Abstract
Harmful cyanobacterial blooms, which force us to develop economical and environmental friendly ways for eutrophication control, are recognized as a nuisance that cause negative impacts on natural resources and humans. In the current study, strain Bacillus amyloliquefaciens T1 with anti-cyanobacterial activity against bloom-forming cyanobacterium Microcystis aeruginosa 905 was isolated from an eutrophication pond in Wuhan, China, and was identified by 16S ribosomal DNA (rDNA) sequence analysis. B. amyloliquefaciens T1 cell-free filtrate at a concentration of 2 % (v/v) showed a strong anti-cyanobacterial effect against M. aeruginosa 905 (initial cell density was 1.0 × 106 cells mL−1), and a reduction of 99.4 % in cell number was observed after incubation for 6 days. The anti-cyanobacterial effect was in accordance with the spore number rather than the cell number of B. amyloliquefaciens T1, suggesting that M. aeruginosa 905 was indirectly affected by the secretion of anti-cyanobacterial active substances. In addition, the interactions of B. amyloliquefaciens T1 with other cyanobacteria and green algae indicated that M. aeruginosa 907, M. aeruginosa 908, M. aeruginosa 912, and M. aeruginosa 7806 were obviously suppressed, while Anabaena flosaquae 1092 and Chlorella pyrenoidosa 415 proved to be not susceptible. Effects of environmental factors showed that the best inhibition efficiency would be achieved at 30 °C and pH 9.0, and the solid B. amyloliquefaciens T1 agent of B. amyloliquefaciens T1 could selectively kill the wild cyanobacteria from a shallow eutrophic pond. Based on the results obtained from this study, the anti-cyanobacterial bacterium B. amyloliquefaciens T1 had a potential application for eutrophication control in the natural environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, eutrophication and harmful cyanobacterial blooms in lakes, rivers, and reservoirs have become a great concern on a global scale (Qin et al. 2006; Qu and Fan 2010; Tang et al. 2012). In particular, blooms caused by toxic cyanobacteria, such as Microcystis, Anabaena, Oscillatoria, and Cylindrospermopsis, result in the production of microcystin that affects ecosystem functioning and creates a significant water quality problem (Gan et al. 2012; Chislock et al. 2013; Paerl et al. 2013; Yang et al. 2014; Zhu et al. 2014). As a fast and efficient method, chemical agents such as copper sulfate, potassium permanganate, hydrogen peroxide, and ozone are used for eutrophication control (Miao et al. 2009; Qian et al. 2010; Fan et al. 2013; Matthijs et al. 2012). However, the use of chemical methods induces secondary pollution which is potentially dangerous in aquatic ecosystems (Qin et al. 2006; Qu and Fan 2010; Tang et al. 2012). Therefore, there is a need to explore ecologically safe ways to control harmful cyanobacterial blooms.
Early studies have shown that bacteria associated with harmful cyanobacterial blooms play an important role in regulating or terminating cyanobacterial blooms, and interactions between cyanobacteria and bacteria have gained increasing attention. A number of bacteria with the ability of inhibiting or biodegrading cyanobacteria have been found, including Aquimarina sp. (Chen et al. 2011), Pseudomonas sp. (Wang et al. 2005; Sakata et al. 2011), Streptomyces sp. (Hua et al. 2009; Kong et al. 2013a, b; Luo et al. 2013; Somdee et al. 2013), and Bacillus sp. (Ahn et al. 2003; Nakamura et al. 2003; Shi et al. 2006; Mu et al. 2007). It is widely recognized that the use of bacteria with anti-cyanobacterial activity represents an effective and environmental friendly option for the control of cyanobacterial and algal blooms (Lovejoy et al. 1998; Hua et al. 2009; Chen et al. 2011; Zhang et al. 2011; Kong et al. 2013a, b; Luo et al. 2013). In spite of the relatively large number of laboratory studies which have focused on isolation and identification of anti-cyanobacterial bacteria and their action modes, identification of anti-cyanobacterial active substances and the application of anti-cyanobacterial bacteria are limited. In addition, information regarding high efficient biodegradation of cyanobacteria and their potential application is very limited to date.
