Abstract
Most commercial algal extracts are produced from brown algae by alkaline hydrolysis; however, little scientific information has been published regarding the details of the production process. In this research, we have investigated the effect of pH (pH 8–12) and temperature (40, 60, and 80 °C) on liquid extract production from the brown alga Macrocystis pyrifera. Production conditions influenced the physicochemical characteristics of the final product as the extract viscosity increased with increasing pH and temperature to a maximum which occurred at pH 10 and 80 °C. This suggests that at higher pH conditions, alginate and other polysaccharides were extracted. All the extracts obtained promoted growth of tomato plants (Solanum lycopersicum) and adventitious root formation in the mung bean cutting bioassay (Vigna radiata), as the pH process was increased during the production of the liquid extracts. The highest auxin-type activity was obtained with the extract produced at pH 11 and 80 °C, while the fastest tomato seedling growth was achieved with the extract produced at pH 12 and 80 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of extracts obtained from marine algae to promote plant growth has been extensively studied since the first product was launched into the market in the 1940s (Craigie 2011). These products enhance the growth and yield of various crops, improve nutrient mobilization, and favor the development of roots, while increasing the plant chlorophyll content and leaf area (Blunden 1991; Crouch and van Staden 1992; Blunden et al. 1997; Turan and Köse 2004; Stirk and van Staden 2006; Khan et al. 2009; Craigie 2011). These effects are attributable to the presence of hormones that occur naturally in seaweeds that also are present in seaweed extracts that regulate plant growth (Crouch and van Staden 1993; Tarakhovskaya et al. 2007). In addition, it is suggested that during the hydrolysis of seaweeds, the polysaccharides are degraded to oligosaccharides which may act as metabolic inducers that trigger the physiological responses mentioned above (Chandía et al. 2004).
Currently, different extraction processes are used to obtain seaweed biostimulants. Depending on the production process, marine algal products can be classified as either extracts or suspensions. The term “extract” is used for products that are produced with some chemicals to hydrolyze the alga, while the term “suspension” refers to those products that are produced without chemicals (Blunden 1991). Suspensions of algae are obtained by physical processes such as cryogenic grinding, homogenization processes, and/or micro-fractionation (Blunden 1991). Kelpak, a commercial seaweed product, is obtained through a process called “cold cell burst” which is performed by gradual reduction of particle size after which the cells are collapsed by pressure shocks (Stirk and van Staden 2006). Most commercial extracts are produced by alkaline hydrolysis using sodium hydroxide, sodium carbonate, or potassium carbonate (Stephenson 1974; Craigie et al. 2007; Craigie 2011; Stirk and van Staden 1997). Other trademarks, in order to increase the value as a natural product, state that their products are produced without the use of acids or alkalis, naming the product an aqueous extract. These extracts also stimulate growth in plants (Kumari et al. 2011).
Extractions can be carried out under alkaline, neutral, or acidic conditions (Booth 1969). Extraction under slightly acidic pH conditions for 10 min at 20 °C produced an extract with the same density (D = 1.04) and concentration of soluble solids (8 %) as when extracted at the same pH at 60 °C for 2 h (Booth 1969). The production of Maxicrop™ extract is carried out using a pressure chamber and alkaline hydrolysis but there is not information about the extraction conditions such as pressure, temperature, pH, or processing time (Stephenson 1974). Little information exists in the literature on the effects of temperature and pH on extraction technology. The aim of our research was to assess the effects of pH and temperature during the production of liquid extracts from Macrocystis pyrifera on their physicochemical properties and plant growth promoting activity.
Materials and methods
Production of the extracts
The giant kelp Macrocystis pyrifera (Linnaeus) C. Agardh was harvested from Santo Tomas, Baja California, Mexico, in March, 2009. The alga was sundried (12 % moisture) and packaged for transportation. It was then milled using a hammer mill to 30-mesh particle size, packaged, and stored at room temperature for later use within the next 3 months.
Two hundred gram of milled seaweed was rehydrated using 1,800 mL of distilled water for 12 h at room temperature (22 °C). Next day, 1,500 mL of distilled water were added to allow the seaweed stirring during the extraction step. The seaweed was placed in a water bath, and the pH and temperature were adjusted to the required experimental conditions. The pH was adjusted with potassium carbonate (solid, technical grade). The experimental design consisted of performing the alkaline hydrolysis extraction under three different pH values (8, 9, and 10) at three different temperatures (40, 60, and 80 °C). Additional extracts were produced at pH 11 and 12, but only at a temperature of 80 °C where potassium hydroxide was used to reach the appropriate pH. Each combination of factors was carried out in triplicate. In all cases, agitation at 800 rpm was applied with an external stirrer type propeller. Temperature and pH were monitored and adjusted if necessary throughout the extraction process.
