Abstract
γ-Aminobutyric acid (GABA) is known as an inhibitory neurotransmitter in human, while in plants, GABA is an intermediate for amino acid metabolism and also is accumulated in response to a wide range of environmental stress. In the present study, GABA accumulation in Aphanothece halophytica was increased 2-fold in mid-log phase cells grown under salt stress (2.0 M NaCl). When mid-log phase cells were subjected to changes in NaCl concentrations and pH for 4 h, the highest GABA accumulation was observed in cells adapted in medium that contained 2.0 M NaCl and that was adjusted to pH 4.0, respectively. The increase of GABA accumulation was accompanied by an increased glutamate decarboxylase activity. Addition of glutamate to growth medium stimulated GABA accumulation under acid stress but had no effect under salt stress. However, the highest GABA accumulation was detected in cells exposed to both high salt and acid stresses combined with the 5 mM glutamate supplementation with an approximately 3-fold increase as compared to the control. The unicellular A. halophytica showed a similarly high content of GABA to that of a filamentous Arthrospira platensis suggesting the possibility of genetic manipulation of the genes of A. halophytica involved in GABA synthesis to increase GABA yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
γ-Aminobutyric acid (GABA), a non-protein four-carbon amino acid, is a valuable component of the free amino acid pool that is widely distributed in nature among prokaryotes and eukaryotes (Bouche and Fromm 2004). In animals, GABA is well known as a major inhibitory neurotransmitter of the nervous system and it has several physiological functions such as hypotensive and diuretic effect (Jiang et al. 2010). GABA in plants probably plays a dual role as both a signaling molecule and a metabolite. Moreover, GABA could be involved in regulating the cytosolic pH and acts as an osmoregulator (Bouche and Fromm 2004). In plants and animals, GABA is mainly metabolized through the GABA shunt pathway which bypasses two steps of the tricarboxylic acid (TCA) cycle. The first step of this shunt is the irreversible α-decarboxylation of glutamate using a pyridoxal 5′-phosphate-dependent glutamate decarboxylase (GAD, EC 4.1.1.15). The second enzyme is GABA transaminase (GABA-T; EC 2.6.1.19) which catalyzes the reversible conversion of GABA to succinic semialdehyde. The last step is irreversible oxidation of succinic semialdehyde to succinate, catalyzed by succinic semialdehyde dehydrogenase (SSADH; EC 1.2.1.16) (Bouche and Fromm 2004; Knoop et al. 2013; Steinhauser et al. 2012; Shelp et al. 1999, 2012). The GABA shunt had been reported to be associated with various physiological responses including the regulation of cytosolic pH, nitrogen metabolism, carbon fluxes into the TCA cycle, deterrence of insects, protection against oxidative stress, osmoregulation, and signaling (Bouche and Fromm 2004).
Cyanobacteria are oxygenic photoautotrophic prokaryotes that can be found in a broad habitat range. They can use solar energy to synthesize biomass-stored chemical energy from simple inorganic compounds. In recent years, cyanobacteria have drawn much attention as a rich source of bioactive compounds and considered as one of the most promising groups of organisms to produce them (Singh et al. 2005; Rastogi et al. 2014). They are also reported as a source of renewable fuel, functional food, and secondary metabolites with potential biotechnological applications, especially in marine cyanobacteria (Nunnery et al. 2010; Khetkorn et al. 2013). The accumulation of GABA has been studied in a wide variety of plant and bacterial species but not much in cyanobacteria. Furthermore, it is rather difficult to compare results from those studies due to the use of different cyanobacterial strains and general growth conditions. Previously, we demonstrated that under high salinity, the presence of glutamate in the growth medium enhanced growth and glycine betaine accumulation of Aphanothece halophytica (Boonburapong et al. 2012). Here, we reported the accumulation of GABA and GAD activity of A. halophytica under various NaCl concentrations and pH, as well as the effect of glutamate supplementation. In addition, we also made a comparative analysis of the GABA content of A. halophytica and other five cyanobacterial strains.
Materials and methods
The halotolerant cyanobacterium, Aphanothece halophytica, originally isolated from the Dead Sea in Israel, was grown in BG11 liquid medium supplemented with Turk Island salt solution containing 0.5 M NaCl. Other cyanobacterial strains, Synechocystis sp. PCC 6803, Synechococcus sp. PCC 7942, Arthrospira platensis, Anabaena siamensis TISTR 8012, and Anabaena sp. PCC 7120, listed in Table 1 were grown in normal BG11 liquid medium. Both media were buffered with 10 mM HEPES-KOH (pH 7.6). The initial cell concentration was adjusted to an OD750 of 0.1, and cultures were grown aerobically under continuous illumination of 40 μmol photons m−2 s−1 with cool white fluorescent lamps from two sides on a rotatory shaker at 160 rpm and 30 °C.