Given the importance of biological control for harmful cyanobacterial blooms, anti-cyanobacterial microorganisms, especially for the rapid and harmless biodegradation of Microcystis with microcystin toxin production, still need to be discovered. The aim of the present study was to isolate strains capable of biodegrading the cyanobacterium Microcystis aeruginosa 905, one of the predominant species involved in harmful cyanobacterial blooms in freshwater lakes of China. Four anti-cyanobacterial bacteria were obtained, and one of these with the most effective anti-cyanobacterial effect was identified by morphological characteristics and 16S ribosomal DNA (rDNA) gene sequence analysis, and the anti-cyanobacterial effects of the anti-cyanobacterial bacterium Bacillus amyloliquefaciens T1 on other cyanobacteria and green algae were studied as well. Additionally, the potential application of this anti-cyanobacterial bacterium was investigated.
Materials and methods
Microcystis aeruginosa 905, M. aeruginosa 907, M. aeruginosa 908, M. aeruginosa 912, Anabaena flosaquae 1092, and a green algae Chlorella pyrenoidosa 415 were obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB), Chinese Academy of Sciences (Wuhan, China); M. aeruginosa 7806 was provided by Professor Brett Neilan from the School of Biotechnology and Biomolecular Sciences, University of New South Wales (Sydney, Australia). Before being used as inoculants, they were cultured for 7 days to reach the log phase, and the culture conditions were as follows: sterilized BG11 medium, 36 μmol photons m−2 s−1 white light, light/dark = 14:10 h, and 25 ± 1 °C (Kong et al. 2013a, b).
Isolation and screening of anti-cyanobacterial bacteria
Subsurface sediment samples were collected from the surface layer (0∼20 cm) of an eutrophic pond in Wuhan, China. Sediment powder (10 g) was suspended in 90 mL sterile water and diluted to a series of concentrations. Approximately 0.1 mL of each dilution was spread on solid beef extract peptone plates and then cultured for 2∼3 days at 37 °C (Shen et al. 2002). Colonies with different morphologies were selected and streaked on new agar plates to obtain purified isolates (Mu et al. 2007). A modified cyanobacterial growth inhibition bioassay was used to isolate anti-cyanobacterial bacteria according to our previous study (Kong et al. 2013b). In brief, 0.2 mL cell-free filtrate from each isolate (cultured at 37 °C with a shaking speed of 180 rpm for 48 h in beef extract liquid medium) was filtered through a 0.22 μm cellulose acetate membrane and was added into 10 mL BG11 medium containing M. aeruginosa 905 at an initial cell density of 1.0 × 106 cells mL−1 and was then cultivated for 7 days under the conditions described above. Positive strains were inoculated into inclined tubes and stored at 4 °C for further study.
Identification of anti-cyanobacterial bacterium T1
Gram staining and analysis of physiological and biochemical characteristics of T1 were performed according to the procedures described by Shen et al. (2002). For the 16S rDNA gene sequencing and phylogenetic analysis, strain T1 was incubated in LB medium at 37 °C for 24 h with a shaking speed of 170 rpm (Julkowska et al. 2005). The cells were collected by centrifugation at 7000×g for 5 min (4 °C). DNA extraction was performed by using the 3S DNA Isolation Kit V2.2 (Biocolor BioScience & Technology Co., Shanghai, China). Fragments of the 16S rDNA from the isolate were amplified by PCR using the primers 27F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGCTACCTTGTTACGACTT-3′) (Mulder et al. 2011), and the amplified fragment was sequenced by AuGCT Biotech Co., Ltd. (Beijing, China) (Zhang et al. 2011). The BLAST procedure was performed using the database at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST).
Cyanobacterial inhibition bioassays
Effects of the cell-free filtrate concentrations
Bacillus amyloliquefaciens T1 was maintained at 4 °C in LB medium (Julkowska et al. 2005), and the fermentation broth was prepared by incubating the seed culture at 37 °C for 36 h at a shaking speed of 170 rpm. Soon afterwards, cell-free filtrate was obtained by centrifuging the fermentation broth at 10,000×g for 10 min and then filtering through a 0.22-μm cellulose acetate membrane (Kong et al. 2013b). The anti-cyanobacterial effects were studied by adding the cell-free filtrate (0 to 5 %, v/v) to 95 mL M. aeruginosa 905 culture at an initial cell number of 1.0 × 106 cells mL−1, brought to a final volume of 100 mL by the addition of beef extract liquid medium (Shen et al. 2002). A negative control was made by adding 5 mL beef extract liquid medium. All treatments and controls were in triplicate and incubated under the conditions described above. Cell numbers of M. aeruginosa 905 were counted every other day.