After reaching the experimental pH and temperature, 300 mL samples were poured from the extraction paste in a 500 mL baker at time zero and at 15 min intervals to 2 h (nine samples). The viscosity of the samples was measured in the laboratory using a Brookfield viscometer Model LVT DV-I. This viscosity is called “process viscosity” and is defined as the viscosity of the extract, including the effect of the alkali-insoluble algal material in the paste (Hernández-Carmona et al. 1999). After the extraction step, the final volume of the paste obtained was measured (from the initial 3,300 mL), and the paste was diluted by adding 0.5 volume of water. The residual seaweed was removed using a continuous centrifuge (Alfa-Sharples mod. PV10104-1) operated at 5,000 rpm with a feed rate of 500 mL min−1. At the end of this step, the total volume of the diluted extract was measured and recorded as the extract yield. Viscosity was measured at 22 °C.
Finally, the pH of the extract was adjusted to 7 with 10 % phosphoric acid. Formaldehyde (0.2 %) was added as a preservative, and the extracts were stored in plastic opaque containers at room temperature. Samples of all extracts produced were coded with the first number as the extraction pH and the second number the extraction temperature as follows: E840, E860, E880, E940, E960, E980, E1040, E1060, E1080, E1180, and E1280.
Auxin-like activity
Samples were sent to the Research Centre for Plant Growth and Development at the University of KwaZulu-Natal, South Africa, where the mung bean bioassay was carried out to measure auxin-like activity. This bioassay quantifies the adventitious root formation of mung bean (Vigna radiata) using indole-3-butyric acid (IBA) as a reference, according to the methodology described by Stirk et al. (2002) modifying pulse treatment to 6 h. Mung bean seeds were surface disinfected using commercial bleach with sodium hypochlorite as the active ingredient at 3 % for 20 min. After being rinsed with sufficient water, the mung bean seeds were sown in moist vermiculite in a germination tray. The seedlings were maintained at a constant temperature of 26 °C, with a light intensity of 100 μmol photons m−2 s−1 provided by both incandescent and fluorescent lights, photoperiod of 16 h light and 8 h dark, and were watered as required. After 10 days, the seedlings that measured between 10 and 12 cm in height and had developed the first two true leaves were cut at the base of the stem. The cotyledons were removed, and the length of the stems was adjusted to 3 cm below the cotyledons.
Solutions of the algal extracts were prepared at 1, 2, 5, 10, 20, and 50 % (v/v) by dilution with distilled water. In addition, solutions of IBA were prepared at concentrations of 10−3, 10−4, 10−5, 10−6, and 10−7 M. Distilled water was used as a control. Five seedlings were placed in a 20-mL vial containing the extract being tested, with four replicates in total. The mung bean cuttings were maintained for 6 h in the solutions and then withdrawn, rinsed with purified water, and placed in vials containing distilled water. The cuttings were maintained for 10 days in a growth cabinet in the conditions described above, after which, the number of adventitious roots formed was counted.
Assessing growth promotion function, using tomato as a model plant
Tomato seeds (Solanum lycopersicum L. var. Rio Grande) were washed for 15 min with neutral liquid detergent (1 % v/v) to break the surface tension and remove any fungicide and then disinfected using commercial bleach with sodium hypochlorite as the active ingredient at 3 % for 20 min. The seeds were dried in the shade and stored in dark bottles at room temperature.
Filter paper (Whatman No. 4) was placed in Petri dishes (10 cm diameter), and 10 tomato seeds were placed on each paper. Ten milliliters of the algal extract diluted with water to a concentration of 1 % was added to each Petri dish. Each treatment was tested with six replicates. Water was used as control to compare the results without nutrients. The Petri dishes were incubated in a culture room at 27 °C, 100 μmol photons m−2 s−1 and a photoperiod of 16 h light and 8 h dark. After 14 days, the following parameters were evaluated: length and fresh and dry weight of stem and roots.
In all cases, the data were tested for normality and homoscedasticity. For comparison of means of multiple groups or treatments, the analysis of variance (ANOVA), the Kruskal–Wallis test, or the multiple comparison test of Tukey’s least significant difference (α = 0.05) was used.
Results and discussion
Physicochemical properties
In liquid seaweed extracts produced at pH 10 and higher, a paste with a high viscosity was formed. Therefore, clarification was a critical step in the production process (Fig. 1). The extract viscosity varied significantly across the range of different treatments (p < 0.05).