Conditions for salt stress and pH changes
A. halophytica cells were cultured under growth condition until mid-log phase before harvesting the cells by centrifugation at 3500×g for 10 min at room temperature, washed twice with fresh medium, and resuspended in desired medium containing various NaCl concentrations and pH before collecting the cells for GABA extraction and GAD activity determination.
GABA extraction and HPLC analysis
GABA was extracted using a modification of the procedure of Bieleski and Turner (1966). Cells were collected, resuspended in 10 mM potassium phosphate citrate buffer (pH 7.6), and homogenized using ultrasonic laboratory homogenizer before the supernatant was collected and dried in a centrivap concentrator Model 7970001 (Labconco). The dried sample was extracted with 600 μL of a mixture of water:chloroform:methanol 3:5:12 (v:v:v), followed by 300 μL of chloroform and 450 μL of water before centrifugation at 10,000×g, 4 °C for 10 min. The upper water-methanol phase was collected, dried, and dissolved in 200 μL of 0.1 N HCl. The solution was filter sterilized through a 45-μm membrane before determination of GABA by HPLC. Quantification of GABA content was performed using a Shimadzu Prominence Ultra-Fast Liquid Chromatography System (Shimadzu Scientific Instruments Inc.) equipped with UV-vis detector. GABA was determined by derivatizing with O-phthalaldehyde (OPA) reagent and separated by reverse phase high-performance liquid chromatography using 4.6 × 150 mm, 5.0 μm Agilent Zorbax Eclipse AAA analytical column and 4.6 × 12.5 mm, 5.0 μm guard column (Agilent Technologies Inc.). HPLC mobile phase conditions were modified from the gradient program described by Henderson et al. (2006) to suit our HPLC and column system. OPA-derivatized amino acids were monitored at 338 nm. Purchased standards of glutamate and GABA (Sigma Chemical Co.) were used for identification and quantification (external standard method). Norvaline was used as internal standard for OPA-derivative amino acids.
Determination of glutamate decarboxylase activity
Crude enzyme was extracted from cells at mid-log phase and incubated at 30 °C for 30 min in assay reaction (total volume 200 μL) containing 50 mM potassium phosphate citrate buffer (pH 5.8), 30 mM L glutamate, 20 μM pyridoxal-5-phosphate, and 1 mM CaCl2. The reaction was terminated by boiling for 10 min before determination of produced GABA using HPLC. Glutamate decarboxylase (GAD) activity was expressed as the amount of produced GABA per min per milligram protein. The protein content was determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Statistical analysis
The results are presented as mean values of three or five replicates (mean ± SE). A one-way analysis of variance was applied to evaluate the significance of data (Prism6, USA).
Results and discussion
GABA accumulation in Aphanothece halophytica under salt stress
Growth and GABA contents of A. halophytica under normal (0.5 M NaCl) and salt stress (2 M NaCl) conditions were monitored for 30 days (Fig. 1a). Under salt stress, A. halophytica cells accumulated higher levels of GABA than under the normal condition, with an approximately 2-fold increase in maximum observed after 10 days of growth. Salt stress decreased growth rate to half of that under normal growth condition. It is apparent that the changes in GABA contents in both cells under normal and salt stress conditions occurred during the log phase of growth. The decline of GABA contents to the initial level was observed when cells entered the stationary growth phase. This suggested the operation of the active GABA shunt pathway of carbon metabolism in A. halophytica during its growth. The profile of GAD activity during the growth of A. halophytica is also shown in Fig. 1b. The activity for both cells under normal and salt stress conditions remained high throughout the entire cultivation period with a tendency of a decrease towards the stationary phase of growth. This provided further support that the operation of GABA shunt pathway in A. halophytica is likely active during its growth. At present, it is unclear why the GAD activity did not correlate with the GABA levels inside the cells. Nevertheless, it should be noted that the conditions of the GAD activity assayed in vitro might not truly represent the conditions prevailing inside the cells at different growth phase, i.e., the availability of glutamate, the pH, etc. At early and late log phases, the enzyme might not fully function in vivo in contrast to the optimal operation of the enzyme at mid-log phase giving rise to high intracellular GABA contents. Earlier work reported that activation of GAD coincided with the entry to stationary phase of growth of Escherichia coli (Castanie-Cornet et al. 1999). However, later work provided evidence that GAD-dependent system is involved in acid response of exponential phase E. coli grown in minimal medium (Šeputienė et al. 2006). Several strains of E. coli when grown in minimal medium also showed active GAD in the exponential growth phase (Bhagwat 2003).
