fatty acids (PUFA) (~70 %, mainly C16:4 and C18:3 α-linolenic) as indicated by the vector loadings to the top right of the ordination plot. Nutrient-replete cultures at 18 ppt had similar profiles to deplete cultures at 8 and 11 ppt and were characterized by higher proportions of omega-6 (ω-6) fatty acids (~18 %) (Pearson product correlation >0.5, data not shown). Actual TFA concentrations (mg g−1 DW) for all species and treatments are listed in S. Tables 1, 2, 3 and 4. Mesotaenium sp. grouped by salinity rather than nutrient status (Fig. 4b, stress 0). Cultures at 2 ppt were characterized by a higher proportion of ω-3 (~30 %, mainly C18:3, α-linolenic) whereas biomass from 8 and 11 ppt had a higher proportion of total PUFA (53–66 %, mainly C18:3) and ω-6 contents (43–53 %, mainly C20:3). The MDS of fatty acid profiles from S. quadricauda yielded groups strongly separated by culture nutrient status (Fig. 4c, stress 0), with a higher proportion of ω-3 (~54 %, mainly C16:4 and C18:3) and PUFA (~67 %) in replete cultures and an increase in proportion of ω-6 (3 % increase, mainly driven by an increase in C18:2) fatty acids in deplete cultures. In Tetraedron sp., fatty acid profiles were affected by both nutrient status and salinity. Replete cultures at 2 ppt showed distinctly different profiles to all other cultures (Fig. 4d, stress 0.01) with the highest proportions of PUFA (57–64 %), lower proportions of saturated fatty acids (SFA) (~20 %) and monounsaturated fatty acids (MUFA) (~12 %) and the highest total ω-3 content (~52 %). Cultures at 8 and 11 ppt were most affected by nutrient depletion as indicated by the large distance between the points in the ordination plot, with an increase in proportion of MUFA contents (15–20 %, mainly C18:1) and decrease in PUFA contents (15–30 %, mainly C16:4 and C18:3). Cultures at 18 ppt were least affected by nutrient status, with nutrient-replete and nutrient-deplete cultures grouping close together with similar FA proportions in both conditions.
Fatty acid quality of Desmodesmus armatus (a), Mesotaenium sp. (b), S. quadricauda (c) and Tetraedron sp. (d) at different salinities and nutrient status. Multidimensional scaling (MDS) ordinations show clusters of samples based on the similarity of their fatty acid profiles (% of total FA). The vector loadings of the specific fatty acids relate to the clusters of samples in the ordination plots (correlations of R > 0.8). The direction and size of each vector indicate the relative abundance of that variable in samples in the corresponding section of the ordination plot
Lipid and fatty acid productivities
Due to high biomass productivity (Fig. 2), Mesotaenium sp. at 2 and 8 ppt showed the highest total lipid (11–13 mg L−1 day−1) and TFA productivities (4–4.5 mg L−1 day−1) (S. Table 5). Nutrient-replete fatty acid productivities were highest from 2 to 11 ppt in all species. Fatty acid productivities were mainly driven by biomass productivities, with the exception of high productivities of C18:1 in Mesotaenium sp. at 18 ppt and C18:2 in Tetraedon sp. at 11 ppt which were driven by a high FA content rather than biomass productivity (S. Table 6). Mesotaenium sp. at all salinities had the highest C16:0, C16:2 and C18:2 productivities, and D. armatus had the highest C16:4 productivity (S. Table 6). The highest C18:1 productivity was observed in Tetraedron sp. at 11 and Mesotaenium sp. at 18 ppt., while the highest C18:3 productivity occurred for D. armatus and Mesotaenium sp. at 2 ppt (S. Table 6).
AA contents
Total protein content (as the sum of AA) and essential AA (EAA) content were highest in D. armatus (309–450 mg g−1 DW and 150–233 mg g−1 DW, respectively) and lowest in Mesotaenium sp. (179–260 mg g−1 DW, 92–135 mg g−1 DW, respectively) (Table 3). Except for Mesotaenium sp. and Tetraedron sp. at 2 ppt and S. quadricauda at 11 ppt, nutrient depletion leads to a decrease in amino acids (AA) in all cultures. With the exception of Tetraedron sp., AA contents at 11 ppt were always lower than at 2 ppt in both nutrient-replete and nutrient-deplete conditions. Although AA content decreased with nutrient depletion, proportions of total AA content (%) remained similar, maintaining a similar profile regardless of conditions. In all species, the predominant EAAs were histidine and leucine with the highest concentrations (34–64 mg g−1 DW and 21–39 mg g−1 DW, respectively), whereas methionine and arginine contents were lowest (5–9 mg g−1 DW and 5–10 mg g−1 DW, respectively). More specifically, the highest histidine contents (64 mg g−1 DW) were observed in D. armatus and Tetraedron sp. and the highest leucine contents (39 mg g−1 DW) in D. armatus. Lysine contents were highest in D. armatus and S. quadricauda (27 mg g−1 DW) (for detailed AA profiles, see Supplementary Tables 7, 8, 9 and 10).