Effects of anti-cyanobacterial bacterium culture time
To study the effect of anti-cyanobacterial bacterium culture time, B. amyloliquefaciens T1 was incubated in 200 mL LB medium at 37 °C and 170 rpm; 10 mL of the culture was sampled at 4- or 2-h intervals over the 36 h of incubation, and the cell and spore numbers of B. amyloliquefaciens T1 were determined. In addition, the removal efficiency of M. aeruginosa 905 for each sample was tested by adding B. amyloliquefaciens T1 cell-free filtrate at a concentration of 2 % (v/v).
Effects of B. amyloliquefaciens T1 on cyanobacteria and green algae
The cyanobacteria M. aeruginosa 905, M. aeruginosa 907, M. aeruginosa 908, M. aeruginosa 912, and A. flosaquae 1092 and the green alga C. pyrenoidosa 415 were diluted to an initial cell density of 1.0 × 106 cells mL−1, and B. amyloliquefaciens T1 cell-free filtrate at a concentration of 2 % (v/v) was added to evaluate the anti-cyanobacterial effects. Controls were made by adding 5 mL beef extract liquid medium. All treatments and controls were in triplicate and incubated under the conditions described above. Cell numbers of cyanobacteria or green algae were counted after incubating for 4 days.
Effects of environmental factors
Environmental factors such as temperature and pH have an important influence on microbial degradation (Nakamura et al. 2003; Mu et al. 2009; Li et al. 2012). In consideration of the potential application of the anti-cyanobacterial bacterium for eutrophication control, the cyanobacterial suspensions (pH = 7.0) were incubated at 25 and 30 °C, while to assess the effect of pH, the cyanobacterial suspensions were adjusted to pH 7.0, 9.0, and 10.0 and incubated at 25 °C. The pH was adjusted by adding 0.1 M NaOH or 0.1 M HCl. All treatments were performed with the addition of 2 % (v/v) cell-free B. amyloliquefaciens T1 filtrate, and the control was as previously mentioned. Cell numbers of M. aeruginosa 905 were counted after incubating for 4 days.
Application of solid B. amyloliquefaciens T1 agent
In order to evaluate the anti-cyanobacterial effect of B. amyloliquefaciens T1 for eutrophication bioremediation, a solid B. amyloliquefaciens T1 agent was prepared as follows: B. amyloliquefaciens T1 fermentation broth was inoculated into liquid fermentation medium (molasses 5 %, corn starch 5 %, urea 0.1 %, KH2PO4 0.05 %, MgSO4 0.02 %, and pH 7.0∼7.5) with an inoculation ratio of 10 % and was cultured at 37 °C for 24 h with 170 rpm, and afterwards, the semifinished product was transferred into a solid fermentation medium (corn starch 10 %, bran 20 %, rice husk 10 %, rape cake 20 %, and tortillas 10 %) with an inoculation ratio of 10 % and was cultured in an incubator (the humidity was about 85 %) at 37 °C for 48 h. Finally, the solid inoculants were dried to constant weight at 55 °C and the solid B. amyloliquefaciens T1 agent (1.20 × 1010 cells g−1) was obtained.
The anti-cyanobacterial effect of solid B. amyloliquefaciens T1 agent on wild cyanobacteria (from a shallow eutrophic pond) was undertaken with different concentrations ranging from 0.1 to 1.0 mg L−1 and was incubated under the conditions mentioned above. The anti-cyanobacterial effect was observed by phase contrast microscope, and cell numbers were counted every other day.
Analytical methods
The cell number of anti-cyanobacterial bacterium was determined using the dilution method of plate counting, while for the determination of spore number, samples were pretreated in a water bath at 80 °C for 10 min and were then determined using the pour plate method. All the plates were incubated at 37 °C for 72 h (Shen et al. 2002). The cell number of cyanobacteria/algae was determined using a hemocytometer. Cell numbers of each treatment were determined three times, and the arithmetical mean (±SD) was obtained.
The removal efficiency of cyanobacteria/algae was calculated according to Eq. (1):
where C 0 and C t are the cell number of the control and test group at initial and time t, respectively (Kong et al. 2013b).
Results
Isolation and identification of bacteria with anti-cyanobacteria activity
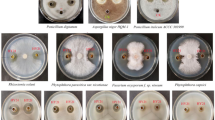
Numerous bacterial strains were isolated from the subsurface sediment samples and were then subjected to cyanobacterial inhibition bioassay for anti-cyanobacterial effects against the toxic cyanobacterium M. aeruginosa 905. As a result of isolation and screening, four strains (R1, T1, A1, and L2) were selected for biodegradation experiments on the basis of their anti-cyanobacterial activity (Fig. 1). With an initial cell-free filtrate concentration of 5 % (v/v), the cyanobacterium M. aeruginosa 905 was biodegraded within 4 days with a removal efficiency of 94.7 ± 5.8 % (strain R1), 97.1 ± 4.3 % (strain T1), 92.2 ± 3.8 % (strain A1), and 90.2 ± 3.9 % (strain L2). Among the four strains, T1 was the most effective and further identified.
