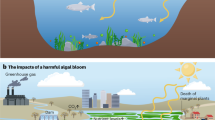
Abstract
Algal blooms have become a major concern in coastal areas and the great lakes of the world. Because of their various consequences for aquatic ecosystems and resources, algal blooms are called “harmful algal blooms” (HABs). HABs often become severely detrimental when they involve one or more toxin-producing microalgae of various taxonomic origins. The accumulation of algal biomass also has deleterious effects on the ecological status of water. However, appropriate management strategies can allow the beneficial utilization of these events by consuming the biomass feedstock in the production of valuable biocommodities, including biofuels, functional food ingredients, UV-absorbing compounds, pharmaceutical products, etc. However, if the algal biomass can be harvested prior to the onset of their death phase, nutrients (carbon, nitrogen, and phosphorus) can also be removed from the ecosystem by harvesting the algal blooms. Great progress has been made in the last decade in monitoring and predicting HABs, and a demand is emerging for persuasive postevent management policies that focus on the potential utilization of these blooms as natural renewable bioresources. This review summarizes various potential applications of nuisance algal blooms and the need for scientific research into their economic and industrial potential. Major algal products with great ecological and economic significance and their contemporary global utilization are analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Algal blooms have become a global epidemic, and the nature and frequency of harmful algal bloom (HAB) outbreaks has changed considerably over the last several decades (Anderson et al. 2012; Ferreira et al. 2011; O’Neil et al. 2012). Recently, countries including the USA, Canada, Greece, Norway, Spain, Portugal, Ireland, China, Japan, and Korea have invested considerable funds and effort into HAB management programs (Kitsiou and Karydis 2011; Shen et al. 2012; Kim et al. 2014; Latimer et al. 2014).
Various legislative authorities throughout the world have been considering the development of technologies to prevent, control, and mitigate HABs and cost-effective ways to reduce or eliminate the risks they pose. For instance, the US Congressional legislation led to the establishment of a National Research Plan for coastal HABs to monitor and assess algal blooms in the oceans, estuaries, and Great Lakes of the USA (HABHRCA 1998; Atmospheric Administration 2014). Such efforts increase our understanding of HAB patterns and interactions, and the scale of algal variability (Cloern and Jassby 2010).
In contrast, algae have huge potential as a potent bioresource, so diverting the biomass of algal blooms to other value-added applications is promising. During the last decade, algae have received increasing attention as a feedstock for pollution remediation and renewable energy production because of their growth rate, as well as their carbohydrate and lipid productivities. They can grow in saline and degraded water, they can utilize waste carbon dioxide and nutrients, and they can produce fuel precursors and high-value biochemicals (Roesijadi et al. 2008; Huesemann and Benemann 2009; Mata et al. 2009; Holdt and Kraan 2011; Kim et al. 2014). Moreover, algal secondary metabolites are biomolecules with great potential for application in various industrial sectors, including in the production of food, pharmaceuticals, and cosmetics (Cardozo et al. 2007; Cornish and Garbary 2010). One important prerequisite for the commercial use of algae is the development of large-scale culture systems with optimized high-density cell culture conditions (Huesemann and Benemann 2009). In this context, nuisance algal blooms can provide a natural, but largely untapped, renewable source of innate algal biomass. It has been estimated that the amount of algae collected from Great Lake blooms can reach thousands of tonnes a day (Zhong et al. 2012). Previous researchers have demonstrated various ways of treating this biomass, including composting and methanization, to recover nuisance algae and combat serious secondary environmental pollution (Yan et al. 2010; Zhong et al. 2012).
Since numerous studies have discussed prevention, control, and mitigation of algal blooms (Anderson 1997; Boesch et al. 1997; Anderson et al. 2012), this study focuses on the possibilities and prospects of the utilization of algal blooms as a natural resource and their various beneficial applications. We also discuss challenges for this application.
Prospects for the beneficial use of algal blooms
Algal blooms are natural phenomena and have distinct ecological significance in aquatic ecosystems. For example, spring phytoplankton blooms in temperate and polar regions are characterized by algae that are subsequently consumed by the zooplankton, ensuring the efficient transfer of energy to higher trophic levels (Rose and Caron 2007). Algal blooms are also potential sources of algal biomass, a valuable natural resource (Maddi et al. 2011). Although the overall yield of algae from large water bodies is quite low, some promising low-cost technologies have recently been reported for the recovery of algal biomass from sources such as lakes (Smith 2011). The algal biomass can be profitably utilized for other viable processes, and the possibility of obtaining bioactive compounds from algal blooms remains largely unexplored (Maddi et al. 2011).
Algal blooms as a natural resource
In general, algae have developed various defense strategies to survive in a competitive environment, which result in a significant level of structural–chemical diversity, involving different metabolic pathways (Barros et al. 2005). Both secondary and primary algal metabolisms have been studied as a prelude to their future rational economic exploitation (Cardozo et al. 2007). In this context, our need to determine how to utilize algal blooms industrially is growing. Algal blooms should be an important source of biocompounds and other industrially useful materials, able to fulfil the increasing demand for algal extracts, as fractions or pure compounds, for economic exploitation. Advances in algal biotechnology have led to significant exploration of the use of novel algal bioactive metabolites in energy, functional foods, pharmaceutical, and cosmetics industries (Waters et al. 2010; Holdt and Kraan 2011; Borowitzka 2013a).
Biofuels
Considering the various ways available for the disposal and resource utilization of algal blooms, harvesting and using them as a “green” fuel source is one of the most promising future options. An algal biomass can provide several different types of renewable biofuels when treated with thermochemical or biochemical/biological technologies, including methane produced by anaerobic digestion, biodiesel derived from algal oil, and photobiologically produced biohydrogen (Chisti 2007; Huesemann and Benemann 2009). Maddi et al. (2011) recently suggested that the nuisance algae obtained from natural blooms can be utilized for the production of liquid fuels with thermochemical methods such as hydrothermal liquefaction (Elliot et al. 2013). Algae from blooms in Maumee Bay of Lake Erie (dominated by Lyngbya spp.) were pyrolyzed in a fixed-bed reactor and the yields, and compositions of the products were compared. The results suggested that it is feasible to convert algal cultures deficient in lipids, such as nuisance algae from natural blooms, into liquid fuels. Similarly, the pyrolysis of nuisance algae (Lyngbya spp. and Cladophora spp.) obtained from eutrophic water bodies provided N-rich biochar, which is a useful competent raw material for the production of biofuels (Chaudhari et al. 2003). Cyanobacterial blooms in large lakes that arise as products of eutrophication have gained attention as promising biomass feedstocks for bio-oil production via pyrolysis. It has recently been estimated that the total potential yield of bio-oil from algal blooms in Dianchi Lake, China can reach 6,800 t year−1 (Hu et al. 2013). Euglena, a common bloom genus in freshwater bodies, has moderately higher amounts of lipids (14–20 % on a dry weight basis) than other microalgae, such as Scenedesmus obliquus (12–14 %), Dunaliella spp. (6–8 %), Spirulina spp. (4–9 %), Anabaena spp. (4–7 %), and Synechococcus spp. (11 %), which is important in the search for sustainable resources for the production of biodiesel (McMichens, anonymous). Management policies involving the application of the “biorefinery” concept could increase the economic value of algal bloom biomasses by improving their suitability for the production of bioenergy-exploiting bioremediation capacities during production and extracting high-value products from them before energy production strategies are applied (Mulbry et al. 2010; Bruhn et al. 2011). This approach would also reduce cost of nuisance algae treatments and should mitigate the eutrophication of lakes (Li et al. 2012; Hu et al. 2013).