Carbohydrate contents
Carbohydrate content was highest in Tetraedron sp. (393–465 mg g−1 DW) and lowest in S. quadricauda (263–367 mg g−1 DW) (Table 3). At 2 ppt, nutrient depletion induced a carbohydrate content increase (20–50 mg g−1 DW) in all species. At 11 ppt, carbohydrate contents were higher than at 2 ppt in all species when nutrient-replete; however, nutrient depletion only induced a further increase in Tetraedron sp. (~40 mg g−1 DW).
Discussion
This study identified species-specific effects of both salinity and nutrient status on growth and biochemical profiles of D. armatus, Mesotaenium sp., S. quadricauda and Tetraedron sp.
Growth
Despite being freshwater species, this study showed that D. armatus, S. quadricauda and Tetraedron sp. are relatively halotolerant, with similar growth up to 11 ppt salinity, whereas Mesotaenium sp. has a lower salinity tolerance with optimal growth up to 8 ppt. Growth responses to salinity have implications for on-site cultivation of these four microalgal species. Although these species tolerated 18-ppt salinity to varying degrees, large-scale cultivation at this salinity will not be viable. Allowing for salinity increases due to evaporation, only D. armatus can potentially be cultured up to 11 ppt. Scenedesmus quadricauda and Tetraedron sp. should ideally be grown between 2 and 8 ppt and Mesotaenium sp. at 2 to potentially 5 ppt, reducing the evaporation margin, after which growth will be compromised. For example, considering average tropical East Queensland evaporation rates (~5 % daily), these findings imply that D. armatus, S. quadricauda and Tetraedron sp. could be cultured in saline groundwater around 5 ppt with daily water replacements for up to ~24 days (~11 ppt) without adverse effects of salinity. In contrast, Mesotaenium sp. in these conditions would be affected by salinity after ~12 days (~8 ppt), consequently requiring complete water replacement every 12 days, which has serious environmental implications depending on water availability at cultivation sites.
Biomass production of D. armatus, Mesotaenium sp. and Tetraedron sp. in this study is difficult to compare due to limited or absence of reports on growth patterns for these species. In general, biomass production was lower than in previous reports for S. quadricauda and other chlorophytes being examined for biotechnological potential (Patil 1991; Tiftickjian and Rayburn 1986; Zhou et al. 2011; Dickinson et al. 2013). Lower biomass content was likely due to light limitation, as the current study was performed under controlled laboratory conditions with an average light intensity of 40 μmol photons m−2 s−1compared to 100–200 μmol photons m−2 s−1(Tiftickjian and Rayburn 1986; Dickinson et al. 2013) or natural daylight (Patil 1991). This is of little concern, as the aim was to identify suitably halotolerant freshwater species, and culture conditions were not optimized to maximize productivity. Furthermore, large-scale cultivation at high population densities is likely to reduce penetrating light intensities to similar levels.
The observed patterns of decreasing growth with increasing salinity are to be expected for freshwater microalgal species, as increasing culture salinity (mainly Na+ and Cl−) may lead to an over-production of ROS which cause not only oxidative stress, enzyme inactivation and reduction of photosynthetic rates (Sudhir and Murthy 2004; Mahajan and Tuteja 2005), but also cellular ionic imbalance and subsequent water loss (Setter and Greenway 1979; Erdmann and Hagemann 2001). Acclimation to high salinities includes three processes: (1) restoration and maintenance of cell turgor and volume, (2) changes in permeability of the cell membrane and regulated uptake (K+) and expulsion (Na+) of ions and (3) the accumulation of osmoprotectant compatible solutes and stress proteins (Brown 1976; Erdmann and Hagemann 2001). Approximately 20 different compatible solutes have been shown to occur in microalgae with variable degrees of osmoprotection and salinity compensation. This has implications for growth, which will decrease, if ATP utilization is predominantly for osmotic regulation and/or nitrogen taken up is utilized for N-based osmoregulatory solute synthesis (e.g. proline, glycine betaine) (Vanlerberghe and Brown 1987; Erdmann and Hagemann 2001).
Nutrient dynamics, specifically nitrogen fluxes, can provide insight into possible osmoregulatory mechanisms, particularly when considered in combination with changes in biochemical profiles. In this study, the similarities in N uptake per unit biomass between salinities and the decrease of AA contents, specifically proline and glycine (Suppl. Tables 7, 8, 9 and 10) with increasing salinity in all species, indicate that these species are unlikely to produce N-containing osmolytes reported in microalgae (glycine betaine and/or proline). Although not specifically studied here, we hypothesize that osmoregulation in these species could be achieved via accumulation of carbohydrates which has been reported for other chlorophyte species, e.g. Chlamydomonas sp., Chlorella emersonii, Dunaliella sp. and Stichococcus bacillaris (Ben-Amotz and Avron 1983; Erdmann and Hagemann 2001). This is supported by increased carbohydrate contents with increasing salinity under nutrient-replete conditions.