Process viscosity of the seaweed liquid extract from M. pyrifera at different conditions of pH and temperature: E840 (○); E860 (△); E880 (□); E940 ( ); E960 (
); E960 ( ); E980 (
); E980 ( ); E1040 (●); E1060 (▲); E1080 (■); E1180 (◊), and E1280 (▽). Different letters indicate significant differences between treatments (p < 0.05) according to ANOVA test followed by Tukey mean comparison
); E1040 (●); E1060 (▲); E1080 (■); E1180 (◊), and E1280 (▽). Different letters indicate significant differences between treatments (p < 0.05) according to ANOVA test followed by Tukey mean comparison
The extracts obtained at pH 11, 80 °C (E1180) presented an initial viscosity of 3,080 mPa·s, higher than those obtained at lower pH conditions, i.e., E1080 (1,535 mPa·s) and E1060 (963 mPa·s). However, as processing time was increased the viscosity decreased in E1180, ending at 1,853 mPa·s after 2 h. Similarly, during the production of the extract at pH 12, initial viscosity values decreased significantly (p < 0.05) from 417 mPa·s to 321 mPa·s at the end of the extraction period (Fig. 1). At the end of the process (120 min), the treatment E1080 produced the highest viscosity (15,506 mPa·s). This value was four times higher than the value obtained during alginate production using the same conditions (pH 10, 80 °C, 2 h) and the same species (3,730 cps; Hernández-Carmona et al. 1999). This increase in viscosity in the present study was mainly due to the solubilization of alginate, as it was demonstrated by Hernández-Carmona et al. (1999), but also by the extraction of other polysaccharides (such as fucoidan and laminaran) and other components that are released when the cellular walls are disrupted. The viscosity was also increased because of the evaporation of water during the extraction process. Changes in viscosity are linked to the degradation of the alginate by depolymerization and are strongly dependent on pH during extraction (Haug et al. 1963, 1967). When extraction was carried out at 68 °C for 5 h, a slow degradation of viscosity occurred in the region of pH 5 to 10 (Haug et al. 1963, 1967). When alginate is subjected to alkaline conditions at elevated temperatures, it is degraded into oligomeric fragments and numerous other breakdown products (Craigie 2011). Some of these are likely to possess biological activity, and so, process conditions can be an important variable in plant response to the extracts. In the present study, the extracts E1180 and E1280 produced the lowest viscosities at the end of the extraction period. When the viscosity was measured at 22 °C, these products also showed lower viscosity values than extracts produced at pH 10 (Fig. 2). Polysaccharides are susceptible to a variety of degradation mechanisms, including free radical depolymerization (oxidation–reduction) and alkaline degradation catalyzed by enzymatic reactions. Degradation occurs by breaking the glycosidic linkages, and generally, the rate of degradation depends on the concentration of reactants and temperature (Holme et al. 2008). Under alkaline conditions, alginate depolymerization is carried out mainly by a beta elimination type reaction (Haug et al. 1963, 1967; Holme et al. 2008).
Viscosity of the final liquid extracts of M. pyrifera measured at 22 °C, T1-E840; T2-E860; T3-E880; T4-E940; T5-E960; T6-E980; T7-E1040; T8-E1060; T9-E1080; T10-E1180, and T12-E1280. Different letters indicate significant differences between treatments (p < 0.05) according to the nonparametric Kruskal–Wallis test followed by the Tukey mean comparison
Extraction time is another factor, which influences the quantity and quality of the alginate and other components extracted from the algae. The maximum alkali treatment time for the extracts obtained in this study was 120 min. Previously, Hernández-Carmona et al. (1996) obtained the highest yield of alginate (27.84 %) from M. pyrifera at pH 10 after 120 min, but it was not statistically different to the yield obtained with the treatment at 90 min (26.53%). This suggests that process time could be reduced to 90 min. However, it has to be corroborated, analyzing the influence of other important components of the seaweed extract. In algal extract production at the industrial level, this difference in time is important due to the high energy cost, and it is therefore important to assess this parameter in future studies.
The viscosity is a variable in the production process of commercial algal extracts that can be used to predict the course of the reaction. A high viscosity must be sought in order to achieve the maximum amount of soluble components. The application of a highly viscous product in the field could lead to problems such as blocking sprinklers or drip irrigation systems. However, this can be solved by diluting the final product to a suitable viscosity before use. Separation of the residual seaweed from a highly viscous extract is a difficult step, but it was solved in the present study using the decanter centrifuge, separating the two fractions at a viscosity never documented before (1,495 ± 83 mPa·s).