Functional food ingredients
The algal metabolites that can be used as functional food ingredients are carotenoids, lipids, proteins, polysaccharides, and phenolics (Cornish and Garbary 2010; Ibañez and Cifuentes 2013). Among these, carotenoids have great potential utility as food colorants, feed supplements, and nutraceuticals. Carotenoids (from all sources) have a combined market value of almost US$1.2 billion (BCC Research 2011). The bloom-forming supralittoral green microalga Dunaliella salina is widely used as a natural source of high-value β-carotene (Hejazi et al. 2004; Raja et al. 2007). Its production is a substantial growth industry, and its commercial utilization is economically viable (Singh et al. 2005), with an estimated global market of US$280 million by 2015 (Ribeiro et al. 2011). β-Carotene is used in food industries as a coloring additive, an antioxidant, and provitamin A (Pisal and Lele 2005). It also has anticancer, antiaging, and immunomodulatory properties (Rock 2002). Pisal and Lele (2005) found that the β-carotene production by D. salina can be increased by various stress parameters, including cell division inhibition, nitrogen starvation, high salinity, and high irradiation with high salinity, and the highest yield (8.28 pg cell−1) was obtained with high irradiation combined with high temperature. D. salina can accumulate up to 14 % of total dry weight as β-carotene, and several commercial facilities for β-carotene production operate in Australia, Israel, and China (Borowitzka 2013b). The largest commercial production plant (∼800 ha in area) in Australia is operated by BASF (Germany) and produces β-carotene extracts and Dunaliella powder for human use and animal feeds. The prices of these products range from US$300 to US$3,000 kg−1 (Borowitzka 1992; Spolaore et al. 2006; Emeish 2012). Grung and Liaaen-Jensen (1993) have also reported a high yield of astaxanthin (75 % of the total carotenoids) from a natural bloom of Euglena sanguinea. The high content of ketocarotenoids (0.7 % of the dry weight) is characteristic of secondary carotenoid production under stressed growth conditions. The annual worldwide aquaculture market for astaxanthin is estimated to be US$226 million per year, with an average price of US$2,500 kg−1 (Hejazi and Wijffels 2004; BCC Research 2011).
Among the various complex compounds present in algae, phycobiliproteins (PBPs) stand out as natural dyes that can be used for food products. Phycobiliproteins are eco-friendly, nontoxic, and noncarcinogenic and are therefore receiving attention in preference to synthetic colorants (Chakdar et al. 2012). Because they are stable at low temperatures (Patel et al. 2004) and in acidic and basic solutions, with some preservative like citric acid (Mishra et al. 2010), they can be used as food colorants in chewing gum, jellies, and health drinks and as coloring agents in sweet confectionaries (Eriksen 2008). Dainippon Ink & Chemicals (Sakura, Japan) developed “Lina Blue,” the phycocyanin extracted from Arthrospira (Spirulina) platensis, for application in various food industries, including chewing gum, ice sherbets, popsicles, candies, soft drinks, dairy products, and wasabi (Chakdar et al. 2012). Purified phycobiliproteins are sold for about US$5,000–30,000 g−1 in a modest US$5–10 million market (Radmer 1996; Sekar and Chandramohan 2008).
The bloom-forming cyanobacterium, Aphanizomenon flos-aquae, has been marketed as a food supplement (Torres et al. 2006). However, some strains of this species have been shown to produce PSP toxins, such as saxitoxin (Pereira et al. 2008). Therefore, the appropriate monitoring and study of the toxin production profiles of various strains of bloom-forming algae is essential (Grobbelaar 2003).
UV-absorbing compounds
Mycosporine-like amino acids (MAAs) are natural compounds found in a wide variety of marine and freshwater organisms, including fungi, bacteria, cyanobacteria, phytoplankton, and macroalgae (Singh et al. 2008a; de la Coba et al. 2009). They are water-soluble, low-molecular-weight (generally <400 Da) compounds composed of either an aminocyclohexenone ring or an aminocyclohexenimine ring carrying nitrogen or amino alcohol substituents (Nakamura et al. 1982) and are particularly characterized by their high UV absorption. However, evidence is accumulating that MAAs may have additional functions: they may act as antioxidant molecules, providing some protection against the photo-oxidative stress induced by reactive oxygen species (ROS) (Dunlap and Yamamoto 1995); they can accumulate as compatible solutes following salt stress; their formation is induced by desiccation or by thermal stress in certain organisms; they may function as accessory light-harvesting pigments in photosynthesis or as an intracellular nitrogen reservoir; and they are involved in fungal reproduction (Oren and Gunde-Cimerman 2007). Furthermore, MAAs can block the formation of most cytotoxic and mutagenic DNA lesions, including cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts (6-4PP), inhibiting mutation and cell death (Misonou et al. 2003). Experiments investigating the photo-degradation and photo-sensitization of several MAAs have demonstrated their possible roles as stable and effective sunscreen compounds (Whitehead and Hedges 2005) and support their commercial exploitation as sun-care products for the protection of skin and other nonbiological materials, e.g., as photostabilizing additives in plastics, paint, and varnish (Bandaranayake 1998). Mycosporine-based sunscreen use has increased significantly in recent decades as a consequence of the perception that UV-B radiation is the main cause of skin cancer and the photoaging process (Maier and Korting 2005). The cosmetic industry has become a profitable business, generating an estimated annual turnover of US$382 billion (Leonard 2011; Łopaciuk and Łoboda 2013). The value of the cosmetic market in the USA is estimated to reach US$78.4 billion in 2016 and US$94.9 billion in 2021 (Rossi et al. 2007). With the increasing market size, this area is potentially very lucrative and the use of MAAs as highly efficient natural UV blockers in sunscreen formulations is commercially attractive (Cardozo et al. 2007; Bhatia et al. 2011). Thus, MAAs are versatile biometabolites with various types of biotechnological potential. Therefore, the large-scale acquisition of UV-absorbing biocompounds is of growing economic importance in the cosmetics and biomedical industries.