For remediation/nutrient provision purposes, S. quadricauda and Tetraedron sp. at 2 ppt had the highest total N uptake, and Mesotaenium sp. and S. quadricauda had the highest phosphate uptake. This has dual implications depending on cultivation site, where, if nutrients have to be added at a cost, strain selection should be towards low-nutrient-consumption species (e.g. D. armatus), and, if nutrient-rich wastewater is available, high-nutrient-uptake species (e.g. Mesotaenium sp. and S. quadricauda) should be selected. The faster phosphate uptake in Mesotaenium sp. and S. quadricauda compared to the other species could be due to higher biomass production, specifically in Mesotaenium sp. or an indication of storage capacity by these species. Microalgae can store phosphate as polyphosphate for later use when external phosphate becomes limiting (Powell et al. 2009). Excessive uptake and polyphosphate storage are either a consequence of nutrient starvation followed by re-exposure (Aitchison and Butt 1973), or ‘luxury uptake’, which does not require prior nutrient starvation (Eixler et al. 2006). In this instance, luxury uptake is most likely, as cultures were maintained nutrient-replete until intentional depletion.
Mesotaenium sp. at low salinities (2 to 8 ppt) had the highest biomass, lipid and FAME productivities using the least nutrients, making it the most suitable species for cultivation when fertilization incurs a cost. For wastewater remediation of nitrate and phosphate, S. quadricauda is the most suitable species showing the highest removal rates of these nutrients. These findings correlate with previous reports that have identified S. quadricauda as an effective species for nutrient-rich wastewater remediation (Martı́nez et al. 2000; Shi et al. 2007; Dickinson et al. 2013).
Biochemical profiles
Total lipid contents were at the lower end of the range reported for other green algae but correspond to previous findings for a number of chlorophyte species (Griffiths and Harrison 2009) (see comparisons in Table 4).
An increase in total lipid and FAs in microalgae following nutrient depletion has been shown in previous studies, e.g. Chlorella vulgaris (Converti et al. 2009) and Scenedesmus subspicatus (Dean et al. 2010), and is often used for large-scale microalgal culture manipulations (Sharma et al. 2012). However, this study shows that the degree of this effect varies between species and salinity conditions. In D. armatus and Mesotaenium sp., neither salinity nor nutrient depletion had any effects on total lipid content, whereas in S. quadricauda and Tetraedron sp., nutrient depletion only had significant effects at higher salinities from 8 and 11 ppt, respectively. This shows that nutrient depletion and/or salinity stress is not universally effective lipid induction methods, supporting previous studies using Chlorella sorokiniana (Griffiths and Harrison 2009), Chlorella sp., Scenedesmus sp., (Rodolfi et al. 2009), Tetraselmis sp. and Nannochloropsis sp. (Reitan et al. 1994).
N limitation under continued photosynthetic carbon acquisition leads to diversion of carbon from growth to storage (Becker 1994; Rodolfi et al. 2009), as production of N-containing compounds such as proteins, nucleic acids and chlorophylls is inhibited, therefore resulting in reduced growth and biomass productivity. Although nutrient depletion for 4 days increased TFA content and was statistically significant in all species, differences were small mainly driven by the small variance between replicate fatty acid samples. As such, larger-nutrient-depletion periods would need to be applied which cannot be recommended as a means to favourably alter the biochemical profiles and productivity of these species due to impacts on cultivation footprints required to sustain biomass yields.
While FA comparisons between studies show a degree of variability, generally due to differences in culture conditions, FA profiles here were similar to those of other chlorophytes, with C16:0, C16:2, C18:1, C18:2 and C18:3 being the predominant fatty acids (Dunstan et al. 1992). More specifically, D. armatus, Mesotaenium sp. and Tetraedron sp. had comparable FA profiles to other reports for these species (Lang et al. 2011) and S. quadricauda FA profiles are comparable to those described for the same species by Ahlgren and Hyenstrand (2003) (compare with Suppl. Tables 1, 2, 3 and 4).
Fatty acid profiles were affected differently between the four species: Mesotaenium sp. was affected by culture salinity, whereas in D. armatus and S. quadricauda, nutrient availability had the greatest influence and Tetraedron sp. was affected by a combination of both. In all species, nutrient depletion induced an increase of SFA and MUFA driven mainly by an increase of C16:0 and C18:1, respectively and a decrease of C18:3 (except Tetraedron sp. at 2 ppt). Similar changes have been reported for a number of green algae, e.g. Botryococcus braunii and the eustigmatophyte Nannochloropsis sp. (Reitan et al. 1994; Zhila et al. 2005; Rodolfi et al. 2009; Su et al. 2011), and are most likely due to the accumulation of neutral lipids such as triacylglycerols, which in the Chlorophyceae, which have been observed to contain mainly C16:0 and C18:1 FA (Becker 1994).
