Abstract
The objective of this study is developing microalgal culture systems to produce biodiesel with low-energy inputs in the ocean. The semi-permeable membrane photobioreactors (SPM-PBRs), which are capable of transferring nutrients dissolved in seawater into the algal broth while containing the cells inside, were operated in a reservoir containing Incheon coastal seawater in the laboratory to observe biomass dynamics, variations in nutrient concentration, pH, and salinity prior to their deployment in the ocean. A green microalga Tetraselmis sp., isolated from Incheon nearshore, was cultivated in a simulated ocean condition under continuous illumination. According to the data obtained from the experiment, microalgal growth was found to be primarily limited by the phosphorus concentration in seawater rather than other major nutrients (carbon and nitrogen) and the pH and salinity of the algal broth, which remained constant. Furthermore, we were able to understand why biomass productivity decreases as the culture progresses. N and P transfer rates through the membranes gradually decreased due to membrane fouling caused by various factors. For an enhancement of nutrient transfer rate and biomass productivity, various SPMs with different nutrient transfer rates and molecular weight cut-offs (MWCOs) were also explored. Biomass productivity increased in proportion to the nutrient transfer rate of the membranes, and the fatty acid content increased from 12 to 30 % at day 0 without a significant change in its composition. With further developments in SPM-PBRs, another technology to simultaneously produce microalgal biomass and reverse eutrophication of the ocean or lakes may be provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the past decades, human societies have been seeking renewable energy sources to replace fossil fuels as fossil reserves are limited and a large amount of fossil fuel emissions contribute to air pollution (Chisti 2007). As petroleum reserves are depleting fast, in order to reduce industry’s dependence on this source, clean technologies aimed at sustainable energy production in future need to be developed urgently (Chapman 2014). Recently, biofuels such as biodiesel and bioethanol, which are generated through photosynthesis, have attracted attention as alternatives to overcome the energy crisis and reduce the emission of greenhouse gases (GHGs) into the atmosphere (Djuric Ilic et al. 2014). Photosynthetic systems in plants and algae possess a unique ability to capture photons from sunlight and generate various type of biomolecules by assimilating CO2 (Huang et al. 2010). However, production of biofuels from edible crops such as corn, soybean, and sugarcane has its own limitations as these crops are sources of food and also require a huge area of land for cultivation (Lam and Lee 2012; Norsker et al. 2011). Among the photosynthetic organisms, microalgae have been recognized as the most promising alternative to the edible crops for biofuel production as they can double more than once per day (Um and Kim 2009), accumulate a large amount of lipids in the cell body (Tran et al. 2010), survive and thrive under harsh environment conditions (Balasubramanian et al. 2011), and require only use of marginal places (e.g., desert, ocean) for their cultivation. Despite the great potential of microalgae to produce renewable and sustainable bioenergy, the sequential processes involved in microalgal bioenergy production such as cultivation, harvesting, drying, extraction, and transesterification are not mature yet. Further, high cost and intensive energy consumption in the production process prevent microalgal bioenergy from replacing fossil fuels as the energy source. In other words, microalgal biofuel production needs to overcome hurdles, and technological advances are required in each step for commercialization (Mata et al. 2010).
The cost and energy inputs for microalgae cultivation account for approximately 40 % of the total cost in the biodiesel production process (Hu et al. 2008). Therefore, commercialization of microalgal biodiesel would depend on reducing the cost and external energy requirements in the cultivation system and maximizing of lipid and biomass productivity with the optimal cultivation strategy. If microalgae can be cultivated at sufficiently low cost in large scales, production of microalgal biofuels, which have much bigger market value than other traditional microalgae-derived products, will be able to bring enormous revenue (Borowitzka 2013; Markou and Nerantzis 2013; Vigani et al. 2015). Recently, as one way to reduce the production cost of microalgae, some attempts have been made to cultivate microalgae in photobioreactors (PBRs) which are constructed using inexpensive plastic materials (Rodolfi et al. 2009; Trent et al. 2012; Willson and Butler 2009).
Many researchers have mentioned that one of the many advantages of microalgal biodiesel production is its requirement of low land area when compared to crop-based biodiesel production (Petkov et al. 2012; Scott et al. 2010; Verma et al. 2010). Although biodiesel production from microalgae requires less area of land, a large area of land is still needed to produce a significant amount of biodiesel to replace fossil fuels. It was estimated that 1.1–2.5 % of the existing US farming area (2–4.5 Mha) should be used to cultivate microalgae for biodiesel production to meet 50 % of all transport fuel usage in the USA (Chisti 2007). However, similar to land crops requiring certain conditions for farming, microalgae also need certain conditions such as sunlight intensity and temperature for good productivity, and not all countries have large areas of land available for microalgae cultivation. For small coastal countries, the ocean, which covers 70 % of the earth’s surface, may be a better place to cultivate microalgae for biodiesel or for other commercial uses. The ocean environment provides a large area for microalgal cultivation, relatively stable temperature with its high heat capacity, culture broth mixing and circulating with waves, dissolved substances potentially usable as nutrients, and seawater that could be used as the culture medium when growing marine microalgae. In particular, the West Sea (Yellow Sea) between Korea and China is suffering from serious eutrophication caused by decades of waste dumping and sewage discharges from the two countries. For example, according to National Fishery Research and Development Institute (Busan, Korea), the levels of total inorganic nitrogen and phosphorus concentrations in Korean west coastal seawater (Incheon) have been observed to be 0.98 ± 0.78 and 0.03 ± 0.02 mg L−1, respectively, during 1999–2007. The high levels of nitrogen and phosphorus, otherwise considered as pollution, can turn into valuable nutrients for microalgal cultivation, eliminating the needs of fertilizer use.