The increasing demand for algal extracts by the commercial sector can be met by exploiting the biomass of algal blooms. Algal blooms usually form floating surface scums that are exposed directly to high solar irradiance, including UV radiation. Therefore, bloom-forming algae must have evolved adaptations to protect their photosynthetic apparatus and other labile cellular constituents from photo-oxidation and direct cell damage (Paerl et al. 1983). One of their strategies to overcome the stress generated by short-wavelength radiation is the accumulation of UV-absorbing/screening compounds as a third line of defense (Singh et al. 2010). MAAs effectively dissipate the absorbed radiation energy as heat, without producing harmful effects on the cytoplasmic targets (Conde et al. 2004). Surface-bloom-forming dinoflagellates, cryptomonads, prymnesiophytes, and raphidophytes are reported to accumulate much higher concentrations of MAAs than other microalgae (Carreto et al. 2001), and the most efficient protection of blooming algae from UV damage in the Great Lakes may be provided by the constant synthesis of MAAs (Liu et al. 2004). More recently, in Kongsfjorden Bay, located in Arctic Svalbard, the total MAA concentrations were higher in the bloom areas (10.75 ± 5.01 μg L−1) than in the outer bay (6.9 ± 2.5 μg L−1) where porphyra-334, in particular, was found in very high concentrations in the bloom area, with an average concentration of 5.07 ± 3.35 μg L−1 (Ha et al. 2012). MAAs significantly facilitate the survival of blooming algae and cyanobacteria as surface scums, and massive blooms can clearly be regarded as potent natural sources of MAAs. The wide occurrence of MAAs and other UV-absorbing compounds has been reported in several bloom-forming cyanobacteria genera (Table 1). Among them, Nodularia spumigena, Nodularia baltica, and Nodularia harveyana (Sinha et al. 2003a) and A. flos-aquae (Torres et al. 2006) are potential natural sources of porphyra-334 (λ max = 334 nm). MAAs also occur in varying compositions in the bloom-forming dinoflagellates Gymnodinium sanguineum (Neale et al. 1998), Alexandrium tamarense, Alexandrium catenella, and Alexandrium minutum (Carreto et al. 2001). The MAA contents per cell and the MAA percentage ratios reported in three Alexandrium species are summarized in Table 2. It has been reported that a mixture of P-334 and shinorine can suppress aging in human skin and may thus be used in cosmetics and toiletries as UV protectants and activators of cell proliferation (Daniel et al. 2004). The ability of these compounds to prevent UV-induced skin damage in vivo (de la Coba et al. 2009) has led to their commercialization as Helioguard 365™, a formulation containing shinorine and porphyra-334 that is used in skin-care and cosmetic products (Balskus and Walash 2010).
Pharmaceutical products
In general, natural products play a valuable role in the drug discovery process (Cragg et al. 1997). Therefore, investigation of new algal secondary metabolites, a different source of natural products, has proved to be a promising area of pharmaceutical study (Cardozo et al. 2007). The bloom-forming cyanobacteria can be used as rich sources of PBPs (phycoerythrin and phycocyanin), which are used extensively as therapeutic agents in oxidative-stress-induced diseases and as fluorescent markers in biomedical research (Glazer 1994; Rodríguez-Sánchez et al. 2012). The accessory light-harvesting pigments, PBPs, are chromoproteins with anti-inflammatory, hepatoprotective, neuroprotective, and antioxidant properties, which could play crucial photodynamic roles during tumor and leukemia treatments (Mishra et al. 2011). They can also be used as fluorescent neoglycoproteins probes in fluorescent labeling of antibodies (Romay et al. 1998; Richa et al. 2011) in diagnostic kits for immunology, cell biology, and biomedical research (Sekar and Chandramohan 2008). Phycobiliprotein conjugates prepared by the covalent attachment of phycoerythrin to allophycocyanin, protein A, or avidin have been developed as fluorescent probes, with wide use in histochemistry, fluorescence microscopy, flow cytometry, and fluorescence immunoassays (Oi et al. 1982; Glazer and Stryer 1983; Glazer 1994). Their various properties, including their high molar absorbance coefficient, high fluorescence quantum yields, large Stokes’ shift, high oligomer stability, and high photostability, make phycobiliproteins very powerful and highly sensitive fluorescent reagents (Chakdar et al. 2012). Furthermore, the oxygen-radical-scavenging properties of phycobiliproteins allow them to be used to treat oxidative-stress-induced neuronal injury in neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases (Rimbau et al. 1999). The prices of phycobiliprotein products are US$3–25 mg−1 for the native pigments, but they can reach US$1,500 mg−1 for certain cross-linked pigments (with antibodies or other fluorescent molecules), and their global market was estimated to be more than US$50 million (Spolaore et al. 2006); Del Campo et al. 2007; Bux 2013). The algal toxins can bioaccumulate in the food chain to very high concentration in mollusks, fish, and other aquatic organisms, causing a health hazard for humans, domestic animals, and wildlife. Toxicological effects can include neurotoxicity, hepatotoxicity, cytotoxicity, and dermatotoxicity (Cardozo et al. 2007). On the other hand, the toxins of some of the bloom-forming algae may be used in some pharmaceutical applications (Waters et al. 2010; Zimba et al. 2010). Cardozo et al. (2007) discussed potential uses of the microcystins (protein phosphatase types 1 and 2A inhibitors), cylindrospermopsin (protein synthesis inhibitor and depletion of reduced glutathione), homo- and anatoxin-a, anatoxin-a(s) (postsynaptic nicotinic agonist and acetylcholinesterase inhibitor), saxitoxins (voltage-dependent sodium channel site 1), and domic acid (glutamate receptors) from freshwater bloom-forming algae including Microcystis, Anabaena, Oscillatoria, Alexandrium, Pseudo-nitzschia, and Nostoc. Recently, Zimba et al. (2010) also characterized the alkaloid toxin euglenophycin (inhibit the growth of the cancer cell) from the bloom-forming species E. sanguinea. Euglena species can also simultaneously produce antioxidant vitamins, such as β-carotene, l-ascorbic acid, and α-tocopherol (Takeyama et al. 1997), which have antioxidant, industrial, and medicinal applications. Moreover, Euglena species are good sources of paramylon, which has strong medicinal (used as an immunostimulant and immunopotentiator), antioxidant, antiviral, and anticancer properties (Kataoka et al. 2002; Nakashima et al. 1994; Quesada et al. 1976). For example, paramylon has recently been shown to protect against acute hepatic injury induced by carbon tetrachloride, based on its antioxidative effects, and it can inhibits the development of atopic-dermatitis-like lesions in mice (Sugiyama et al. 2010). The biggest challenge will be to harvest algal blooms for pharmaceutical applications. However, the harvest must be made prior to the algal bloom causing poisoning and even death of surrounding organisms. In addition, the target compounds must be harvested and extracted without releasing them to the surrounding waters (see “Challenges and future directions”).