Growth, GABA contents, and GAD activity of Aphanothece halophytica under normal and salt-stress conditions. Cells were grown in the medium containing 0.5 M NaCl (normal) or 2 M NaCl (salt stress). At various time intervals, aliquots of the culture were determined for OD750 (■, normal; □, salt stress), GABA content (upward-pointing filled triangle ▲, normal; upward-pointing blank triangle △, salt stress) and GAD activity (filled circle ●, normal; blank circle ○, salt stress). The data are means and standard errors of the means, n = 5
Effect of different NaCl concentrations and pHs on GABA accumulation and GAD activity in Aphanothece halophytica
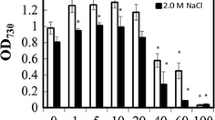
GABA accumulation was investigated under various external concentrations of NaCl and pH values. Cells were pre-cultured under the normal growth condition until mid-log phase before subjecting them to different stress conditions for 4 h. Figure 2a shows that GABA accumulation was increased about 1.7-fold when the concentration of NaCl was increased from 0.5 to 2.0 M NaCl. This result is in agreement with the previous reports in many plants showing the increased accumulation of GABA with an increase of NaCl concentration. For example, the cultivar seedling roots of soybean showed about an 11- to 17-fold increase of GABA when cells were subjected to 50–150 mM NaCl as compared with control without supplementation of NaCl (Xing et al. 2007). In tomato suspension, an increase of GABA accumulation was observed when cells were treated with 140 mM NaCl (Bolarín et al. 1995). With regard to cyanobacteria, we also found that the increase of NaCl (2–350 mM) and sorbitol (4–300 mM) in the growth medium of Synechocystis sp. PCC 6803 resulted in an increase of both GAD activity and GABA contents (Kanwal et al. 2014). When NaCl concentration was increased up to 2.5 and 3.0 M, a decrease in intracellular GABA contents of A. halophytica was observed (Fig. 2a). This was probably due to either the utilization of GABA via GABA shunt pathway under severe salt stress or gad gene was repressed or GAD enzyme activity was inhibited by high intracellular Na+. Similar observations have been reported for Synechocystis sp. PCC 6803 where salt stress by 550 mM NaCl resulted in a decrease in GABA content (Kanwal et al. 2014).
GABA accumulation in Aphanothece halophytica under various NaCl concentrations (a) and pH values (b). Cells at mid-log phase were subjected to different concentrations of NaCl and pHs and incubated for 4 h before determination of GABA accumulation. The data are means and standard errors of the means, n = 5
Changes in pH ranging from 3 to 11 were studied for the effect on GABA accumulation in A. halophytica. Figure 2b shows that acidic condition with the pH lowered to 4.0 stimulated greater accumulation of GABA than did neutral pH, the highest content of GABA was observed at pH 4.0 with an approximately 1.3-fold increase as compared to that of the normal growth condition at pH 7.6. Interestingly, GABA accumulation declined under alkaline pH despite the fact that the alkaline condition can support growth of A. halophytica with optimal growth occurring at pH 9.0. Our results of acid-induced GABA accumulation are in line with the previous results in E. coli where GAD activity was induced upon exposure to acid stress (Ma et al. 2002). Moreover, many plant cultivars showed high GAD activity and increased level of GABA in response to cytosolic acidification (Snedden et al. 1995, 1996). In cyanobacteria, there has been no report on the effect of acid stress on GABA accumulation except for the effect of low pH on cell physiology and survival of Synechocystis sp. PCC 6308 (Huang et al. 2002).