In this study, for utilization of various marine resources in microalgal culture, semi-permeable membrane (SPM)-PBRs, capable of transferring nutrients dissolved in seawater into the PBRs while the cells are contained inside, were tested for their feasibility for microalgal biodiesel production. In addition, their biomass and biodiesel productivities were evaluated as a function of mass transfer properties of the SPMs. Recent membrane technologies have been widely applied to waste treatments, culturing and harvesting microalgae, desalination of seawater, purification of specific compounds (e.g., proteins, amino acids, chemicals), and separations (Gao and Zhang 2013; Honda et al. 2012; Jeong et al. 2010; Zhang et al. 2010; Zhao et al. 2013). Some microalgae cultivation systems that use membranes for CO2 fixation, N and P removal in various wastewaters, and biodiesel production have been reported (Honda et al. 2012; Trent et al. 2012). For the first time, the application of membrane for marine microalgal culture in ocean by absorbing essential nutrition dissolved in seawater for cells is reported in this research. We also tested SPM-PBRs with different permeability in natural seawater for investigating the possibility of microalgal cultivation. The contents and compositions of biodiesel produced from SPM-PBRs were compared in all the cases.
Materials and methods
The green microalga, Tetraselmis sp. KCTC12236BP, was locally isolated from coastal seawater in Ganghwa Island, Incheon, Korea. The natural seawater (NSW), taken from Incheon nearshore or artificial seawater (ASW), mixed with reagents, consisting of 30 g L−1 NaCl, 0.66 g L−1 KCl, 8.48 g L−1 MgCl2·6H2O, 1.9 g L−1 CaCl2·2H2O, 6.318 g L−1 MgSO4·7H2O, and 0.18 g L−1 NaHCO3, was used as the basis of culture media. For the source of nitrogen and phosphorus, f/2-Si medium, which consists of 75 mg L−1 NaNO3, 5 mg L−1 NaH2PO4·H2O, 3.15 g L−1 FeCl3·6H2O, 4.36 g L−1 Na2EDTA·2H2O, 180 mg L−1 MnCl2·4H2O, 22 mg L−1 ZnSO4·7H2O, 10 mg L−1 CoCl2·6H2O, 9.8 mg L−1 CuSO4·5H2O, and 6.3 mg L−1 Na2MoO·2H2O, was additionally added to ASW or NSW. Seed cultures were prepared by suspending a single colony from a master plate in a 250 mL Erlenmeyer flask containing 100 mL of ASW and f/2-Si medium. The seed culture flask was cultured in an illuminated shaking incubator (model KMC-8480SF, Vision Scientific Co. Ltd., Daejeon, Korea) at a constant light intensity of 50 μmol photons m−2 s−1 and then scaled up to a 2 L bubble column (BC)-PBR at a light intensity of 100 μmol photons m−2 s−1 with 2 % (v/v) continuous CO2 bubbling at 0.1 vvm (Lee et al. 2006).
Semi-permeable membranes and photobioreactor operation
SPMs made of cellulose were purchased from Spectumlabs (Spectrum Laboratories, USA). Four different tubing type membranes (product number 132725, 132675, 132566, and 132544) were used for the experiment without any modification. Specifications of the SPMs are summarized in Table 1. SPMs were immersed in deionized water for 24 h to remove impurities and to swell the membranes before use. After one side was clipped by a plastic enclosure for filling with microalgae and culture medium aseptically in a clean bench, other side was also clip-sealed (Fig. 1). Then, the prepared SPM-PBRs were submerged at 0.05 m below the seawater surface in an acrylic rectangular reservoir (1 m in length × 0.5 m in width × 0.3 m in height), which is capable of holding a maximum of 150 L water, and filled with 30 L of Incheon NSW. The seawater in the reservoir was agitated with a paddle to circulate the water and to prevent cells from settling in the PBRs. To keep nutrient gradients across the membrane, the seawater in the reservoir was replaced three times a day, and pH, temperature, and salinity were measured right before and after the replacement for keeping the culture condition and seawater quality constant. Light was supplied continuously by two 55 W fluorescent lamps 200 μmol photons m−2 s−1.
Measurement and analysis of samples
To investigate the variations in nutrients, NO3 −, NH4 +, and PO4 3− concentrations in culture broth and seawater were analyzed by an autoanalyzer (TRAACS 2000, Bran+Luebbe, Germany) after the samples were filtered through a 0.2 μm membrane syringe filter (Minisart, Sartorius Germany). The total inorganic carbon (TIC) concentrations in water were analyzed by a TOC analyzer (Multi N/C 3000, Analytical Jena, Germany). The activities of carbonate species were also calculated based on carbonate equilibrium relationship by multiplying with salinity, pH, and temperature values (Wolf-Gladrow 2001). The pH and salinity were monitored before and after replacing the seawater in the reservoir and also right after sampling by a pH meter (720P, Istek, Seoul, Korea) with an epoxy pH electrode and by an EC meter (EC-40 N, Istek, Korea) with a glass electrode.