Other applications
Apart from the applications described above, nuisance algal blooms can be used in numerous other ways. For instance, blooms of diatom are natural sources of siliceous shells, which are widely used as filter substances and mild abrasives. Diatomite, a porous and lightweight sedimentary rock resulting from the accumulation and compaction of diatom valves, has a range of commercial applications, including food, beverage, pharmaceutical, chemical, and agricultural industries, because of its low density, high porosity, low thermal conductivity, high melting point, and chemical inertia (Lopez et al. 2005). Diatom valves are also being developed to produce specific nanostructures with a range of applications (Chauton et al. 2014) including for use in drug delivery applications (Aw et al. 2012) and solar devices (Jeffryes et al. 2011).
Algal feeds are particularly valuable for seafood species with fastidious dietary requirements that cannot be met by formulations based on traditional agricultural commodity products, such as corn, soybeans, and fish- and food-processing by-products (Metting 1996; Borowitzka 1997). Algae also provide essential amino acids, fatty acids, or other unidentified growth factor requirements and add natural coloration to aquaculture feeds. Algae have also been proposed as an apt feed for aquaculture, allowing the development of the fatty acid profiles in fish and seafood desired by consumers (Ibañez and Cifuentes 2013).
The sale of dried algal biomass as an organic fertilizer could also provide a significant source of revenue (Mulbry et al. 2006). The use of blue-green algae as soil-conditioning supplements and as biofertilizers for rice cultivation has already been reported (Metting 1996). Algal biofertilizers can provide nitrogen and mobilize inorganic phosphates, thereby improving the soil structure. The widespread use of algal materials in lieu of inorganic fertilizers in urban/suburban areas could reduce fertilizer-related nutrient losses from these areas (Mulbry et al. 2008). However, the presence of toxin-producing algal species constitutes a great concern in this context.
Nitrogen-rich biochar obtained from the pyrolysis of eutrophic nuisance Lyngbya spp. is a useful soil supplement to improve soil quality and as feedstock for the production of valuable materials including carbon fibers, activated carbon, and carbon nanotubes (Lehmann and Joseph 2009). Other algal metabolites receiving increasing attention are the exopolymeric substances from certain cyanobacteria, which are rich in monosaccharides (Klock et al. 2007) that are useful gumlike substances in the textile and cosmetic industries. Nichols et al. (1993) reported that diatom blooms are excellent sources of essential polyunsaturated fatty acids (PUFAs, 40–50 % of total fatty acids), with potential commercial uses.
Carbon, nitrogen, and phosphorus remediation
Seasonal blooms of microscopic algae in the ocean are reported to absorb enormous quantities of CO2, much like terrestrial forests. The potential for CO2 capture by microalgae has been estimated to be 10–50 times greater than that of terrestrial plants (Li et al. 2008). In fact, excessive algal growth can contribute to the oceanic uptake of CO2, amounting globally to about one third of the CO2 emitted each year from burning fossil fuels (Sayre 2010; Mahadevan et al. 2012). However, the biggest challenge in microalgae is increasing the intervals of CO2 sequestration. Since John Martin suggested the “iron hypothesis” and said “give me half a tanker of iron, and I’ll give you an ice age,” ocean iron fertilization has been considered as a potential solution for global climate change and examined in different locations (Martin 1990; Sedwick and DiTullio 1997; Buesseler et al. 2008; Raiswell et al. 2008; Smetacek et al. 2012). Ocean iron fertilization involves stimulating net phytoplankton growth by releasing iron to certain parts of the surface ocean, such as high-nutrient low-chlorophyll regions, so that the bloom biomass sink to the deep sea floor. Thus, iron-fertilized microalgal blooms may sequester carbon for timescales of centuries in ocean bottom water and for longer in the sediments. This hypothesis is still under debate and has been reviewed by a number of researchers (see Woods Hole Oceanographic Institution 2008; Smetacek et al. 2012).
Algal blooms in coastal zones, ponds, or lakes have been considered as one of the biggest environmental problems. The sinking of algae to the bottom in shallow waters delivers biomass to microbes, with decomposition consuming oxygen, leading to hypoxia and anoxia. However, the increased intervals of CO2 sequestration may be achieved if the algal biomass can be harvested prior to the onset of their death phase and utilized in environmentally friendly ways (e.g., biofuel, food ingredients, cosmeceutical compounds, fertilizer etc.; Wosnitza and Barrantes 2006; Kuo 2010; Maddi et al. 2011). Kuo (2010) estimated that 1,350–2,700 t of nitrogen can be removed by harvesting 23,000 t of algal biomass during spring blooms (April–June) in the Gulf of Mexico. His estimate was based on tissue nitrogen contents of 5.8–11.6 % (Veldhuis et al. 2005). Considering the Redfield ratio, 9,000–18,000 t of carbon and 85–170 t of phosphorus can also be removed by harvesting algal blooms in this region. The nitrogen removal estimate of Kuo (2010) was 0.25–0.50 % of the daily nitrogen loading in the Gulf of Mexico. This modelling suggests that harvesting algal biomass during spring blooms could reduce hypoxia if, again, harvested prior to the algae death phase.
Challenges and future directions
The biggest challenge for useful applications of algal blooms is cost-effective harvesting and further concentration of the algal biomass before they cause problems in surrounding environments, so the biomass can be processed into value-added products. Microalgal harvesting methods have been extensively reviewed, and there are number of ways to harvest algae, such as sedimentation, flocculation, flotation, centrifugation, and filtration or a combination of any of these (Milledge and Heaven 2013; Pragya et al. 2013). In general, the optimal harvesting method should not be species-specific, should use as few chemicals and minimal energy as possible, and, preferably, should not release intracellular material (Chen et al. 2011). However, there are a number of difficulties facing the harvesting and recovery of microalgal biomass due to the nature of microalgal cells, low concentrations of biomass, and high costs (Pahl et al. 2013). In the case of harvesting HAB species, biomass quantity is not an implement although the other two factors remain problematic. In particular, HAB species may present problems associated with the likely release of toxins to the environment during the harvesting process, which could cause various impacts to ecosystems. In some toxin-producing cyanobacteria, microcystins can be synthesized within the cells and released to the surrounding waters when the cells are lysed or become old and leaky (Sangolkar et al. 2006). In this case, two steps of harvesting process may be used. The algal biomass can be initially concentrated by about 30-fold with a very low-cost harvesting process; then, further centration processing can be conducted on land afterward. The cell lysis may occur during the concentration process, therefore no negative impact to the ecosystem from the harvesting and concentration process (Huesemann and Benemann 2009). Despite the fact that harvesting can never be the simple solution to mitigate HAB events, a wide range of technologies have been employed to treat water sources affected by cyanobacterial toxins, including the use of powdered activated carbon (PAC), coagulation/clarification, etc. However, these conventional treatments are typically only effective when the problem of cyanobacterial toxin is relatively minor (Ghernaout et al. 2010). It has been suggested that the highest level of removal of unwanted materials can be achieved by postclarification ozonation followed by granulated activated carbon filtration (Ghernaout et al. 2010). Other promising technologies to remove variable cyanobacterial loading that may prove to be cost-effective includes dissolved air floation and titanium oxide/UV oxidation combined with membrane filtration (Ghernaout et al. 2010).