In the present study, cells at mid-log phase were treated under different NaCl concentrations and pH values for various times before determination of GABA and in vitro GAD activity by HPLC. The results demonstrated that A. halophytica slightly accumulated GABA under normal growth condition (containing 0.5 M NaCl, pH 7.6) up to 4 h (Fig. 3a). Interestingly, cells under high salt stress (2.0 M NaCl) showed significantly enhanced GABA accumulation. In contrast, cells without NaCl supplementation showed no accumulation of GABA as evidenced by a decrease in GABA content within 1 h of incubation (Fig. 3a). Surprisingly, glutamate decarboxylase (GAD), an enzyme catalyzing GABA synthesis from glutamate, was not much induced by high salt (2 M NaCl) when compared to the 0.5 M NaCl condition (Fig. 3b). This suggested that GAD activity played little or no role in GABA accumulation under high salt stress. It remains to be further investigated whether GAD is downregulated in A. halophytica under salt stress. In the absence of NaCl, GAD activity was also increased but at a lower rate than those in the presence of NaCl.
GABA accumulation and GAD activity in Aphanothece halophytica subjected to different NaCl concentrations and pHs for various times. GABA accumulation and GAD activity in mid-log phase cells adapted in medium containing different concentrations of NaCl (a, b) or different medium pH (c, d) for various times up to 4 h. The data are means and standard errors of the means, n = 5
Under acidic pH condition, GABA accumulation was strongly induced within 1 h (Fig. 3c) suggesting that GABA accumulation was a short-term adaptive response to acidic stress. Synechocystis sp. PCC 6308 stressed under acid pH medium had the ability to increase pH of the medium from 4 to 6 within 5 min, where pH 6 or above is a preferable pH for cell growth after pH stress (Huang et al. 2002). Furthermore, GAD was likely a major enzyme used for induction of GABA accumulation under acid stress in A. halophytica as evidenced by a good correlation between GAD activity and GABA accumulation when cells were stressed under acid pH for a longer time (Fig. 3d). The increased GABA accumulation in A. halophytica is a beneficial adaptive response to acid stress. The accompanying increase of GAD activity which consumes intracellular H+ under acid stress would raise the intracellular pH towards the favorable neutral pH, thus alleviating the toxic acidification inside the cells.
Exogenous glutamate can enhance GABA accumulation under acid stress
In plants, GABA is primarily synthesized through the proton-consuming GAD activity in the cytosol using glutamate as a substrate. Up to now, GABA metabolism in cyanobacteria remains elusive. In the present study, GAD activity in A. halophytica was detected as mentioned above. Previously, we showed that A. halophytica contained a sodium-dependent glutamate transporter, ApGltS, that functioned as glutamate transporter (Boonburapong et al. 2012). Thus, the presence of glutamate in the medium may induce higher GABA accumulation in A. halophytica. As expected, an increased exogenous glutamate resulted in an increased GABA accumulation when cells were under either acid stress (0.5 M NaCl, pH 4.0) or both acid and salt stress (2.0 M NaCl, pH 4.0) (Fig. 4a, panels 3 and 4). Under the latter condition, A. halophytica accumulated the highest GABA content when 5 mM glutamate was present in the medium. Supplemented glutamate, after being taken up by the cells, is unlikely to undergo the usual conversion to 2-oxoglutarate, an intermediate of the TCA cycle, by the action of a reversible alanine aminotransferase. This is supported by the fact that cyanobacteria lack the 2-oxoglutarate dehydrogenase activity (Steinhauser et al. 2012) and this necessitates the operation of GABA shunt pathway comprising GAD and GABA transaminase (GABA-T) as well as the newly discovered succinic semialdehyde dehydrogenase, SSDH (Zhang and Bryant 2011) reactions resulting in the formation of succinate which then joins the TCA cycle. The accumulated 2-oxoglutarate, because of the lack of 2-oxoglutarate dehydrogenase activity, would rather enhance the formation of glutamate and this would further increase GABA content by the GAD activity presuming that both GABA-T and SSDH were less active under acidic pH 4.0. In contrast, supplementation of glutamate at a slightly alkaline pH of 7.6 hardly affected GABA accumulation in normal cells (Fig. 4a, panel 1). This suggested that under this condition, the GABA formed by GAD activity was further processed by GABA-T and SSDH to yield succinate which then entered the usual TCA cycle. On the other hand, exogenous glutamate had little effect on an increase of GABA level under salt stress (Fig. 4a, panel 2). This was likely due to the preferential utilization of glutamate for the synthesis of glycine betaine needed as an osmolyte under salt stress. Under salt stress, a higher proportion of glutamate would be diverted to the synthesis of glycine betaine rather than to GABA synthesis via the glyoxylate pathway mediated by glutamate/glyoxylate aminotransferase leading to the synthesis of glycine (Eisenhut et al. 2008). This glycine would then undergo a 3-step methylation to form glycine betaine (Waditee et al. 2003). The increased accumulation of an osmolyte glycine betaine was previously shown in A. halophytica grown at high salinity (Reed et al. 1984; Ishitani et al. 1993). Previously we showed that the presence of high concentration of glutamate up to 50 mM in high salt medium stimulated the growth of A. halophytica and the presence of glutamate in the medium resulted in a significant increase of intracellular glycine betaine (Boonburapong et al. 2012). Taken together, it seems that glutamate could have a pivotal role in A. halophytica to be utilized for the synthesis of GABA or glycine betaine depending on the type of stress, i.e., glutamate is utilized for GABA synthesis under acid stress and for glycine betaine synthesis under salt stress.