Previous reports for the effects of increasing salinities on microalgal FA profiles have shown similar patterns to nutrient depletion with increases of C18:1 contents in B. braunii (Rao et al. 2007; Zhila et al. 2011), Isochrysis sp., D. salina (Ben-Amotz et al 1985) and Desmodesmus abundans (Xia et al. 2014) and increases of C16:0 in B. braunii (Rao et al. 2007; Zhila et al. 2011), Nannochloropsis oculata and Nitzschia frustulum (Renaud and Parry 1994). However, reports on the effects of sodium chloride on microalgal fatty acids are scarce and often contradictory (Zhila et al. 2011). Furthermore, it is also unclear if fatty acid composition plays a role in microalgal osmoregulation (Renaud and Parry 1994). A primary role of fatty acids in algae is related to functions of cell membranes and metabolic processes (Guschina and Harwood 2006). The degree of membrane fatty acid unsaturation is also a significant parameter in algal adaptation to environmental conditions. Fatty acid changes in response to high salinities are required to maintain membrane fluidity and prevent destruction (Zhila et al. 2011).
The distinct effect of salinity on Mesotaenium sp FA profiles are difficult to explain and put into the context of current literature. The high concentrations of C16:0 and C18:1 at 18 ppt are potentially due to the inhibited growth at this salinity having similar effects to nutrient limitation (see above) which results in the accumulation of TAG containing C16:0 and C18:1 FA (Ben-Amotz et al. 1985).
Microalgal PUFAs have a promising biotechnological market for food, feed and material applications (Lligadas et al. 2010; Pulz and Gross 2004). Examples include the following oleic acid (C18:1, ω-9), linoleic (C18:2, ω-6), α-linolenic (C18:3, ω-3) and stearidonic acid (C18:4, ω-3). Oleic acid (C18:1) can be used to produce fatty acid-derived diols and polyols, from which polyurethanes can be synthesized through polyaddition reactions with organic isocyanates (Lligadas et al. 2010). In this study, C18:1 content was significantly increased in all species by a combination of nutrient depletion and high salinities (11–18 ppt). The highest C18:1 content was observed for Tetraedron sp. at 11 ppt (54 mg g−1 DW) and Mesotaenium sp. at 18 ppt (35 mg g−1 DW). Both could be potential candidates for bioplastic manufacturing; however, Mesotaenium sp. would require a two-step cultivation process (Su et al. 2011), with biomass production at 2 ppt followed by salinity stress (18 ppt).
For dietary applications, linoleic acid (C18:2, ω-6) and α-linolenic acid (C18:3, ω-3) are essential nutrients for immune system function and tissue regeneration processes (de Jesus Raposo et al. 2013). They are also important precursors for other ω-6 and ω-3 FAs (Guil-Guerrero 2007), with distinct cellular functions (Simopoulos 2002). An imbalance in ω-6 and ω-3 FA ratios in current ‘western diets’ has been linked to a range of diseases such as cardiovascular disorders, diabetes, obesity, inflammatory processes, increased susceptibility to viral infections, certain types of cancer, autoimmune disorders, rheumatoid arthritis, asthma and depression (Simopoulos 2002; Guil-Guerrero 2007). Consequently, a ~1:1 ω-6/ω-3 uptake ratio has been recommended to ensure good health and normal development. This is an important consideration when identifying novel feed and FA sources. Desmodesmus armatus, S. quadricauda and Tetraedron sp. have low ω-6/ω-3 (generally <0.4:1) ratios and could therefore be beneficial as ω-3 nutritional supplements. Mesotaenium sp., on the other hand, had a particularly high ω-6/ω-3 ratio at salinities above 8 ppt (3–10:1) driven by a high C18:2 content, making it a possible candidate for pharmacological applications in the topical treatment of skin hyperplasias (Proksch et al. 1993).
Stearidonic acid (SA, C18:4) has also been shown to possess a number of health benefits and bioactive properties to prevent a range of conditions including certain cancers, arthritis and thrombosis (Guil-Guerrero 2007). Microalgae have previously been suggested as a potential source of SA (Guil-Guerrero 2007). In this study, C18:4 was present in D. armatus and Tetraedron sp.; however, only D. armatus at lower salinities contained notable amounts (7 mg g−1 DW) of this FA. Depending on the viability of targeting this FA for health purposes, D. armatus is therefore a suitable candidate for further research to improve C18:4 productivity yields.
Lipid and fatty acid productivities were generally low in this study compared to the same species in other studies (Rodolfi et al. 2009; Zhou et al. 2011), which is likely due to the low growth rates, as actual total lipid and fatty acid contents were comparable to previous studies (Dunstan et al. 1992; Ahlgren and Hyenstrand 2003; Rodolfi et al. 2009; Zhou et al. 2011). Consequently, future research should focus on increasing biomass productivity.