Recently, electronic yarns have been synthesized using bovine serum albumin as the electrostatic glue between commercial yarns and graphene oxide (GO) (Yun et al. 2013). Electronic yarns are considered as the flexible conducting wires for wearable electronics. Moreover, a GO/nylon-6 fabric composed of randomly oriented nanofibers was also fabricated using an electro-spinning method (Kim et al. 2012). Since the diameter of nanofiber can be controlled by an applied bias voltage during electrospinning, it is possible to synthesize the fabric with the various pore sizes. In this respect, physical adsorption of algae may be achieved by a porosity control. Moreover, chemisorption of algae using the GO-based fabric is also expected because GO easily interacts with different functional groups.
After technical feasibility, economics is the critical issue. Even when considering the most efficient algae biomass production methods and the current technology of biomass to oil conversion rate (∼33 %), the price of algae derived biofuels has been estimated to be over 20 times higher than the current oil price (Huesemann and Benemann 2009). If harvested from algal blooms, the production cost may be significantly reduced; however, the harvesting costs should be very high because the occurrence of algal blooms may be sporadic and its continued use may not be feasible. Therefore, future studies should focus on cost- and technology-effective methods that are appropriate for harvesting algal cells under specific bloom conditions.
Conclusions
Algae have several existing and potential applications, ranging from human and animal nutrition to cosmetics manufacture and the production of high-value molecules such as fatty acids, pigments, and other biometabolites. Among the 10,000 species of microalgae thought to exist, only a few thousand strains are kept in collections, the chemical contents of a few hundred have been investigated, and just a handful are cultured in industrial quantities for various purposes (Olaizola 2003). Currently, most microalgal production occurs in outdoor culture, together with the closed-system commercialization of Haematococcus in Japan and Israel and of Chlorella in Germany (Spolaore et al. 2006). The most striking aspect of algal blooms is the large quantity of biomass that is generated naturally. The ecological, economic, and health risks of algal blooms have been widely studied in recent decades, with particular attention to their toxic effects on other aquatic organisms and humans (Anderson 1997; Boesch et al. 1997; Anderson et al. 2012). However, very few attempts have been made to explore the beneficial aspects of these phenomena. It seems that harvesting algal blooms is a versatile approach that will not only provide an abundance of biomass but will also counter eutrophication and restore the esthetic qualities of natural water bodies. Nuisance algal blooms can thus be utilized potentially as solar-powered natural cell factories for the production of various metabolites with enormous economic value. However, this aspect of algal blooms is still largely unconsidered and unexplored. Therefore, more comprehensive research is required to effectively utilize algal blooms.
References
Anderson DM (1997) Turning back the harmful red tide. Nature 388:513–514
Anderson DM, Cembella AD, Hallegraeff GM (2012) Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annu Rev Mar Sci 4:143–176
Aw MS, Simovic S, Yu Y, Addai-Mensah J, Losic D (2012) Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol 223:52–58
Balskus EP, Walash CT (2010) The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329:1653–1656
Bandaranayake WM (1998) Mycosporines: are they nature’s sunscreens? Nat Prod Rep 15:159–172
Barros MP, Pinto E, Sigaud-Kutner TCS, Cardozo KHM, Colepicolo P (2005) Rhythmicity and oxidative/nitrosative stress in algae. Biol Rhythm Res 36:67–82
BCC Research (2011) The global market for carotenoids. http://www.bccresearch.com/report/carotenoids-global-market-fod025d.html. Accessed 3 Sept 2014
Bhandari RR, Sharma PK (2011) Photosynthetic and biochemical characterization of pigments and UV-absorbing compounds in Phormidium tenue due to UV-B radiation. J Appl Phycol 23:283–292
Bhatia S, Garg A, Sharma K, Kumar S, Sharma A, Purohit AP (2011) Mycosporine and mycosporine-like amino acids: a paramount tool against ultra violet irradiation. Pharmacol Rev 5:138–146
Boesch DF, Anderson DM, Horner RA, Shumway SE, Tester PA, Whitledge TE (1997) Harmful algal blooms in coastal waters: options for prevention, control, and mitigation. NOAA Coastal Ocean Program Decision Analysis Series No. 10. NOAA Coastal Ocean Office, Silver Spring, MD. 46 pp + appendix
Böhm GA, Pfleiderer W, Böger P, Scherer S (1995) Structure of a novel pigment from the terrestrial cyanobacterium Nostoc commune. J Biol Chem 270:8536–8539
Borowitzka MA (1992) Algal biotechnology products and processes-matching science and economics. J Appl Phycol 4:267–279
Borowitzka MA (1997) Algae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Borowitzka MA (2013a) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Borowitzka MA (2013b) Dunaliella: Biology, production, and markets. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. John Wiley & Sons, Ltd, pp 359–368
Bruhn A, Dahl J, Nielsen HB, Nikolaisen L, Rasmussen MB, Markager S, Olesen B, Arias C, Jensen PD (2011) Bioenergy potential of Ulva lactuca: biomass yield, methane production and combustion. Biores Technol 102:2595–2604
Buesseler KO, Doney SC, Karl DM, Boyd PW, Fei C, Coale KH, De Baar JWH, Falkowski PG, Johnson KS, Lampitt RS, Michaels AF, Nagvi SWA, Smetacek V, Takeda S, Watson AJ (2008) Ocean iron fertilization—moving forward in a sea of uncertainty. Science 319:162
Bux F (2013) Biotechnological applications of microalgae: biodiesel and value-added products. CRC, Boca Raton
Cardozo KH, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E (2007) Metabolites from algae with economical impact. Comp Biochem Physiol C 146:60–78
Carreto JI, Carignan MO, Montoya NG (2001) Comparative studies on mycosporine-like amino acids, paralytic shellfish toxins and pigment profiles of the toxic dinoflagellates Alexandrium tamarense, A. catenella, and A. minimum. Mar Ecol Prog Ser 223:49–60
Chakdar HC, Jadhav SD, Dhar DW, Pabbi S (2012) Potential applications of blue green algae. J Sci Ind Res 71:13–20