The cells of strain T1 were short rod shaped, and oval spores were observed in the cells, and the bacterium was Gram-positive. The strain could grow at 50 °C and pH 5.7 and utilize nitrate as an electron acceptor. However, growth under anaerobic conditions was negative. This bacterium was able to utilize glucose, xylose, arabinose, mannitol, lactose, and sucrose, but not glycerol (Table 1).
Additionally, the 16S rDNA sequences of strain T1 (1402 bp) were determined and submitted to the GenBank database (accession No. GU359043.1). A phylogenetic tree was constructed based on 16S rDNA sequences (Fig. 2) and similarity calculations following the phylogenetic analysis, showing that strain T1 was most closely related to B. amyloliquefaciens DSM7 with a 100 % similarity in nucleic acid sequence homology. Based on the results of the morphological and biochemical characteristics and 16S rDNA sequences, this anti-cyanobacterial bacterium was identified as B. amyloliquefaciens T1.
Anti-cyanobacterial effect of B. amyloliquefaciens T1 on M. aeruginosa 905
Cyanobacterial inhibition bioassay was carried out at various concentrations of the cell-free filtrate ranging from 0.5 to 5 % (v/v) to study the anti-cyanobacterial effects of B. amyloliquefaciens T1 on M. aeruginosa 905. A 1 % cell-free filtrate could effectively inhibit the growth of M. aeruginosa 905 with a removal efficiency of 60.3 % was observed on day 2 (Fig. 3). In the case of 0.5 % treatment group, the removal efficiency of M. aeruginosa 905 on day 2, 4, and 6 was only 16.3, 41.5, and 43.3 %, respectively, indicating that a 0.5 % concentration was not effective for M. aeruginosa 905 inhibition. More importantly, the cell density of treatment groups with 1 to 5 % cell-free filtrate addition was 0.85 ± 0.091 × 106, 0.08 ± 0.003 × 106, 0, and 0 cells mL−1 on day 6, giving a removal efficiency of 94.0, 99.4, 100, and 100 %, respectively. Moreover, no growth of M. aeruginosa 905 was observed over 1 week.
Anti-cyanobacterial bacterium culturing and cyanobacteria removal
Oval spores were produced when the B. amyloliquefaciens T1 was incubated in LB medium. In order to evaluate the effect of B. amyloliquefaciens T1 cell growth on M. aeruginosa 905, the time course for the anti-cyanobacterial bacterium cell growth and oval spore production was followed (Fig. 4). As the fermentation time went on, the cell number of B. amyloliquefaciens T1 increased sharply from 0.23 ± 0.02 × 108 to 2.47 ± 0.13 × 108 cells mL−1 in the first 20 h and then began a slow decline, whereas the spore number increased progressively with culture time, with a maximal production of 1.23 ± 0.05 × 103 spores mL−1 after 26 h (Fig. 4). The M. aeruginosa 905 removal efficiency was 98.4 ± 4.7 % and remained stable above 98 %. It was obvious that the removal efficiency was increased with increasing of spore number (from 16 to 26 h), suggesting that the anti-cyanobacterial effect was better correlated with the spore number of B. amyloliquefaciens T1 rather than the cell number and that the anti-cyanobacterial active substances might be in the cell-free filtrate.
Anti-cyanobacterial effect of B. amyloliquefaciens T1 on other cyanobacteria and green algae
The effects of B. amyloliquefaciens T1 cell-free filtrate on other harmful cyanobacteria (M. aeruginosa 907, M. aeruginosa 908, M. aeruginosa 912, M. aeruginosa 7806, A. flosaquae 1092) and the green alga C. pyrenoidosa 415 were investigated over a period of 4 days (Fig. 5). Growth of all the Microcystis species was significantly inhibited by the B. amyloliquefaciens cell-free filtrate (ANOVA, p < 0.01), achieving removal efficiencies of 76.9 ± 3.1 % (M. aeruginosa 907), 78.2 ± 2.2 % (M. aeruginosa 908), 72.9 ± 3.0 % (M. aeruginosa 912), and 85.1 ± 1.8 % (M. aeruginosa 7806). On the contrary, the growth of A. flosaquae 1092 was not affected, while the growth of C. pyrenoidosa 415 was promoted, reaching a cell density of 5.87 ± 0.48 × 106 cells mL−1, which was nearly 1.5 times than that of the control (p < 0.05).