AA profiles in this study were similar to previous reports for Chlorella sp. and Scenedesmus sp. (Brown and Jeffrey 1992; Ahlgren and Hyenstrand 2003), except for histidine concentrations which were considerably higher in species in this study (up to 6.5 % of DW). The decrease of AA concentrations observed in all species in this study following nutrient depletion has been extensively documented and is most likely due to the diversion from protein production to carbohydrate or lipid production in the absence of N for protein synthesis (Flynn 1990; Rodolfi et al. 2009; Mata et al. 2010). As mentioned earlier and as documented in other microalgal species (Brown and Hellebust 1978; Greenway and Setter 1979; Vanlerberghe and Brown 1987), the species in this study do not use AA-based osmoregulation to combat salinity stress, as indicated by salinity-induced decreases in AA content, particularly proline and glycine.
Feed protein quality is determined by AA digestion and absorption by animals and their respective AA requirements for metabolic processes. In general, the ideal protein source for an organism contains the same AA content and AA proportions as the organism itself (Brown and Jeffrey 1992; De Silva et al. 2012). Limitation of one or more specific AAs restricts growth and results in the inability to utilize other essential AAs (De Silva et al. 2012) which becomes problematic when formulating feeds for farmed animals as certain EAAs are often limiting, e.g. methionine, lysine and threonine in fish, shrimp, cattle, swine and poultry feeds (D’Mello 1993; Kung Jr and Rode 1996; Rawles et al. 2013; Nunes et al. 2014). Currently, optimizing animal feed protein quality is not only carried out by supplementing feed with synthetic AAs but can also be achieved by AA blending from other sources with high concentrations of target AAs, e.g. plants, algae and insects (Boland et al. 2013), or as a by-product of biotechnological processes such as biofuel production (Williams and Laurens 2010). This study has shown that D. armatus had the highest AA contents and would be the most suitable species for AA production, in particular, lysine or as a feed supplement for species where lysine is often limiting, e.g. giant clam (Tridacna gigas) aquaculture (Brown 1991).
The carbohydrate content increase following N-depletion is due to the diversion of carbon from protein synthesis to carbohydrate and lipid production (see above). This increase is consistent with previous studies showing a carbohydrate increase following N depletion in Scenedesmus obliquus (Ho et al. 2012). Although this study did not specifically focus on carbohydrate production and composition in the four study species, microalgae are a potential source of sugars such as xylose, arabinose, mannose, galactose, glucose and, the less common, sugars rhamnose, fucose and uronic acids (Cheng et al. 2011; Ho et al. 2012; Krienitz et al. 1999), with an interesting potential for commercialization (Draaisma et al. 2013).
Conclusions
While all species cultured at salinities of 2–18 ppt, Mesotaenium sp. was the least salinity tolerant and D. armatus was the most halotolerant species of the dominant microalgae isolated from ash dam water of a Queensland coal-fired power station. Nitrogen uptake rates correlated with biomass irrespective of salinity, which together with decreased levels of proline and glycine at higher salinities suggest that salinity tolerance in these species is not achieved by glycine betaine or proline accumulation, as described for some other chlorophytes. Increased carbohydrate contents suggest instead that carbohydrate-based osmoregulatory mechanisms could be involved in salinity acclimation. The total lipid content data of the examined species suggest that neither increased salinity nor nitrogen depletion should be viewed as a universal mechanism to increase total lipids or fatty acids, as D. armatus and Mesotaenium sp. did not respond significantly to either treatment and S. quadricauda and Tetraedron sp. were only significantly affected by higher salinities. This is further corroborated by the finding that the FA profile was predominantly influenced by salinity in Mesotaenium sp., by nutrient status in D. armatus and S. quadricauda and by a combination of the two in Tetraedron sp. In general though, the isolated species responded to nutrient limitation with an increase in SFA and MUFA, particularly C16:0 and C18:1, which is well known from the literature. Generally, D. armatus, S. quadricauda and Tetraedron sp. were characterized by low ω-6/ω-3 ratios making them potential candidates for ω-3 supplements. In contrast, Mesotaenium sp. was characterized by a ω-6/ω-3 ratio of 2–10:1, making it unsuitable for diet supplementation with ω-3 FAs, yet it could be a pharmacological candidate for the topical treatment of skin hyperplasias.