Chaudhari TS, Dalai KA, Bakhshi NN (2003) Production of hydrogen and/or syngas via steam gasification of biomass-derived chars. Energy Fuels 17:1062–1067
Chauton MS, Skolem LMB, Olsen LM, Vullum PE, Walmsley J, Vadstein O (2014) Titanium uptake and incorporation into silica nanostructures by the diatom Pinnularia sp. (Bacillariophyceae). J Appl Phycol doi:10.1007/s10811-014-0373-8:1–10
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Cloern JE, Jassby AD (2010) Patterns and scales of phytoplankton variability in estuarine-coastal ecosystems. Estuar Coast Shelf Sci 33:230–241
Conde FR, Churio MS, Previtali CM (2004) The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem Photobiol Sci 3:960–967
Cornish ML, Garbary DJ (2010) Antioxidants from macroalgae: potential applications in human health and nutrition. Algae 25:155–171
Cragg GM, Newman DJ, Snader KM (1997) Natural products in drug discovery and development. J Nat Prod 60:52–60
Daniel S, Cornelia S, Fred Z (2004) UV-A sunscreen from red algae for protection against premature skin aging. Cosmet Toilet Manuf Worldwide pp. 39–143
de la Coba F, Aguilera J, de Gálvez MV, Álvarez M, Gallego E, Figueroa FL, Herrera E (2009) Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J Dermatol Sci 55:161–169
Del Campo JA, García-Gonzáles M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Dunlap WC, Yamamoto Y (1995) Small-molecule antioxidants in marine organisms: antioxidant activity of mycosporine-glycine. Comp Biochem Physiol B 112:105–114
Elliott DC, Neuenschwander GC, Hart TR (2013) Hydroprocessing biooil and products separation for coke production. ACS Sustain Chem Eng 1:389–392
Emeish S (2012) Production of natural β-carotene from Dunaliella living in the Dead Sea. Jordan J Earth Env Sci 4:23–27
Eriksen NT (2008) Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
Ferreira JG, Andersen JH, Borja A, Bricker SB, Camp J, da Silva MC, Garces E, Heiskanen AS, Humborg C, Ignatiades L, Lancelot C, Menesguen A, Tett P, Hoepffner N, Claussen U (2011) Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar Coast Shelf Sci 93:117–131
Ferroni L, Klisch M, Pancaldi S, Häder D-P (2010) Complementary UV-absorption of mycosporine-like amino acids and scytonemin is responsible for the UV-insensitivity of photosynthesis in Nostoc flagelliforme. Mar Drugs 8:106–121
Garcia-Pichel F, Castenholz R (1993) Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl Environ Microbiol 59:163–169
Ghernaout B, Ghernaout D, Saiba A (2010) Algae and cyanotoxins removal by coagulation/flocculation: a review. Desal Water Treat 20:133–143
Glazer AN (1994) Phycobiliproteins—a family of valuable, widely used fluorophores. J Appl Phycol 6:105–112
Glazer AN, Stryer L (1983) Fluorescent tandem phycobiliprotein conjugates. Emission wavelength shifting by energy transfer. Biophys J 43:383–386
Grobbelaar JU (2003) Quality control and assurance: crucial for the sustainability of the applied phycology industry. J Appl Phycol 15:209–215
Grung M, Liaaen-Jensen S (1993) Algal carotenoids 52; secondary carotenoids of algae 3; carotenoids in a natural bloom of Euglena sanguinea. Biochem Systemat Ecolol 21:757–763
Ha S-Y, Kim Y-N, Park M-O, Kang S-H, Kim H-C, Shin KH (2012) Production of mycosporine-like amino acids of in situ phytoplankton community in Kongsfjorden, Svalbard arctic. J Photochem Photobiol B114:1–14
HABHRCA (1998) Harmful algal blooms and Hypoxia Research and Control Act of 1998. US Congress, Washington
Hejazi MA, Holwerda E, Wijffels RH (2004) Milking microalga Dunaliella salina for beta-carotene production in two-phase bioreactors. Biotechnol Bioeng 85:475–481
Hejazi MA, Wijffels RH (2004) Milking of microalgae. Trends Biotechnol 22:189–194
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed; functional food applications and legislation. J Appl Phycol 23:543–597
Hu Z, Zheng Y, Yan F, Xiao B, Liu S (2013) Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): product distribution and bio-oil characterization. Energy 52:119–125
Huesemann MH, Benemann JR (2009) Biofuels from microalgae: review of products, processes and potential, with special focus on Dunaliella sp. In: Ben-Amotz A, Polle JEW, Subba Rao DV (eds) The alga Dunaliella: biodiversity, physiology, genomics, and biotechnology. Science, New Hampshire, pp 445–474
Jeffryes C, Campbell J, Li H, Jiao J, Rorrer G (2011) The potential of diatom nanobiotechnology for applications in solar cells, batteries, and electroluminescent devices. Energy Env Sci 4:3930–3941
Ibañez E, Cifuentes A (2013) Benefits of using algae as natural sources of functional ingredients. J Sci Food Agricult 93:703–709
Karsten U, Maier J, Garcia-Pichel F (1998) Seasonality in UV-absorbing compounds of cyanobacterial mat communities from an intertidal mangrove flat. Aquat Microbl Ecol 16:37–44
Kataoka K, Muta T, Yamazaki S, Takeshige K (2002) Activation of macrophages by linear (1→3)-beta-d-glucans. Implications for the recognition of fungi by innate immunity. J Biol Chem 277:36825–36831
Kim CH, Kim B-H, Yang KS (2012) TiO2 nanoparticles loaded on graphene/carbon composite nanofibers by electrospinning for increased photocatalysis. Carbon 50:2472–2481
Kim JK, Kraemer GP, Yarish C (2014) Field scale evaluation of seaweed aquaculture as a nutrient bioextraction strategy in Long Island Sound and the Bronx River Estuary. Aquaculture 433:148–156
Kitsiou D, Karydis M (2011) Coastal marine eutrophication assessment: a review on data analysis. Environ Internat 37:778–801
Klock J-H, Wieland A, Seifert R, Michaelis W (2007) Extracellular polymeric substances (EPS) from cyanobacterialmats: characterization and isolation method optimization. Mar Biol 152:1077–1085
Kuo CT (2010) Harvesting natural algal blooms for concurrent biofuel production and hypoxia mitigation. MS Thesis, University of Illinois at Urbana-Champaign
Latimer JS, Tedesco MA, Swanson RL, Yarish C, Stacey PE, Garza C (2014) Long island sound: prospects for the urban sea. Springer, New York, 558 pp
Lehmann J, Joseph S (eds) (2009) Earthscan. UK, London
Leonard C (2011) Global beauty industry trends 2011, Skin Inc. http://www.skininc.com/spabusiness/trends/126516783.html. Accessed 3 Sept 2014 http://www.skininc.com/magazine/
Li Y, Horsman M, Wu N, Lan CQ, Dubios-Calero N (2008) Biofuels from microalgae. Biotechnol Prog 24:815–820