Effects of temperature and pH
Environmental factors show great influence on the growth of organisms and the biodegradation of cyanobacteria. In the control groups at 25 °C and pH 9.0, cell numbers of M. aeruginosa 905 were 9.54 ± 1.31 × 106 and 9.27 ± 0.81 × 106 cells mL−1, respectively, which were much higher than the other conditions (Fig. 6). The results shown in Fig. 6 show that B. amyloliquefaciens T1 could be used at temperatures from 25 to 30 °C and pH from 7.0 to 10.0 with the best inhibition efficiency achieved at 30 °C and pH 9.0. Thus, the anti-cyanobacterial bacterium B. amyloliquefaciens T1 had the potential application for eutrophication control in the natural environment.
Application of solid B. amyloliquefaciens T1 agent
Aiming to confirm the anti-cyanobacterial effect of B. amyloliquefaciens T1, the growth of wild cyanobacteria from a shallow eutrophic pond was examined by adding solid B. amyloliquefaciens T1 agent at concentrations of 0.1, 0.5, and 1.0 mg L−1. The result indicated that at 0.1 g L−1, there was no anti-cyanobacterial effect, but higher concentrations showed a dose-dependent effect (Table 2). Within 24 h after the addition of solid B. amyloliquefaciens T1 agent, the cyanobacteria began to flocculate and the cyanobacterial suspension became yellow after incubation for 60 h, indicating that a dosage of 0.5 mg L−1 (or above) had a significant anti-cyanobacterial effect.
Control cyanobacteria and cyanobacteria with 0.5 mg L−1 solid B. amyloliquefaciens T1 agent were observed by phase contrast microscopy (Fig. 7). It was obvious that the control cells had an intact cell wall and the plasma membrane was close to the cell wall, demonstrating the integrity of cell structure (Fig. 7a). However, alterations in cyanobacterial cell morphology were observed during solid B. amyloliquefaciens T1 agent exposure (Fig. 7b–d). Compared with the control, the cell membrane was gradually destroyed and the plasma membrane began to detach from the cells in some cases when it is exposed to solid B. amyloliquefaciens T1 agent for 60 h (Fig. 7b) and the cyanobacterial cells were absolutely destroyed (Fig. 7c), and the cell organelles were released after 80 h of incubation (Fig. 7d).
Discussion
Biodegradation is an important pathway for removing poisonous and harmful pollutants from the environment (Tang et al. 2012; Zhu et al. 2014). Therefore, screening and application of anti-cyanobacterial bacteria may be important to the eventually successful implementation for cyanobacteria inhibition. Hua et al. (2009) studied M. aeruginosa 905 biodegradation in conical flasks and showed that the culture broth of Streptomyces sp. NT0401 (5 %, v/v) could degrade nearly 90 % after incubation for 5 days. A recent study showed that Streptomyces sp. L74 cell-free medium (10 %, v/v) exhibited a 41.0 % algicidal rate on M. aeruginosa 905 on day 2 (Luo et al. 2013). Bacillus sp. strains also have been reported as M. aeruginosa-biodegrading bacteria in the past few years (Ahn et al. 2003; Nakamura et al. 2003; Shi et al. 2006; Mu et al. 2007). For example, Bacillus cereus demonstrated the ability of lysing the bloom-forming cyanobacterium M. aeruginosa and other cyanobacteria but required cell-cell contact (Shi et al. 2006). In the present study, B. amyloliquefaciens T1 was isolated from a eutrophic pond and the laboratory investigation demonstrated that only 1 % (v/v) of the cell-free filtrate was required to effectively suppress the growth of Microcystis (Fig. 3). In comparison with previous studies, the removal efficiency of 94.0 % obtained from our study indicated that M. aeruginosa 905 was more sensitive to B. amyloliquefaciens T1 than other Bacillus sp. strains (Ahn et al. 2003; Nakamura et al. 2003; Shi et al. 2006; Mu et al. 2007). The high sensitivity of M. aeruginosa 905 to B. amyloliquefaciens T1 may be attributed to the characteristics of secreta (Luo et al. 2013) or partly because of species differences within cyanobacteria and diatoms (Kang et al. 2005; Paul and Pohnert 2011). Although previous studies have indicated that harmful cyanobacteria or algae such as M. aeruginosa (Chen et al. 2011), Aphanizomenon flos-aquae (Shi et al. 2006), Heterosigma akashiwo (Wang et al. 2005), and Karlodinium veneficum and Gyrodinium instriatum (Pokrzywinski et al. 2012) could be inhibited by microorganisms, the removal effects of M. aeruginosa 905 in laboratory investigation by the strain B. amyloliquefaciens T1 was relatively more efficient compared with these studies.