C:N:P mass ratio in Tetraselmis sp. was determined by an elemental analyzer (Elemental Analyzer, Thermo Fisher Scientific, USA) for C, N, and an inductively coupled plasma mass spectrometer (ELAN6100, PerkinElmer, USA) for P, respectively. From the obtained mass ratio data of Tetraselmis sp., we could calculate the estimated biomass productivity in a SPM-PBR by multiplying the rate of nutrients transferred into the culture.
The cell concentration and the average cell size were preciously measured with a Coulter counter (Multisizer 3, Beckman Coulter, USA). The various data from Coulter counter were collected by Multisizer 3 software, and they were then exported to an Excel spreadsheet to calculate the cell concentration, cell size distribution, average cell size, and fresh cell weight. The ratio of fresh cell weight (gFCW) to dry cell weight was 3.34 ± 0.05.
Measurements of membrane permeability coefficient for NO3 − and PO4 3−
For an accurate estimation of nutrient transfer rate through each membrane used in the study, we calculated membrane permeability coefficient (K N and K P) for NO3 − and PO4 3−. To acquire K values, a membrane with a surface area of 0.0041 m2, containing 0.03 L of filter-sterilized NSW, was submerged in a 2.5 L rectangular reservoir containing 2 L of filtered seawater with NO3 − or PO4 3− concentrations of 0.05, 0.1, 0.2, and 0.4 g L−1. Then, variations in the nutrient levels in the membrane were observed over time with slow-stirring by a magnetic stirrer. As time passed, the nutrient concentration in SPM proportionally increased until it was in equilibrium with that in the reservoir. We could achieve a linear relationship between membrane retention time in reservoir and N or P concentration in the SPM. The rate of nutrient accumulation in SPMs can be expressed as (Lee and Hing 1989):
where V is the liquid volume inside SPM (L), K is the membrane permeability coefficient (m2 min−1), A is the area of membrane (m2), D is the thickness of membrane (m), C out and C in (nutrient concentration in and out of the SPM, respectively, with C out > C in ). After integrating Eq. (1), the K values for NO3 − and PO4 3− could be obtained by plotting ln (C out − C in ) × DV A−1 over time. K values, obtained as function of nutrient concentration in reservoir, remained fairly constant irrespective of the change in N and P concentrations in the outer reservoir. The K values are summarized in Table 2 and were used for estimating biomass productivity based on the rates of nutrients diffusing through the membrane and the mass ratio in Tetraselmis sp.
Analysis of fatty acid composition and content
Samples were pretreated for fatty acid analysis. After centrifugation, the cells were lyophilized. An aliquot amount of dried samples were dissolved in 2 mL of a freshly prepared mixture of acetyl chloride and methanol (5:100, v/v), with nonadecanoic acid (19:0) as an internal standard, to a concentration of 1 mg L−1. Vials containing samples with reactants were completely sealed before the reactions. Transesterification reaction was performed at 80 °C for 1 h under pure nitrogen gas stream and darkness. After the transesterification reaction, the converted fatty acid methyl esters were extracted by addition of 1 mL hexane. Fatty acid methyl esters (FAMEs) were analyzed by a gas chromatograph equipped with flame ionization detector (Acme 6000 GC, Younglin, Anyang, Korea) with HP-INNOW WAX column (100 m length, 0.25 mm diameter, 0.2 μm film thickness) under chromatographic conditions as follows: carrier gas, helium; flow rate, 3 mL min−1; injector temperature, 25 °C; detector temperature, 300 °C; initial temperature, 140 °C; initial duration time, 2 min; temperature elevation rate, 4 °C min−1; final temperature, 240 °C; final duration time, 5 min; and resulting in a total heating time of around 32 min. The FAMEs were identified by comparing their retention time with those of FAME standards mixture (F.A.M.E. Mix C4-C24, Supelco, USA) (Tran et al. 2010).
Experimental setup and operation
Three experiments were performed to study the biomass and biodiesel productivities in the SPM-PBRs. In the first experiment, 0.0288 m2 of SPMs (B) were used to build SPM-PBRs, and Tetraselmis sp. was cultivated in 400 mL of culture broth. In the second experiment, the effect of biofouling on the SPM-PBRs ability to supply nutrition was investigated. After the first experiment, the membrane of one SPM-PBR was replaced with new membrane, and the cultivation was continued for four more days. The effect of different mass transfer rates on the microalgal biomass and fatty acid productivity was investigated in the third experiment. SPM-PBRs were built with 0.010 m2 of four SPMs (A, B, C, and D) and contained 0.1 L of culture broth. The experiments were performed as duplicates. The durations of the first, second, and third experiments were 11, 4, and 12 days, respectively. The temperature inside the reservoir was maintained at 20 ± 1 °C throughout the experimental periods.