Li R, Zhong Z, Jin B, Zheng A (2012) Selection of temperature for bio-oil production from pyrolysis of algae from lake blooms. Energy Fuels 26:2996–3002
Liu Z, Häder D-P, Sommaruga R (2004) Occurrence of mycosporine-like amino acids (MAAs) in the bloom-forming cyanobacterium Microcystis aeruginosa. J Plankton Res 26:963–966
Łopaciuk A, Łoboda M (2013) Global beauty industry trends in the 21st century. Management, knowledge and learning international conference 2013. Active Citizenship by Knowledge Management & Innovation. http://www.toknowpress.net/ISBN/978-961-6914-02-4/papers/ML13-365.pdf. . Accessed 3 Sept 2014
Lopez PJ, Desclés J, Allen AE, Bowler C (2005) Prospects in diatom research. Curr Opin Biotechnol 16:180–186
Maddi B, Viamajala S, Varanasi S (2011) Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Biores Technol 102:11018–11026
Mahadevan A, D’Asaro E, Lee C, Perry MJ (2012) Eddy-driven stratification initiates North Atlantic spring phytoplankton blooms. Science 337:54–58
Maier T, Korting HC (2005) Sunscreens—which and what for? Skin Pharmacol Physiol 18:253–262
Martin JH (1990) Glacial-interglacial CO2 changes: the iron hypothesis. Paleoceanography 5:1–13
Mata TM, Martins AA, Caetano N (2009) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Matsui K, Nazifi E, Kunita S, Wada N, Matsugo S, Sakamoto T (2011) Novel glycosylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc commune. J Photochem Photobiol B 105:81–89
Metting B (1996) Biodiversity and application of microalgae. J Ind Microbiol 17:477–489
Milledge JJ, Heaven S (2013) A review of the harvesting of micro-algae for biofuel production. Rev Env Sci Biotech 12:165–178
Mishra SK, Shrivastav A, Mishra S (2010) Effect of preservatives for food grade C-phycoerythrin isolated from marine cyanobacteria Pseudanabaena sp. Int J Biol Macromol 47:597–602
Mishra SK, Shrivastav A, Mishra S (2011) Preparation of highly purified C-phycoerythrin from marine cyanobacterium Pseudanabaena sp. Prot Express Purif 80:234–238
Misonou T, Saitoh J, Oshiba S, Tokitomo Y, Maegawa M, Inoue Y, Hori H, Sakurai T (2003) UV absorbing substance in the red alga Porphyra yezoensis(Bangiales, Rhodophyta) blocks thymine photodimer production. Mar Biotechnol 5:194–200
Mulbry W, Kondrad KP (2010) Scrubbing the bay: nutrient removal using small algal turf scrubbers on Chesapeake Bay tributaries. Ecol Eng 36:536–541
Mulbry W, Kondrad S, Pizarro C, Kebede-Westhead E (2008) Treatment of dairy manure effluent using freshwater algae: algal productivity and recovery of manure nutrients using pilot-scale algal turf scrubbers. Biores Technol 99:8137–8142
Mulbry W, Kondrad S, Pizarro P (2006) Biofertilizers from algal treatment of dairy and swine manure effluents: characterization of algal biomass as slow release fertilizer. J Veg Sci 12:107–125
Mushir S, Fatma T (2011) Ultraviolet radiation-absorbing mycosporine-like amino acids in cyanobacterium Aulosira fertilissima: environmental perspective and characterization. Curr Res J Biol Sci 3:165–171
Nakamura H, Kobayashi J, Hirata Y (1982) Separation of mycosporine-like amino acids in marine organisms using reverse-phase high performance liquid chromatography. J Chromatogr 250:113–118
Nakashima H, Ohshiro Y, Miyano N, Yamamoto N, Ichikawa S, Kondo H, Takeda M, Sakagami H (1994) Synergistic inhibition of human immunodeficiency virus type 1 (HIV-1) replication in vitro by sulphated paramylon and 3′-azido-2′,3′-dideoxythymidine (AZT). Lett Appl Microbiol 18:24–16
Neale PJ, Banaszak AT, Jarriel CR (1998) Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): mycosporine-like amino acids protect against inhibition of photosynthesis. J Phycol 34:928–938
Nichols DS, Nichols PD, Sullivan CW (1993) Fatty acid, sterol and hydrocarbon composition of Antarctic sea ice diatom communities during the spring bloom in McMurdo Sound. Antarctic Sci 5:271–278
NOAA (National Oceanic and Atmospheric Administration) (2014) Economic impacts of harmful algal blooms. http://www.cop.noaa.gov/stressors/extremeevents/hab/current/econimpact_08.pdf. Accessed 3 Sept 2014
Oi VT, Glazer AN, Stryer L (1982) Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol 93:981–986
Olaizola M (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol Eng 20:459–466
O’Neil JM, Davis TW, Burford MA, Gobler CJ (2012) The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14:313–334
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269:1–10
Oren A (1997) Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol J 14:231–240
Paerl HW, Tucker J, Bland PT (1983) Carotenoid enhancement and its role in maintaining blue-green algal (Microcystis aeruginosa) surface blooms. Limnol Oceanogr 28:847–857
Pahl S, Lee A, Kalaitzidis T, Ashman P, Sathe S, Lewis D (2013) Harvesting, thickening and dewatering microalgae biomass algae for biofuels and energy. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 165–185
Patel A, Pawar R, Mishra S, Sonawane S, Ghosh PK (2004) Kinetic studies on thermal denaturation of C-phycocyanin. Ind J Biochem Biophys 41:254–257
Pereira P, Onodera H, Andrinolo D, Franca S, Araújo F, Lagos N, Oshima Y (2008) Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae, isolated from Montargil reservoir, Portugal. Toxicon 8:1689–1702
Pisal DS, Lele SS (2005) Carotenoid production from microalga, Dunaliella salina. Ind J Biotechnol 4:476–483
Ploutno A, Carmeli S (2008) Prenostodione, a novel UV-absorbing metabolite from a natural bloom of the cyanobacterium Nostoc species. J Nat Prod 64:544–545
Pragya N, Pandey KK, Sahoo PK (2013) A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew Sustain Energy Rev 24:159–171
Quesada A, Vincent WF, Lean DRS (1999) Community and pigment structure of Arctic cyanobacterial assemblages: the occurrence and distribution of UV-absorbing compounds. FEMS Microbiol Ecol 28:315–323
Quesada LA, De-Lustig ES, Marechal LR, Belocopitow E (1976) Antitumor activity of paramylon on sarcoma-180 in mice. Gan Gann 67:455–459
Radmer RJ (1996) Algal diversity and commercial algal products. Mar Biotechnol 46:263–270
Raiswell R, Benning LG, Tranter M, Tulaczyk S (2008) Bioavailable iron in the Southern Ocean: the significance of the iceberg conveyor belt. Geochem Transact 9:7–15
Raja R, Hemaiswarya S, Rengasamy R (2007) Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biotechnol 74:517–523
Rastogi RP, Richa SSP, HäderD-P SRP (2010) Mycosporine-like amino acids profile and their activity under PAR and UVR in a hot-spring cyanobacterium Scytonema sp. HKAR-3. Aust J Bot 58:286–293