Algicidal bacteria are intended to work selectively against cyanobacteria (Ahn et al. 2003; Chen et al. 2011; Kong et al. 2013a, b), marine diatoms (Paul and Pohnert 2011, 2013), dinoflagellates (Pokrzywinski et al. 2012), and Phaeocystis (Zheng et al. 2013). Bacillus fusiformis, an algicidal bacterium isolated from a sewage treatment plant, could suppress the growth of M. aeruginosa, Chlorella, and Scenedesmus (Mu et al. 2007). Our study revealed that the cell-free filtrate of B. amyloliquefaciens T1 also showed anti-cyanobacterial effects against other Microcystis strains such as M. aeruginosa 907, M. aeruginosa 908, M. aeruginosa 912, M. aeruginosa 7806, and M. aeruginosa 905 (Fig. 1). In contrast, A. flosaquae 1092 was not susceptible while growth of the green alga C. pyrenoidosa 415 was promoted by the addition of the cell-free filtrate. Similar results were obtained with the algicidal bacterium Kordia algicida which showed algicidal effects against the marine diatoms Skeletonema costatum, Thalassiosira weissflogii, and Phaeodactylum tricornutum, while Chaetoceros didymus was shown to be not susceptible (Paul and Pohnert 2011). Another study found that Pseudomonas putida HYK0203-SK02 appeared to have algicidal activity against a wide range of phytoplankton such as Synechococcus hantzschii (80.4 %), M. aeruginosa (70.0∼71.4 %), Aulacoseira granulata (31.2 %), Chlorella sp. (30.3 %), and Anabaena cylindrica (41.6 %), whereas the diatom Cyclotella sp. was stimulated (Kang et al. 2005). Additionally, P. putida CH-22, isolated from Lake Chaohu of Anhui Province, China, also caused drastic reductions in M. aeruginosa (89.3 %), Microcystis wesenbergii (76.5 %), Microcystis viridis (70.3 %), A. flos-aquae (61.6∼71.3 %), and Chlorella ellipsoidea (48.5 %) (Zhang et al. 2011).
Apart from the biodiversity of harmful cyanobacterial blooms in aquatic ecosystems, environmental factors such as temperature and pH influence the activity of anti-cyanobacterial bacteria. Ochrobactrum sp. FDT5 was recently found to be effective at reducing the growth of M. aeruginosa at 30 °C and pH 7.6 (Mu et al. 2009). It was also reported that the removal efficiency of M. aeruginosa (in terms of Chl a) was 75.9, 83.6, and 78.3 % at pH 6.5, 7.5, and 8.5, respectively, demonstrating that pH 7.5 was the best for the anti-cyanobacterial effect of this bacterium (Li et al. 2012). However, compared with pH 7.5, the condition of pH 10.1 was beneficial for the anti-cyanobacterial bacterium B. cereus and 100 % lysis of M. viridis was attained in 24 h with the dosage of 1:1 (v/v) (Nakamura et al. 2003). Moreover, over the temperature range of 3 to 30 °C, both 25 and 30 °C were suitable for M. viridis removal (Nakamura et al. 2003). We also found that the optimal temperature and pH for M. aeruginosa 905 biodegradation by B. amyloliquefaciens T1 was 25 °C and pH 9.0.
As illustrated above, the elimination of harmful cyanobacterial blooms might depend on the population abundance and species composition of the cyanobacteria (Matthijs et al. 2012). Therefore, finding the right dosage is another critical issue for the potential application of B. amyloliquefaciens T1. The experiment using wild cyanobacteria from a shallow eutrophic pond indicated that a concentration of more than 0.5 mg L−1 solid B. amyloliquefaciens T1 agent would absolutely suppress the cyanobacterial growth, which was consistent with a previous report that the H2O2 concentration for field application was much higher than that in the laboratory study (Matthijs et al. 2012). Additionally, the reason for the higher dosage required could be due to the presence of A. flosaquae and C. pyrenoidosa, which were not biodegraded by B. amyloliquefaciens T1 (Fig. 5). On the basis of this information, we recommend the application a dosage of more than 1.0 mg L−1 solid B. amyloliquefaciens T1 agent for field samples from a wide range of Microcystis-dominated lakes or reservoirs. Although B. amyloliquefaciens T1 was isolated from an eutrophic pond, the side effects such as ecological risk on aquatic organisms should be considered; moreover, the released products from harmful cyanobacteria when biodegraded such as microcystins and nutrients should also be removed at the same time.