Results
Nitrogen and phosphorus were trace elements compared with carbon in the Incheon NSW (Table 3). Figure 2 shows concentrations of major nutrition (C, N, and P) and culture condition (pH and salinity) in and out of SPM-PBRs during the cultivation period. The pH and salinity remained constant in the range of 7.8–8.2 and 2.8–3.0 %, respectively, during the course of culture (Fig. 2a). The dominant form of CO2 was HCO3 − (over 80 % of total TIC). The dynamics of HCO3 − concentration in culture broth and seawater showed an identical pattern until day 8. TIC concentration was 1–2 mg C L−1 lower in the SPM-PBR, whereas in the surrounding seawater, it was constant at 28–30 mg C L−1 during the whole culture period (Fig. 2b).
Total inorganic nitrogen (TIN) and total inorganic phosphorus (TIP) concentrations in and out of the PBRs exhibited different characteristics from TIC. Concentrations of both ions gradually decreased as the cultivation progressed. A comparison of TIN and TIP concentrations at day 0 and day 11 shows that TIN and TIP concentrations in the culture decreased by 33 and 36 %, respectively (Fig. 2c). These changes indicate that TIN and TIP concentrations in culture are more likely to be the limiting factors. In other words, mass transfer of these ions would determine the biomass productivity.
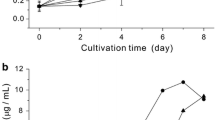
The growth of Tetraselmis sp. was dependent on the phosphorus transfer rate. Nitrogen and phosphorus accounted for 6.58 ± 1.10 and 1.05 ± 0.25 % of the microalga. Theoretical biomass productivities based on nitrogen and phosphorus transfer rates were calculated as 0.111 ± 0.021 and 0.065 ± 0.012 gFCW L−1 day−1, respectively. Figure 3 shows the theoretical and actual growth over the culture period. The overall biomass productivity was 0.07 gFCW L−1 day−1 for the cultivation period. However, daily biomass productivity decreased from 0.079 ± 0.005 to 38 ± 0.8 mg L−1 day−1 after 8 days of cultivation. The reduction in biomass productivity was caused by lowered nitrogen and phosphorus permeability (Fig. 4). K N and K P at day 11 were reduced by 26.2 ± 3.0 and 36.5 ± 3.5 %, respectively, compared with those at day 0.
Profiles of fresh cell weight cultured in natural seawater and estimated growth based on maximum N or P transfer rate through the membrane. Assumptions in estimated growth based on N or P transfer rate through the membrane. All of transferred N, P into the SPM-PBR are used up by cells and integrated into algal biomass. Mass ratios of N, P in Tetraselmis sp. were 6.58 ± 1.10 and 1.05 ± 0.25 % of their constituent, respectively. Fresh cell weight can be converted into dry cell weight by assuming cell’s water content (70 %), vice versa
In order to confirm whether decreased productivity was caused by membrane fouling or not, one of the cultures was transferred to a new SPM and cultivated in seawater for 4 days (Fig. 5). The average biomass productivity in the SPM-replaced PBR during days 11–15 was found to be 0.071 gFCW L−1 day−1, similar to the values obtained during day 0–11 and estimated productivity based on P mass transfer rate (0.065 ± 0.012 gFCW L−1 day−1), whereas that in non-replaced PBR decreased by 40.9 %. After the measurements of K N and K P at day 15, the values were further reduced by 65.5 ± 5.0 and 48.6 ± 2.9 %, respectively.
After 15 days of cultivation in seawater using SPM-PBR in the laboratory, we harvested cells from PBRs (SPM replaced and non-replaced) to analyze fatty acid composition and productivity. Figure 1 shows the SPM-PBRs at the beginning and end of cultivation. Table 4 shows fatty acid profiles, content, and productivity in Tetraselmis sp. No significant differences were seen in the fatty acid composition regardless of SPM replacement and the culture method. The composition was the same with cells grown in a 0.4 L BC-PBR (Table 4). Palmitic acid (C16:0; 28–30 %) was the dominant component, and oleic acid (C18:1, 23–27 %) was the second dominant. Other fatty acids were ranked in following order: linolenic acid (C18:3; 20–22 %), hexadecatetraenoic acid (C16:4; 11–16 %), and linoleic acid (C18:2; 7–10 %).
For enhanced biomass and fatty acid productivity, cells were cultivated in various SPM-PBRs with different nutrient transfer rates. Membrane permeability coefficients (K N and K P) were in a decreasing order from D, C, B, toward A (Table 2). However, actual N and P transfer rates were in a decreasing order from D, C, A, toward B. The volumetric biomass productivities obtained from SPMs for 11 days and estimated productivities based on N and P transfer rates are presented in Fig. 6. Biomass productivities in various SPMs obtained were 0.119 ± 0.010 (A), 0.078 ± 0.010 (B), 0.154 ± 0.029 (C), and 0.161 ± 0.016 (D) gFCW L−1 day−1, respectively. The biomass productivities were correlated with the productivities based on P transfer rates rather than N transfer rates in this experiment, same with the previous experiment. The compositions and contents of fatty acids obtained from our experiments are summarized in Table 5. Fatty acid compositions from different SPM-PBRs showed no significant differences, while fatty acid content increased to 25–27.5 % at day 15 from 11.5 % at day 0.