Effect of exogenous glutamate concentration on GABA (a) and intracellular glutamate accumulation (b) of Aphanothece halophytica under normal and stress conditions. Mid-log phase cells were adapted for 4 h in medium containing 0.5 M NaCl (normal growth) or 2.0 M NaCl (salt stress) or adjusted with acidic pH (pH 4.0), or with both 2.0 M NaCl and acidic pH. The medium was supplemented with various concentrations of exogenous glutamate as indicated. The data are means and standard errors of the means, n = 5
Figure 4a, panel 3, shows that in the absence of exogenous glutamate acid stress caused lower GABA content than did salt stress shown in panel 2. However, addition of glutamate caused little change in GABA content under salt stress (panel 2) in contrast to an observed increase in GABA content under acid stress (panel 3) and under the combined salt and acid stress (panel 4). This suggested the stimulative effect of glutamate on GABA accumulation under acid stress but not under salt stress. Interestingly, cells under both high salt and acid stress supplemented with increasing glutamate concentrations revealed a much higher content of GABA than those under other conditions. The maximum GABA accumulation was 8.03 ± 0.25 nmol.g−1 DW, a 3-fold increase, when cells were subjected to both salt and acid stresses in the presence of 5 mM glutamate compared to that under normal growth condition with no glutamate supplementation (Fig. 4a).
The effect of exogenous glutamate on intracellular glutamate content of A. halophytica under different stresses was also determined. Figure 4b shows that exogenous glutamate at increasing concentration caused an increase in glutamate contents in cells under all conditions tested. It is worth noting that without glutamate supplementation cells under acid stress for 4 h accumulated high intracellular glutamate content under both normal and salt stress conditions. The increased intracellular glutamate content due to exogenous glutamate was seen in cells exposed to acid stress in the presence of 5 mM glutamate with maximum glutamate content of 1.58 ± 0.10 μmol.g−1 DW.
GABA accumulation in Aphanothece halophytica and other cyanobacteria
The accumulation of GABA has been studied in a wide variety of plant and bacterial species but not much investigation was done in cyanobacteria. Furthermore, it is rather difficult to compare results from those studies due to the use of different cyanobacterial strains, general growth conditions, and methods for analysis. In the present study six cyanobacterial strains were tested for their ability to accumulate GABA. Table 1 shows that the efficiency of GABA accumulation depends on the cyanobacterial strain. However, under normal growth condition A. halophytica has the capacity to accumulate about 2- to 4-fold more GABA than other tested cyanobacterial strains except for Arthrospira platensis. Furthermore, A. halophytica could double GABA content under salt stress. Although the GABA content in A. halophytica remains at low level, several environmental stress conditions as well as genetic manipulation of the genes related with GABA shunt pathway can be employed to further increase the efficiency of GABA accumulation in this salt-stress tolerant cyanobacterium.
Conclusion
The present study demonstrated that the halotolerant cyanobacterium Aphanothece halophytica at mid-log phase of growth accumulated GABA with a 2-fold increase under salt-stress condition. Our data also indicated that GABA accumulation was more responsive to salt stress than acid stress. Nevertheless, the highest GABA content was obtained when cells were adapted under both salt and acid stress with the supplementation of 5 mM glutamate for 4 h, an approximately 3-fold increase compared to the control.