Previous studies on the action modes of anti-cyanobacterial bacteria showed that these were by direct and indirect interactions (Lovejoy et al. 1998; Mayali and Doucette 2002; Chen et al. 2011; Zheng et al. 2013). It was obvious that the anti-cyanobacterial substances were secreted by B. amyloliquefaciens T1 and existed in the cell-free filtrate, demonstrating an indirect interaction mode. However, we have not studied the anti-cyanobacterial mechanism, nor have we isolated and identified the anti-cyanobacterial substances and the potential toxicity caused by microcystin. Notwithstanding its limitations, this study does suggest that harmful cyanobacteria such as M. aeruginosa could effectively be inhibited by B. amyloliquefaciens T1.
References
Ahn CY, Joung SH, Jeon JW, Kim HS, Yoon BD, Oh HM (2003) Selective control of cyanobacteria by surfactin-containing culture broth of Bacillus subtilis C1. Biotechnol Lett 25:1137–1142
Chen WM, Sheu FS, Sheu SY (2011) Novel l-amino acid oxidase with algicidal activity against toxic cyanobacterium Microcystis aeruginosa synthesized by a bacterium Aquimarina sp. Enzym Microb Technol 49:372–379
Chislock MF, Doster E, Zitomer RA, Wilson AE (2013) Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat Educ Knowl 4(4):10
Fan J, Ho L, Hobson P, Brookes J (2013) Evaluating the effectiveness of copper sulphate, chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res 47:5153–5164
Gan NQ, Xiao Y, Zhu L, Wu ZX, Liu J, Hu CL, Song LR (2012) The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ Microbiol 14:730–742
Hua XH, Li JH, Li JJ, Zhang LH, Cui Y (2009) Selective inhibition of the cyanobacterium, Microcystis, by a Streptomyces sp. Biotechnol Lett 31:1531–1535
Julkowska D, Obuchowski M, Holland I, Seror S (2005) Comparative analysis of the development of swarming communities of Bacillus subtilis 168 and a natural wild type: critical effects of surfactin and the composition of the medium. J Bacteriol 187:65–76
Kang YH, Kim JD, Kim BH, Kong DS, Han MS (2005) Isolation and characterization of a bio-agent antagonistic to diatom, Stephanodiscus hantzschii. J Appl Microbiol 98:1030–1038
Kong Y, Xu XY, Zhu L (2013a) Cyanobactericidal effect of Streptomyces sp. HJC-D1 on Microcystis auruginosa. PLoS One 8(2):e57654
Kong Y, Zou P, Yang Q, Xu XY, Miao LH, Zhu L (2013b) Physiological responses of Microcystis aeruginosa under the stress of antialgal actinomycetes. J Hazard Mater 262:274–280
Li HJ, Hao ML, Liu JX, Chen C, Fan ZQ, Wang XR (2012) Effect of pH on biologic degradation of Microcystis aeruginosa by alga-lysing bacteria in sequencing batch biofilm reactors. Front Environ Sci Eng 6:224–230
Lovejoy C, Bowman J, Hallegraeff G (1998) Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodium, and Heterosigma. Appl Environ Microbiol 64:2806–2813
Luo J, Wang Y, Tang S, Liang J, Lin W, Luo L (2013) Isolation and identification of algicidal compound from Streptomyces and algicidal mechanism to Microcystis aeruginosa. PLoS One 8(10):e76444
Matthijs HC, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, Talens R, Huisman J (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res 46:1460–1472
Mayali X, Doucette GJ (2002) Microbial community interactions and population dynamics of an algicidal bacterium active against Karenia brevis (Dinophyceae). Harmful Algae 1:277–293
Miao HF, Tao WY (2009) The mechanisms of ozonation on cyanobacteria and its toxins removal. Sep Purif Technol 66:187–193
Mu RM, Fan ZQ, Pei HY, Yuan XL, Liu SX, Wang XR (2007) Isolation and algae-lysing characteristics of the algicidal bacteria B5. J Environ Sci 19:1336–1340
Mu RM, He YJ, Liu SX, Wang XR, Fan ZQ (2009) The algicidal characteristics of one algae-lysing FDT5 bacterium on Microcystis aeruginosa. Geomicrobiol J 26:516–521