Discussion
Salinity, pH, and TIC concentration in the cultures were maintained at the constant levels with the NSW in the reservoir (Fig. 2a, b). Normally, in microalgal culture, cells on metabolizing carbon, nitrogen, and phosphorus ions by photosynthesis produce hydroxide and O2 as metabolites. Thus, the pH in autotrophic microalgal culture commonly starts to increase in proportion to growth rate and algal concentration in the absence of pH control or CO2 gas supplement. pH is controlled by introducing CO2-enriched air. CO2 from flue gases is sometimes utilized in conventional algal culture systems (e.g., PBRs, raceway pond) (Kim et al. 2011; Yun et al. 1996). In the SPM-PBR, pH and salinity could be maintained at the same level as the surrounding environment without making any adjustment because of the proton ion and salt concentration gradients across the membrane. The dominant form of CO2 was HCO3 − (over 80 % of total TIC), which marine microalgae can readily uptake in seawater because of its basic pH value (Fig. 2b) (Wolf-Gladrow 2001). Because of the ionic gradients formed across the membrane by carbon uptake by cells, carbon rapidly diffused into the SPM-PBR until equilibrium was reached, replenishing the carbon utilized by the cells. Although carbon mass ratio in algal cell is about 50 %, a drastic variation or decline of carbon did not occur in the SPM-PBRs due to its relative abundance compared to other ions.
Profiles of TIN and TIP concentrations in and out of the PBRs exhibited different characteristics from TIC. Unlike the TIC concentration, TIN and TIP concentrations were decreased over time (Fig. 2c). After 11 days of cultivation, the concentrations of TIN and TIP were reduced by 33 % and 36 %, respectively (Fig. 4). These variations indicate that TIN and TIP concentrations in culture are more likely to be the limiting factors. In other words, mass transfer of these ions would determine biomass productivity. The C:N:P mass ratio in Tetraselmis sp. was 47:6.5:1.05, and it would be ideal if the C:N:P mass ratio in the Incheon seawater was the same with the mass ration in the microalgae (Mandalam and Palsson 1998). However, the mass ratio of C:N:P in Incheon seawater was 970:23:2, and by comparing the mass ratios in the microalgae and seawater, we found that TIP concentration in seawater was seriously limited. It has been shown that microalgae can uptake phosphorus more than they need for algal growth (Powell et al. 2009), but in the present study, the phosphorus content in Tetraselmis sp. did not vary when they were cultivated in nutrient-sufficient medium or seawater. Therefore, we determined that biomass productivity in the SPM-PBRs is mainly determined by the transferred TIP concentration through the SPM per unit time.
The growth of Tetraselmis sp. showed a linear biomass increase in seawater containing 0.68 ± 0.04 mg N L−1 and 0.060 ± 0.004 mg P L−1 (Fig. 3). As described above, cell growth in SPM-PBRs depends upon the concentration of P. Thus, algal growth could be dependent on the amount of P transferred into the PBR assuming that transferred P is consumed by cells and integrated into the biomass. Theoretically, the maximum increase in the biomass based on the maximum P transfer rate under the given membrane properties (area and thickness), culture volume, and P concentration difference across the membrane (referred to Eq. (1)) could be written as
where P Max. is the theoretical maximum transferred P concentration through the membrane (mg P L−1), C P is the average phosphorus content in Tetraselmis sp. (%), and X Max is the theoretical maximum increase in biomass concentration by transferred P (g L−1).
The calculated maximum biomass production rate (gFCW L−1 day−1) based on N or P transfer rate obtained from Eq. (2) and N and P contents in Tetraselmis sp. were 0.111 ± 0.021 and 0.065 ± 0.012 gFCW L−1 day−1, respectively. Estimated maximum and actual growth were plotted together in Fig. 3 for a comparison. The actual growth was linear, which is typically found in culture with limited substances. This pattern in algal culture is frequently observed due to light limitation in high-density culture (Richmond 2004). However, increasing light intensity did not improve the growth further (data not shown). Moreover, a supplement of 200 μmol photons m−2 s−1 should be enough to support such a slow-growing culture, and when the cells are cultivated in a 0.4 L BC-PBR (0.9 m in long, 0.05 m in inner diameter) with enriched nutrient supplements, growth at 1 g L−1 did not decelerate in comparison with the rate at an exponential phase (data now shown). These additional evidences confirm that the culture in the SPM-PBR is in a P-limited environment. The actual biomass growth never exceeded or followed the estimated growth based on the maximum N transfer rate during the whole culture period. This clearly corresponds to the reports that emphasized the importance of P concentration in algal-bloomed seawater and inland water (Carpenter 2008; Glibert et al. 2004). Unexpectedly, daily productivity decreased after 8 days of cultivation. We suspected that a reduction in the TIN and TIP concentrations was the reason and replaced the SPM to examine if the drop in biomass productivity was a result of changes in the nutrient transfer rates of a membrane.