References
Bhagwat AA (2003) Regulation of the glutamate-dependent acid-resistance system of diarrheagenic Escherichia coli strains. FEMS Microbiol Lett 227:39–45
Bieleski RL, Turner NA (1966) Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem 17:278–293
Bolarín MC, Santa-Cruz A, Cayuela E, Pérez-Alfocea F (1995) Short-term solute changes in leaves and roots of cultivated and wild tomato seedlings under salinity. J Plant Physiol 147:463–468
Boonburapong B, Laloknam S, Yamada N, Incharoensakdi A, Takabe T (2012) Sodium-dependent uptake of glutamate by novel ApGltS enhanced growth under salt stress of halotolerant cyanobacterium Aphanothece halophytica. Biosci Biotechnol Biochem 76:1702–1707
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535
Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci U S A 105:17199–17204
Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2006) Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent application note 5980-1193EN:1–10
Huang JJ, Kolodny NH, Redfearn JT, Allen MM (2002) The acid stress response of the cyanobacterium Synechocystis sp. strain PCC 6308. Arch Microbiol 177:486–493
Ishitani M, Takabe T, Kojima K, Takabe T (1993) Regulation of glycinebetaine accumulation in the halotolerant cyanobacterium Aphanothece halophytica. Funct Plant Biol 20:693–703
Jiang B, Fu Y, Zhang T (2010) Gamma-aminobutyric acid. In: Bioactive Proteins and Peptides as Functional Foods and Nutraceuticals. Wiley-Blackwell, pp 121–133
Kanwal S, Rastogi R, Incharoensakdi A (2014) Glutamate decarboxylase activity and gamma-aminobutyric acid content in Synechocystis sp. PCC 6803 under osmotic stress and different carbon sources. J Appl Phycol 26:2327–2333
Khetkorn W, Khanna N, Incharoensakdi A, Lindblad P (2013) Metabolic and genetic engineering of cyanobacteria for enhanced hydrogen production. Biofuels 4:535–561
Knoop H, Gründel M, Zilliges Y, Lehmann R, Hoffmann S, Lockau W, Steuer R (2013) flux balance analysis of cyanobacterial metabolism: the metabolic network of Synechocystis sp. PCC 6803. PLoS Comput Biol 9:e1003081
Ma Z, Richard H, Tucker DL, Conway T, Foster JW (2002) Collaborative regulation of Escherichia coli glutamate-dependent acid resistance by two AraC-like regulators, GadX and GadW (YhiW). J Bacteriol 184:7001–7012
Nunnery JK, Mevers E, Gerwick WH (2010) Biologically active secondary metabolites from marine cyanobacteria. Curr Opin Biotechnol 21:787–793
Rastogi RP, Sinha RP, Incharoensakdi A (2014) The cyanotoxin-microcystins: current overview. Rev Environ Sci Biotechnol 13:215–249
Reed RH, Richardson DL, Warr SR, Stewart WD (1984) Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol 130:1–4
Šeputienė V, Daugelavičius A, Sužiedėlis K, Sužiedėlienė E (2006) Acid response of exponentially growing Escherichia coli K-12. Microbiol Res 161:65–74
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 193–194:130–135
Singh S, Kate BN, Banerjee UC (2005) Bioactive compounds from cyanobacteria and microalgae: an overview. Crit Rev Biotechnol 25:73–95
Snedden WA, Arazi T, Fromm H, Shelp BJ (1995) Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol 108:543–549
Snedden WA, Koutsia N, Baum G, Fromm H (1996) Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J Biol Chem 271:4148–4153
Steinhauser D, Fernie AR, Araujo WL (2012) Unusual cyanobacterial TCA cycles: not broken just different. Trends Plant Sci 17:503–509
Waditee R, Tanaka Y, Aoki K, Hibino T, Jikuya H, Takano J, Takabe T, Takabe T (2003) Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica. J Biol Chem 278:4932–4942
Xing SG, Jun YB, Hau ZW, Liang LY (2007) Higher accumulation of gamma-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol Biochem 45:560–566
Zhang S, Bryant DA (2011) The tricarboxylic acid cycle in cyanobacteria. Science 334:1551–1553
Acknowledgments
This work was supported by the Office of the Higher Education Commission (OHEC), Thailand, through a grant in the program “Strategic Scholarships for Frontier Research Network for the Ph.D. program, Thai Doctoral degree,” the 90th Anniversary of Chulalongkorn University Ratchadaphiseksomphot Endowment Fund for a Ph.D. scholarship (to BB), and the National Research University project (WCU-013-FW-57) from OHEC (to AI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonburapong, B., Laloknam, S. & Incharoensakdi, A. Accumulation of gamma-aminobutyric acid in the halotolerant cyanobacterium Aphanothece halophytica under salt and acid stress. J Appl Phycol 28, 141–148 (2016). https://doi.org/10.1007/s10811-015-0523-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0523-7