Ribeiro BD, Berreto DW, Coelho AAZ (2011) Technological aspects of β-carotene production. Food Bioprocess Technol 4:693–701
Richa RRP, Kumari S, Singh KL, Kannuajiya VK, Singh G, Kesheri M, Sinha RP (2011) Biotechnological potential of mycosporine-like amino acids and phycobiliproteins of cyanobacterial origin. Biotechnol Bioinform Bioeng 1:159–171
Rimbau V, Camins A, Romay C, Gonzalez R, Pallas M (1999) Protective effect of C-phycocyanin against kainic acid induced neuronal damage in rat hippocampus. Neurosci Lett 276:75–78
Rock CL (2002) Carotenoids and cervical, breast, ovarian and colorectal cancer. Epidemiology and clinical trials. Pure Appl Chem 74:1451–1459
Rodríguez-Sánchez R, Ortiz-Butrón R, Blas-Valdivia V, Hernández-García A, Cano-Europa E (2012) Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. Food Chem 135:2359–2365
Roesijadi G, Copping AE, Huesemann MH, Forster J, Benemann JR (2008) Techno-economic feasibility analysis of offshore seaweed farming for bioenergy and biobased products. Battelle Pacific Northwest Division Report, number PNWD-3931
Romay C, Armesto J, Remirez D, González R, Ledon N, García I (1998) Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflam Res 47:36–41
Rose JM, Caron DA (2007) Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnol Oceanogr 52:886–895
Rossi E, Prlic A, Hoffman R (2007) A study of the European cosmetics industry. Final report prepared for the European Commission, Directorate General for Enterprise and Industry
Sangolkar NL, Maske SS, Chakrabarti T (2006) Methods for determining microcystins (peptide hepatotoxins) and microcystin-producing cyanobacteria. Water Res 40:3485–3496
Sayre R (2010) Microalgae: the potential for carbon capture. Bioscience 60:722–727
Sedwick PN, DiTullio GR (1997) Regulation of algal blooms in Antarctic shelf waters by the release of iron from melting sea ice. Geophys Res Lett 24:2515–2518
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Shen L, Xu H, Guo X (2012) Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors 12:7778–7803
Singh G, Babele PK, Sinha RP, Tyagi MB, Kumar A (2013) Enzymatic and non-enzymatic defense mechanisms against ultraviolet-B radiation in two Anabaena species. Process Biochem 48:796–802
Singh S, Bhushan KN, Banerjee UC (2005) Bioactive compounds from cyanobacteria and microalgae: an overview. Crit Rev Biotechnol 25:73–95
Singh SP, Häder D-P, Sinha RP (2010) Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies. Age Res Rev 9:79–90
Singh SP, Kumari S, Rastogi RP, Singh KL, Sinha RP (2008a) Mycosporine-like amino acids (MAAs): chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Ind J Exp Biol 46:7–17
Singh SP, Klisch M, Sinha RP, Häder D-P (2008b) Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochem Photobiol 84:1500–1505
Sinha RP, Ambasht NK, Sinha JP, Häder D-P (2003a) Wavelength dependent induction of a mycosporine-like amino acid in a rice-field cyanobacterium, Nostoc commune: role of inhibitors and salt stress. Photochem Photobiol Sci 2:171–176
Sinha RP, Ambasht NK, Sinha JP, Klisch M, Häder D-P (2003b) UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacteria). J Photochem Photobiol B 71:51–58
Sinha RP, Klisch M, Häder D-P (1999) Induction of a mycosporine-like amino acid (MAA) in the rice-field cyanobacterium Anabaena sp. by UV irradiation. J Photochem Photobiol B 52:59–64
Smetacek V, Klaas C, Strass VH, Assmy P (2012) Deep carbon export from a southern ocean iron-fertilized diatom bloom. Nature 487:313–319
Smith AS (2011) Harmful algal blooms: action plans for scientific solutions. Committee on Science, Space, and Technology. US Government Printing Office, Washington
Sommaruga R, Chen Y, Liu Z (2009) Multiple strategies of bloom-forming Microcystis to minimize damage by solar ultraviolet radiation in surface waters. Microbiol Ecol 57:667–674
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Subramanian A, Carpenter EJ, Karentz DFalkowski PG (1999) Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol Oceanogr 44:608–617
Sugiyama A, Hata S, Suzuki K, Yoshida E, Nakano R, Mitra S, Arashida R, Yet A (2010) Oral administration of paramylon, a β-1,3-d-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J Veter Med Sci 72:755–763
Takeyama H, Kanamaru A, Yoshino Y, Kakuta H, Kawamura Y, Matsunaga T (1997) Production of antioxidant vitamins, β-carotene, vitamin C, and vitamin E by two-step culture of Euglena gracilis Z. Biotechnol Bioeng 53:185–190
Torres A, Enk CD, Hochberg M, Srebnik M (2006) Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem Photobiol Sci 5:432–435
Veldhuis MJW, Timmermans KR, Croot P, van der Wagt B (2005) Picophytoplankton; a comparative study of their biochemical composition and photosynthetic properties. J Sea Res 53:7–24
Waters L, Hill RT, Place AR, Hamann MT (2010) The expanding role of marine microbes in pharmaceutical development. Curr Opin Biotechnol 21:780–786
Whitehead K, Hedges JI (2005) Photodegradation and photosensitization of mycosporine-like amino acids. J Photochem Photobiol B 80:115–121
Woods Hole Oceanographic Institution (2008) Should we fertilize the ocean to reduce greenhouse gasses? Oceanus 46
Wosnitza TMA, Barrantes JG (2006) Utilization of seaweed Ulva sp. in Paracas Bay (Peru): experimenting with compost. J Appl Phycol 18:27–31
Yan Q, Zhao MX, Miao HF, Ruan WQ, Song RT (2010) Coupling of the hydrogen and polyhydroxyalkanoates (PHA) production through anaerobic digestion from Taihu blue algae. Biores Technol 101:4508–4512
Yun YJ, Hong WG, Kim WJ, Jun Y, Kim BH (2013) A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv Materials 25:5701–5705
Zhong W, Zhang Z, Luo Y, Qiao W, Xiao M, Zhang M (2012) Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour Technol 114:281–286
Zimba PV, Moeller PD, Beauchesne K, Lane HE, Triemer RE (2010) Identification of euglenophycin—a toxin found in certain euglenoids. Toxicon 55:100–104
This work was supported by Incheon National University and Future Marine Project of Ministry of Oceans and Fisheries.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.K., Kottuparambil, S., Moh, S.H. et al. Potential applications of nuisance microalgae blooms. J Appl Phycol 27, 1223–1234 (2015). https://doi.org/10.1007/s10811-014-0410-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0410-7