Mulder IE, Schmidt B, Lewis M, Delday M, Stokes CR, Bailey M, Aminov RI, Gill BP, Pluske JR, Mayer CD, Kelly D (2011) Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity. PLoS One 6(12):e28279
Nakamura N, Nakano K, Sugiura N, Matsumura M (2003) A novel cyanobacteriolytic bacterium, Bacillus cereus, isolated from a eutrophic lake. J Biosci Bioeng 95:179–184
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 65:995–1010
Paul C, Pohnert G (2011) Interactions of the algicidal bacterium Kordia algicida with diatoms: regulated protease excretion for specific algal lysis. PLoS One 6(6):e21032
Paul C, Pohnert G (2013) Induction of protease release of the resistant diatom Chaetoceros didymus in response to lytic enzymes from an algicidal bacterium. PLoS One 8(3):e57577
Pokrzywinski KL, Place AR, Warner ME, Coyne KJ (2012) Investigation of the algicidal exudate produced by Shewanella sp. IRI-160 and its effect on dinoflagellates. Harmful Algae 19:23–29
Qian HF, Yu SQ, Sun ZQ, Xie XC, Liu WP, Fu ZW (2010) Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol 99:405–412
Qin BQ, Yang LY, Chen FZ, Zhu GW, Zhang L, Chen YY (2006) Mechanism and control of lake eutrophication. Chin Sci Bull 51:2401–2412
Qu JH, Fan MH (2010) The current state of water quality and technology development for water pollution control in China. Crit Rev Environ Sci Technol 40:519–560
Sakata T, Yoshikawa T, Nishitarumizu S (2011) Algicidal activity and identification of an algicidal substance produced by marine Pseudomonas sp. C55a-2. Fish Sci 77:397–402
Shen P, Fan XR, Li GW (2002) Microbiology test. Higher Education, Beijing
Shi S, Liu Y, Shen Y, Li G, Li D (2006) Lysis of Aphanizomenon flos-aquae (Cyanobacterium) by a bacterium Bacillus cereus. Biol Control 39:345–351
Somdee T, Sumalai N, Somdee A (2013) A novel actinomycete Streptomyces aurantiogriseus with algicidal activity against the toxic cyanobacterium Microcystis aeruginosa. J Appl Phycol 25:1587–1594
Tang XQ, Wu M, Yang WJ, Yin W, Jin F, Ye M, Currie N, Scholz M (2012) Ecological strategy for eutrophication control. Water Air Soil Pollut 223:723–737
Wang X, Gong L, Liang S, Han X, Zhu C, Li Y (2005) Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae 4:433–443
Yang F, Zhou YL, Yin LH, Zhu GC, Liang GY, Pu YP (2014) Microcystin-degrading activity of an indigenous bacterial strain Stenotrophomonas acidaminiphila MC-LTH2 isolated from Lake Taihu. PLoS One 9(1):e86216
Zhang H, Yu ZL, Huang Q, Xiao X, Wang X, Zhang FY, Wang XQ, Liu YD, Hu CX (2011) Isolation, identification and characterization of phytoplankton-lytic bacterium CH-22 against Microcystis aeruginosa. Limnologica 41:70–77
Zheng X, Zhang B, Zhang J, Huang L, Lin J, Li X, Zhou Y, Wang H, Yang X, Su J, Tian Y, Zheng T (2013) A marine algicidal actinomycete and its active substance against the harmful algal bloom species Phaeocystis globosa. Appl Microbiol Biotechnol 97:9207–9215
Zhu L, Wu YL, Song LR, Gan NQ (2014) Ecological dynamics of toxic Microcystis spp. and microcystin-degrading bacteria in Dianchi Lake, China. Appl Environ Microbiol 80:1874–1881
Acknowledgments
This study was financially supported by a grant from the National High Technology Research and Development Program of China (863 Program) (No. 2013AA102805-04) and the Science and Technology R & D Program of Wuhan, China (No. 201120637175-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Yu and Yun Kong contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Yu, J., Kong, Y., Gao, S. et al. Bacillus amyloliquefaciens T1 as a potential control agent for cyanobacteria. J Appl Phycol 27, 1213–1221 (2015). https://doi.org/10.1007/s10811-014-0402-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0402-7