Biofouling, which could reduce the function of a membrane, is caused by the attachment of bacteria, microalgae, and metabolites from microorganisms on to the membrane, among other reasons (Katebian and Jiang 2013). The nutrient mass transfer of the membrane is a key determinant of biomass productivity in SPM-PBRs. Figure 4 shows a comparison of the membrane permeability coefficient for NO3 − and PO4 3− measured at day 0, 11, and 15. K N and K P at day 11 were reduced by 26.2 ± 3.0 and 36.5 ± 3.5 %, respectively, compared with those at day 0. Buckwalter et al. (2013) in their study of developing the dewatering process in microalgal culture broth using forward osmosis membrane in seawater described that the impact of ocean fouling did not affect the dewatering performance of membrane. However, this constant dewatering efficiency may result from high salt gradient between wastewater and seawater. The SPM-PBR system differs from that used by Buckwalter et al. (2013) as a low salt gradient exists across the membrane. A nutrient gradient is formed when the equilibrium of nutrient concentrations between culture broth in the SPM and the surrounding seawater is disrupted by consumption of nutrients by microalgae. Thus, the sum of nutrient gradients formed by algal activity would be much smaller than the dewatering process with municipal wastewater or medium for freshwater microalgae. In order to confirm whether decreased productivity was caused by membrane fouling or not, one of the culture broth was transferred to a new SPM and cultivated in seawater for 4 days. After replacing the membrane, biomass productivity was recovered, as 0.071 gFCW L−1 day−1, similar to the values obtained during day 0–11 and estimated productivity based on P mass transfer rate (0.065 ± 0.012 g L−1 day−1). On the other hand, biomass productivity was reduced by 40.9 % in non-replaced PBR corresponding to the reduction of K P value (36.5 ± 3.5 %). After 15 days of cultivation, values of K N and K P were further reduced by 65.5 ± 5.0 and 48.6 ± 2.9 %, respectively. These results indicate that membrane should be periodically cleaned or replaced to maintain mass transfer and to maximize biomass productivity based on membrane properties.
The fatty acid analyses of Tetraselmis sp. showed that the carbon chain lengths varied from 16 to 18, and the ratio of unsaturated and saturated fatty acid was 7:3 in all the analyses. These profiles were similar patterns in comparison with other reports regarding the same strain (Bondioli et al. 2012). For balancing between oxidative stability and low-temperature kinematic viscosity of biodiesel, high content of monounsaturated fatty acids (MUFAs) rather than polyunsaturated fatty acids (PUFAs) and saturated fatty acids (SFAs) is desired (Stansell et al. 2012). Based on this, the fatty acid composition from Tetraselmis sp. should be modified to increase the content of MUFA and decrease contents of PUFAs and SFAs. The total fatty acid content as percentage of dry cell weight showed remarkable increases: 27.5 % (non-replaced) and 29.9 % (replaced), at day 15 in comparison with 12 % obtained at day 0. Enhanced fatty acid content in Tetraselmis sp. can be explained by many reports that have studied the effects of specific nutrients limiting the growth or starvation in microalgal culture on fatty acid contents (Huang et al. 2013; Lin and Lin 2011). The concentration of nitrogen and phosphorus in algal culture has been recognized as an important parameter among various environmental factors such as temperature, pH, salinity, and CO2 affecting lipid content, fatty acid composition, growth rate, and cell density because N and P are essential substances involved in the biosynthesis of chlorophyll, DNA, RNA, protein, etc (Bondioli et al. 2012). Thus, a lack of or a low amount of these substrates in culture may lead to changes in metabolism within the cells. To N- and P-limited conditions, high lipid or carbohydrate accumulation was considered as typical responses (Cakmak et al. 2012). Furthermore, it has been often found that growth rate of algae decreased when cells started to accumulate lipids (Mujtaba et al. 2012). Although N- and P-deficient condition could induce high lipid accumulation in the algal cell body, the overall biomass and lipid productivity may be lower when cells are cultivated under the condition than under nutrient-rich conditions. Similar results on this stain were also reported by Bondioli et al. (2012). From their study, the combination of N and P starvation was found to have more influence on lipid accumulation than N starvation alone in the culture. As described earlier, the required major nutrient ratio (C:N:P) in seawater was severely unbalanced and limited for algal culture. The relative abundance of available carbon source compared to N and P in seawater would be able to drive the algal metabolism toward synthesizing hydrocarbon compounds like fatty acids rather than others. Fatty acid productivity of 6.1 mg L−1 day−1 from the SPM-PBR is low compared to that from the BC-PBR, which is 16.0 mg L−1 day−1. To improve biomass and fatty acid productivity, SPMs with different nutrient transfer rates were used in the next experiment.