The overall species responses from this study can now be used to produce a species selection matrix to target species for scaled production based on their salinity tolerance and plasticity in biochemical composition (Table 5). This study confirmed that S. quadricauda is an ideal candidate for environmental services, such as nitrogen and phosphate remediation, as it had the highest uptake rates. This study further identifies that the organism would be suitable across a salinity range of 2 < 11 ppt. D. armatus and Mesotaenium sp., on the other hand, stood out for biomass production under nutrient-poor conditions from 2 < 18 and 2 < 8 ppt, respectively. Such situations are typically encountered when producing carbon dioxide-supplemented biomass at coal-fired power stations in Australia where large amounts of nutrients and/or nutrient-rich water sources are generally unavailable. Both algae also had the highest FA content with a profile suitable for lipid-based biofuel production for on-site consumption. Tetraedron sp. and, Mesotaenium sp., in particular (5.4 % of DW, 45 % of TFA), excelled in accumulation of C18:1 at 8 < 11 and >11 < 18 ppt, respectively, a valuable precursor for bio-degradable plastic production. It needs to be recognized though that such production would require a two-step approach, where biomass accumulation would require cultivation at 2 ppt with subsequent salt stress used to shift the FA profile in favour of C18:1 accumulation, the feasibility of which still requires demonstration. D. armatus also has demonstrated pharmaceutical potential through accumulation of stearic acid when cultured at 2 ppt. While biomass yields and productivities are yet to be demonstrated on site, with regard to freshwater requirements, the results of this study suggest that D. armatus, S. quadricauda and Tetraedron sp. are sufficiently salinity tolerant to only require freshwater make-up water after 24-day cultivation based on East Queensland daily evaporation rates and salinity concentrations of available water sources, while Mesotaenium sp. could only be cultivated for 12 days under the same conditions. These results have major implications for cultivation site and product range selection for these new isolates.
References
Ahlgren G, Hyenstrand P (2003) Nitrogen limitation effects of different nitrogen sources on nutritional quality of two freshwater organisms, Scenedesmus quadricauda (Chlorophyceae) and Synechococcus sp. (Cyanophyceae). J Phycol 39:906–917
Aitchison PA, Butt VS (1973) Relation between synthesis of inorganic polyphosphate and phosphate uptake by Chlorella vulgaris. J Exp Bot 24:497–510
Andersen RA, Berges JA, Harrison PJ, Watanabe MM (2005) Recipes for freshwater and saltwater media. In: Andersen RA (ed) Algal culturing techniques. Elsevier, London, pp 429–538
Aravantinou AF, Theodorakopoulos MA, Manariotis ID (2013) Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol 147:130–134
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, New York
Ben-Amotz A, Avron M (1983) Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol 37:95–119
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Boland MJ et al (2013) The future supply of animal-derived protein for human consumption. Trends Food Sci Technol 29:62–73
BOM (2006) Average pan evaporation December. Bureau of Meteorology, http://www.bom.gov.au/jsp/ncc/climate_averages/evaporation/index.jsp?period=dec#maps
Borowitzka M, Moheimani N (2013) Sustainable biofuels from algae Mitigat Adaptat. Strat Global Change 18:13–25
Brown AD (1976) Microbial water stress. Bact Rev 40:803–846
Brown LM (1982) Photosynthetic and growth responses to salinity in a marine isolate of Nannochloris bacillaris (Chlorophyceae). J Phycol 18:483–488
Brown MR (1991) The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol 145:79–99
Brown LM, Hellebust JA (1978) Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can J Bot 56:676–679
Brown MR, Jeffrey SW (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 1. Amino acids, sugars and pigments. J Exp Mar Biol Ecol 161:91–113
Brune DE, Lundquist TJ, Beneman JR (2009) Microalgal biomass for greenhouse gas reductions: potential for replacement of fossil fuels and animal feeds. J Env Eng 135:1136–1144
Carvalho AP, Meireles LA, Malcata FX (1998) Rapid spectrophotometric determination of nitrates and nitrites in marine aqueous culture media. Analusis 26:347–351
Chan A, Salsali H, McBean E (2014) Nutrient removal (nitrogen and phosphorous) in secondary effluent from a wastewater treatment plant by microalgae. Can J Civil Eng 41:118–124
Cheng Y-S, Zheng Y, Labavitch JM, Vander Gheynst JS (2011) The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella Process. Biochem 46:1927–1933
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure Australian. J Ecol 18:117–143
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought Global. Env Change 19:292–305
Dawson CJ, Hilton J (2011) Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus. Food Policy 36(Suppl 1):S14–S22.
de Jesus Raposo MF, de Morais RMSC, de Morais AMMB (2013) Health applications of bioactive compounds from marine microalgae. Life Sci 93:479–486
De Silva S, Turchini G, Francis D (2012) Nutrition. In: Lucas JS, Southgate PC (eds) Aquaculture: farming aquatic animals and plants. Second edn. Blackwell, Oxford, pp 164–187
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Biores Technol 101:4499–4507
Dickinson KE, Whitney CG, McGinn PJ (2013) Nutrient remediation rates in municipal wastewater and their effect on biochemical composition of the microalga Scenedesmus sp. AMDD Algal Res 2:127–134
D’Mello JPF (1993) Amino acid supplementation of cereal-based diets for non-ruminants animal feed. Sci Technol 45:1–18
Dortch Q, Clayton JR Jr, Thoresen SS, Ahmed SI (1984) Species differences in accumulation of nitrogen pools in phytoplankton. Mar Biol 81:237–250
Draaisma RB, Wijffels RH, Slegers PM, Brentner LB, Roy A, Barbosa MJ (2013) Food commodities from microalgae. Curr Opin Biotech 24:169–177
Dunstan GA, Volkman JK, Jeffrey SW, Barrett SM (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. J Exp Mar Biol Ecol 161:115–134
Eixler S, Karsten U, Selig U (2006) Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 45:53–60
Erdmann N, Hagemann M (2001) Salt acclimation of algae and cyanobacteria: a comparison. In: Rai LC, Gaur JP (eds) Algal Adaptation to environmental stresses. Springer, Berlin, pp 323–361
Flynn KJ (1990) Composition of intracellular and extracellular pools of amino acids, and amino acid utilization of microalgae of different sizes. J Exp Mar Biol Ecol 139:151–166
Gao Y, Yang M, Wang C (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484–491
Garcia-Moscoso JL, Obeid W, Kumar S, Hatcher PG (2013) Flash hydrolysis of microalgae (Scenedesmus sp.) for protein extraction and production of biofuels intermediates. J Supercrit Fluids 82:183–190
Gosch BJ, Magnusson M, Paul NA, de Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts global change. Biol Bioenergy 4:919–930
Greenway H, Setter TL (1979) Accumulation of proline and sucrose during the 1st hours after transfer of Chlorella emersonii to high NaCl. Aus J Plant Physiol 6:69–79
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Guil-Guerrero JL (2007) Stearidonic acid (18:4n-3): metabolism, nutritional importance, medical uses and natural sources. Eur J Lipid Sci Technol 109:1226–1236
Guiry MD (2012) How many species of algae are there? J Phycol 48:1057–1063
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hart B, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K (1991) A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210:105–144
Ho S-H, Chen C-Y, Lee D-J, Chang J-S (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Imamoglu E, Dalay MC, Sukan FV (2009) Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnol 26:199–204
Kirst GO (1989) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol 41:21–53
Krienitz L, Takeda H, Hepperle D (1999) Ultrastructure, cell wall composition, and phylogenetic position of Pseudodictyosphaerium jurisii (Chlorococcales, Chlorophyta) including a comparison with other picoplanktonic green algae. Phycologia 38:100–107
Kung L Jr, Rode LM (1996) Amino acid metabolism in ruminants animal feed. Sci Technol 59:167–172
Lang IK, Hodac L, Friedl T, Feussner I (2011) Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol 11:124
Lim DK, Garg S, Timmins M, Zhang ES, Thomas-Hall SR, Schuhmann H, Li Y, Schenk PM (2012) Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS One 7(7), e40751
Lligadas G, Ronda JC, Galia M, Cadiz V (2010) Oleic and undecylenic acids as renewable feedstocks in the synthesis of polyols and polyurethanes. Polymers 2:440–453
MacIntyre HL, Cullen JJ (2005) Using cultures to investigate the physiological ecology of microalgae. In: Andersen RA (ed) Algal culturing techniques. Elsevier, London, pp 287–326
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Malerba ME, Connolly SR, Heimann K (2012) Nitrate-nitrite dynamics and phytoplankton growth: formulation and experimental evaluation of a dynamic model Limnol Oceanogr 57
Martı́nez ME, Sánchez S, Jiménez JM, El Yousfi F, Muñoz L (2000) Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol 73:263–272
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review Renew. Sust Energy Rev 14:217–232
Muller D, Forster D, Magert HJ, Grewe C, Griehl C (2005) Astaxanthin accumulation under specific stress conditions in Scenedesmus strains. Phycologia 44:39–39
Nunes AJP, Sá MVC, Browdy CL (2014) Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 431:20–27
Olofsson M, Lamela T, Nilsson E, Bergé J-P, del Pino V, Uronen P, Legrand C (2014) Combined effects of nitrogen concentration and seasonal changes on the production of lipids in Nannochloropsis oculata. Mar Drugs 12:1891–1910
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Molec Biol Rev 63:334–348
Patil HS (1991) The role of Ankistrodesmus falcatus and Scenedesmus quadricauda in sewage purification. Bioresour Technol 37:121–126
Peck AJ, Hatton T (2003) Salinity and the discharge of salts from catchments in Australia. J Hydrol 272:191–202
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae - defining the polyphosphate dynamics. Water Res 43:4207–4213
Proksch E, Holleran WM, Menon GK, Elias PM, Feingold KR (1993) Barrier function regulates epidermal lipid and DNA synthesis Brit. J Dermatol 128:473–482
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