Sequential experiments using the SPM demonstrated that microalgal cultivation by utilizing nutrients in seawater was feasible by means of SPM-PBRs and that phosphorus was a critical limiting factor in determining biomass productivity. The obtained results show that increased P permeability of membrane would lead to higher biomass production. For enhanced biomass and fatty acid productivity, cells were cultivated in various SPM-PBRs with different nutrient transfer rates. At this time, we started with 0.1 L working volume and 0.010 m2 of membrane area in each reactor to prevent geometrical differences between SPMs due to different width of the membranes (referred to “Materials and Methods”). In these SPMs, the permeability coefficient was not the only factor determining the mass transfer rates. Although the K N and K P values were higher in B (6–8 kDa) than in A (3.5 kDa), actual N and P transfer rates were faster in A due to the thickness of the membranes, which affects mass transfer rate as shown in Eq. (1). The SPM-PBR with membrane D was expected to have the highest biomass productivity because the nutrient transfer rates were higher than other membranes. As expected, the biomass productivities were correlated to K P values (Fig. 6). Increased nutrient transfer rates of the membranes allowed a faster delivery of nutrients so that the residual nutrients inside the SPM-PBR were replenished as soon as the cells consumed them. Eventually, membranes with higher transfer rates would yield higher biomass productivity. This result confirms that P concentration may be the key parameter for an accurate evaluation of SPM-PBR system operating in the ocean.
Increase in lipid content was found in all SPM-PBRs. This increased lipid content is also found in nutrient-depleted culture of Tetraselmis sp. (20–30 %, data not shown). Although membranes with higher nutrient permeability were used for enhanced biomass and fatty acid production, fatty acid contents were the same as shown by the results from the first deployment in seawater. Daily N and P transfer rates were 37.3 and 23.6 % higher in D than in B. However, daily transfer rate of D was still not enough to satisfy the μmax value (0.77 ± 0.03 day−1) of Tetraselmis sp. and N:P stoichiometric mass ratio was unbalanced in culture broth in spite of augmented P transfer rate. Therefore, the algal culture experienced limitation and starvation of specific nutrients, which possibly led to accumulation of carbon compounds such as lipids or carbohydrates. As expected, the maximum fatty acid productivity of 13.3 mg L−1 day−1 was obtained from membrane D, which was 2.1 times higher than the value obtained from membrane B.
For developing a system to produce biomass and biodiesel from microalgae with low-energy input in the ocean, possibility of SPM-PBRs were examined. Cultivation of Tetraselmis sp. using SPM-PBRs in NSW demonstrated that algal biomass production by transferring necessary nutrition seawater into SPM while containing the cells inside was feasible. The pH and salinity were controlled to be at constant levels as those in seawater by diffusion. Among the major nutrients (C, N, and P), P was the key nutrient to determine biomass productivity based on the mass ratio of N and P in seawater and Tetraselmis sp. Testing SPMs with different mass transfer rates showed that biomass productivity increases in proportion to the permeability of the membrane. Fatty acid compositions were not different between cells cultured in SPM-PBRs and those under optimal conditions in the laboratory. However, fatty acid contents increased to 27.5–29.9 % due to relatively limited N and P concentrations in seawater. Although SPM-PBRs were found to be feasible for the production of microalgae by absorbing pollutants related to eutrophication and algal blooms in the ocean, biomass productivity obtained in this study was still too low (Maximum productivity: 0.161 gFCW L−1 day−1 with membrane D) to produce a reasonable quantity of biomass or biodiesel. However, SPM with pore size up to 2000 kDa MWCO (the value in correspondence with 0.2 μm) could be used for microalgal cultivation without bacterial contaminations (bacterial cell size 0.2–30 μm) (Pearce 2007). Since the highest MWCO used in this study was only 50 kDa (D), theoretical nutrient transfer rate of a membrane with MWCO of 2000 kDa could be 40 times higher, assuming that the membrane properties (area and thickness) are the same with membrane D, which would greatly enhance the biomass and fatty acid productivity in the SPM-PBRs. As membrane technology has been increasingly applied in various fields, more advanced membrane suitable for microalgae cultivation are likely to be available in the near future. This study is the first step in the development of SPM-PBR systems that could enable pollutants dissolved in ocean such as C, N, and P to turn into sources of clean bioenergy. Further research on scale-up, seasonal, and regional productivity evaluation by deployments of PBR in ocean and techno-economic analysis would provide valuable information toward the realization of replacing a meaningful amount of fossil fuels with microalgal biofuels.