Rawles SD, Fuller SA, Beck BH, Gaylord TG, Barrows FT, McEntire ME (2013) Lysine optimization of a commercial fishmeal-free diet for hybrid striped bass (Morone chrysops x M. saxatilis). Aquaculture 396–399:89–101
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty-acid and lipid content of marine microalgae. J Phycol 30:972–979
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture 2. Effects of salinity on growth, gross-chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Richardson JTE (2011) Eta squared and partial eta squared as measures of effect size in educational research. Educa Res Rev 6:135–147
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotech Bioeng 102:100–112
Salama E-S, Abou-Shanaba RA, Kim JR, Lee S, Kim SH, Oh SE, Kim HC, Roh HS, Jeon BH (2014) The effects of salinity on the growth and biochemical properties of Chlamydomonas mexicana GU732420 cultivated in municipal wastewater. Env Technol 35:1491–1498
Setter TL, Greenway H (1979) Growth and osmoregulation of Chlorella emersonii in NaCl and neutral osmotica. Aust J Plant Physiol 6:47–60
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids Biomed. Pharmacotherapy 56:365–379
Sims GG (1978) Rapid estimation of carbohydrate in formulated fish products - protein by difference. J Sci Food Agric 29:281–284
Stephens E, Ross IL, Mussgnug JH, Wagner LD, Borowitzka MA, Posten C, Kruse O, Hankamer B (2010) Future prospects of microalgal biofuel production systems. Trends Plant Sci 15:554–564
Su CH, Chien LJ, Gomes J, Lin YS, Yu YK, Liou JS, Syu RJ (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Sudhir P, Murthy SDS (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Tiftickjian JD, Rayburn WR (1986) Nutritional requirements for sexual reproduction in Mesotaenium kramstai (Chlorophyta). J Phycol 22:1–8
Van Veldhoven PP, Mannaerts GP (1987) Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 161:45–48
Vanlerberghe GC, Brown LM (1987) Proline overproduction in cells of the green alga Nannochloris bacillaris resistant to azetidine 2 carboxylic group. Plant Cell Env 10:251–257
von Alvensleben N, Stookey K, Magnusson M, Heimann K (2013) Salinity tolerance of Picochlorum atomus and the use of salinity for contamination control by the freshwater cyanobacterium Pseudanabaena limnetica. PLoS One 8(5), e63569
Williams PJB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics energy. Env Sci 3:554–590
Xia L, Rong J, Yang H, He Q, Zhang D, Hu C (2014) NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour Technol 161:402–409 d
Zhila NO, Kalacheva GS, Volova TG (2005) Influence of nitrogen deficiency on biochemical composition of the green alga Botryococcus. J Appl Phycol 17:309–315 d
Zhila NO, Kalacheva GS, Volova TG (2011) Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kütz IPPAS H-252. J Appl Phycol 23:47–52
Zhou WG, Li YC, Min M, Hu B, Chen P, Ruan R (2011) Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour Technol 102:6909–6919
Acknowledgments
The project was supported by the Advanced Manufacturing Cooperative Research Centre (AMCRC), funded through the Australian Government’s Cooperative Research Centre Scheme, grant number 2.2.2. The funders had no role in study design, data collection and analysis or preparation of the manuscript and have provided permission to publish. This research is part of the MBD Energy Research and Development program for Biological Carbon Capture and Storage. Nicolas von Alvensleben was supported by an AMCRC PhD scholarship.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
S. Table 1
Effect of salinity and culture nutrient status (replete/deplete) on Desmodesmus armatus fatty acid profiles (FA content (mg g-1 DW)) (DOCX 18 kb)
S. Table 2
Effect of salinity and culture nutrient status (replete/deplete) on Mesotaenium sp. fatty acid profiles (FA content [mg g-1 DW]) (DOCX 17 kb)
S. Table 3
Effect of salinity and culture nutrient status (replete/deplete) on Scenedesmusquadricauda fatty acid profiles (FA content [mg g-1 DW]) (DOCX 16 kb)
S. Table 4
Effect of salinity and culture nutrient status (replete/deplete) on Tetraedron sp. fatty acid profiles (FA content [mg g-1 DW]) (DOCX 17 kb)
S. Table 5
Total lipid and total FAME productivities [mg L-1 day-1] ofDesmodesmus armatus, Mesotaeniumsp., Scenedesmus quadricauda and Tetraedron sp. at 2, 8, 11 and 18 ppt salinity. Productivities were derived from biomass productivities during the exponential growth phase. (DOCX 15 kb)
S. Table 6
Individual FAME productivities [mg L-1 day-1] ofDesmodesmus armatus, Mesotaeniumsp., Scenedesmus quadricauda and Tetraedron sp. at 2, 8, 11 and 18 ppt salinity. Productivities were derived from biomass productivities during the exponential growth phase. (DOCX 17 kb)
S. Table 7
Amino acid profiles [mg g-1 DW] of Desmodesmus armatus at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
S. Table 8
Amino acid profiles [mg g-1 DW] of Mesotaeniumsp. at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
S. Table 9
Amino acid profiles [mg g-1 DW] of Scenedesmus quadricauda at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
S. Table 10
Amino acid profiles [mg g-1 DW] of Tetraedronsp. at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
von Alvensleben, N., Magnusson, M. & Heimann, K. Salinity tolerance of four freshwater microalgal species and the effects of salinity and nutrient limitation on biochemical profiles. J Appl Phycol 28, 861–876 (2016). https://doi.org/10.1007/s10811-015-0666-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0666-6