References
Balasubramanian L, Subramanian G, Nazeer TT, Simpson HS, Rahuman ST, Raju P (2011) Cyanobacteria cultivation in industrial wastewaters and biodiesel production from their biomass: a review. Biotechnol Appl Biochem 58:220–225
Bondioli P, Della Bella L, Rivolta G, Chini Zittelli G, Bassi N, Rodolfi L, Casini D, Prussi M, Chiaramonti D, Tredici MR (2012) Oil production by the marine microalgae Nannochloropsis sp. F&M-M24 and Tetraselmis suecica F&M-M33. Bioresour Technol 114:567–572
Borowitzka M (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25:743–756
Buckwalter P, Embaye T, Gormly S, Trent JD (2013) Dewatering microalgae by forward osmosis. Desalination 312:19–22
Cakmak T, Angun P, Ozkan AD, Cakmak Z, Olmez TT, Tekinay T (2012) Nitrogen and sulfur deprivation differentiate lipid accumulation targets of Chlamydomonas reinhardtii. Bioeng Bugs 3:343–346
Carpenter SR (2008) Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci U S A 105:11039–11040
Chapman I (2014) The end of peak oil? Why this topic is still relevant despite recent denials. Energy Policy 64:93–101
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Djuric Ilic D, Dotzauer E, Trygg L, Broman G (2014) Introduction of large-scale biofuel production in a district heating system-an opportunity for reduction of global greenhouse gas emissions. J Clean Prod 64:552–561
Gao N, Zhang S (2013) Phenolphthalein-based cardo poly(arylene ether sulfone): preparation and application to separation membranes. J Appl Polym Sci 128:1–12
Glibert PM, Heil CA, Hollander D, Revilla M, Hoare A, Alexander J, Murasko S (2004) Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar Ecol Prog Ser 280:73–83
Honda R, Boonnorat J, Chiemchaisri C, Chiemchaisri W, Yamamoto K (2012) Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour Technol 125:59–64
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Huang X, Huang Z, Wen W, Yan J (2013) Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J Appl Phycol 25:129–137
Jeong E, Kim HW, Nam JY, Ahn YT, Shin HS (2010) Effects of the hydraulic retention time on the fouling characteristics of an anaerobic membrane bioreactor for treating acidified wastewater. Desalin Water Treat 18:251–256
Katebian L, Jiang SC (2013) Marine bacterial biofilm formation and its responses to periodic hyperosmotic stress on a flat sheet membrane for seawater desalination pretreatment. J Membr Sci 425–426:182–189
Kim HW, Marcus AK, Shin JH, Rittmann BE (2011) Advanced control for photoautotrophic growth and CO2-utilization efficiency using a membrane carbonation photobioreactor (MCPBR). Environ Sci Technol 45:5032–5038
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30:673–690
Lee YK, Hing HK (1989) Supplying CO2 to photosynthetic algal cultures by diffusion through gas-permeable membranes. Appl Microbiol Biotechnol 31:298–301
Lee HS, Seo MW, Kim ZH, Lee CG (2006) Determining the best specific light uptake rates for the lumostatic cultures in bubble column photobioreactors. Enzym Microb Technol 39:447–452
Lin Q, Lin J (2011) Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour Technol 102:1615–1621
Mandalam RK, Palsson BØ (1998) Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol Bioeng 59:605–611
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283
Norsker NH, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production—a close look at the economics. Biotechnol Adv 29:24–27
Pearce G (2007) Introduction to membranes: filtration for water and wastewater treatment. Filtr Sep 44:24–27
Petkov G, Ivanova A, Iliev I, Vaseva I (2012) A critical look at the microalgae biodiesel. Eur J Lipid Sci Technol 114:103–111
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae—defining the polyphosphate dynamics. Water Res 43:4207–4213
Richmond A (2004) Biological principles of mass cultivation. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Wiley-Blackwell, Oxford, pp 125–177
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Stansell GR, Gray VM, Sym SD (2012) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24:791–801
Tran HL, Kwon JS, Kim ZH, Oh Y, Lee CG (2010) Statistical optimization of culture media for growth and lipid production of Botryococcus braunii LB572. Biotechnol Bioprocess Eng 15:277–284
Trent J, Wiley P, Tozzi S, McKuin B, Reinsch S (2012) Research spotlight: the future of biofuels: Is it in the bag? Biofuels 3:521–524
Um BH, Kim YS (2009) Review: a chance for Korea to advance algal-biodiesel technology. J Ind Eng Chem 15:1–7
Verma NM, Mehrotra S, Shukla A, Mishra BN (2010) Prospective of biodiesel production utilizing microalgae as the cell factories: a comprehensive discussion. Afr J Biotechnol 9:1402–1411
Vigani M, Parisi C, Rodríguez-Cerezo E, Barbosa MJ, Sijtsma L, Ploeg M, Enzing C (2015) Food and feed products from micro-algae: market opportunities and challenges for the EU. Trends Food Sci Technol 1-12
Willson B, Butler J (2009) Low-cost photobioreactors for production of algae-biofuels, Paper presented at AIChE annual meeting, Nashville, TN
Wolf-Gladrow Zeebe RE (2001) CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier, Amsterdam, pp 4–11
Yun YS, Park JM, Yang JW (1996) Enhancement of CO2 tolerance of Chlorella vulgaris by gradual increase of CO2 concentration. Biotechnol Tech 10:713–716
Zhang X, Hu Q, Sommerfeld M, Puruhito E, Chen Y (2010) Harvesting algal biomass for biofuels using ultrafiltration membranes. Bioresour Technol 101:5297–5304
Zhao R, Satpradit O, Rijnaarts HHM, Biesheuvel PM, van der Wal A (2013) Optimization of salt adsorption rate in membrane capacitive deionization. Water Res 47:1941–1952
Acknowledgments
This research was supported by Maritime Biotechnology Program funded by Ministry of Oceans and Fisheries of Korean Government (Project No.: PJT200255) and Manpower training program for ocean energy from the same ministry, for which authors are grateful.
In addition, Hanwool Park was supported by Global Ph.D Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project No.: 2013032623).
Author information
Authors and Affiliations
Corresponding author
Additional information
Z-Hun Kim and Hanwool Park contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, ZH., Park, H., Ryu, YJ. et al. Algal biomass and biodiesel production by utilizing the nutrients dissolved in seawater using semi-permeable membrane photobioreactors. J Appl Phycol 27, 1763–1773 (2015). https://doi.org/10.1007/s10811-015-0556-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0556-y