Auxin-like activity
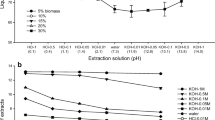
In the mung bean bioassay, extracts elicited increased rooting activity as the pH and temperature process conditions increased (Fig. 3). Most of the extracts showed a statistically significant increase in rooting compared to the control when tested at a concentration of 5 % and higher, except for extract E840 that only showed a significant increase in rooting at a concentration of 50 % (p < 0.05). The extract that produced significantly increased rooting at the lowest concentration i.e. 1 % was E1180 (p < 0.05). At this concentration, E1180 elicited activity equivalent to 10−4 M IBA. Extract E1280 showed significantly increased rooting at a concentration of 2 % (p < 0.05). This was equivalent to activity between 10−5 and 10−4 M IBA. Extracts with low viscosity (E840, E860 E940, E960, and E1040) showed a trend to increase their rooting activity as the extract concentration increased. The extracts with the highest process viscosity (E980, E1060, and E1080) did not increase rooting activity as the concentration increased above 5 %, with rooting remaining constant (Fig. 3).
Auxin-like activity of the extracts of M. pyrifera produced at different pH and temperature, and a standard curve for the adventitious root formation with IBA as reference. The mark (asterisk) indicates statistically significant differences (p < 0.05) according to the nonparametric Kruskal–Wallis test followed by Tukey mean comparison
Stirk and van Staden (1997) stated that the most active extract for rooting was Kelpak, which produced optimal rooting activity at 1 % concentration. This activity was equivalent to 10−6–10−5 M IBA. The least active extract was Marinure, where optimum rooting was obtained at 50 % concentration, also being equivalent to 10−6–10−5 M IBA. Both extracts are produced by alkaline hydrolysis, and although the bio stimulant affectivity may be pH and temperature dependent, nothing has been published so far about that. Some of the extracts evaluated in the present study showed higher activity than the IBA control even at 5 % concentration. Similarly, 5 % was also the optimal concentration in extracts from M. pyrifera obtained through the process known as “cold cell burst” and using the same bioassay (Stirk et al. 2004). Although the extracts E1180 and E1280 showed similar rooting activity as the other extracts, when the concentration was increased to 20 and 50 %, there was a significant decrease in their rooting activity (Fig. 3). This may be due to the increase of bacterial growth.
Indole-3-acetic acid (IAA) is the most frequently occurring auxin in land plants (Bartel 1997). In seaweeds, it has been isolated from different species (Lobban and Harrisson 1997), but there are only a few reports of its presence in commercial extracts. Kingman and Moore (1982) reported the presence of IAA in a commercial extract from Ascophyllum nodosum, in a concentration of 50 mg g−1 (dry matter). IAA was also found in the commercial extract Maxicrop (Sanderson et al. 1987). Crouch et al. (1992) reported the presence of IAA from the auxin-type active fractions in the extract obtained from Ecklonia maxima. Within these active fractions, indole-3-carboxylic acid, indole-3-aldehyde, and other indole compounds were also reported. In extracts of both M. pyrifera and E. maxima obtained by the process of “cold cell burst”, the concentration of IAA was reduced as a function of storage time at 54 °C for 14 days. This was attributed to the susceptibility of this hormone to temperature degradation (Stirk et al. 2004).
Most plants keep IAA in an inactive conjugate form (Bartel 1997), where IAA may be linked to compounds such as sugars, esters, amides, amino acids, and peptides. The formation of these conjugates may be reversible, and IAA can be released by hydrolysis. Their function is primarily storage, transportation, protection against oxidation, and/or catabolism (Woodward and Bartel 2005). Alkaline hydrolysis can be used to obtain IAA in its active form (Avery et al. 1941, 1942; Ueda and Bandurski 1969). The possibility of the existence of new molecules in algal extracts with auxin-like activity is high. For example, the activity of an IAA-like compound with a chloride (Cl) radical is much higher than IAA in some bioassays (Reinecke 1999). There are many reports on purification of organohalogen compounds in macroalgae (Gribble 2003), and their biological activity needs to be investigated.
Growth promotion
Most of the algal extracts obtained in this study increased stem length of tomato seedlings. The average fresh weight of seedling stems treated with the extracts E1060, E1080, E1180, and E1280 was statistically higher than the control treatment (p < 0.05; Fig. 4). However, the average fresh weight of the root did not show statistically significant differences (p = 0.058; Fig. 4). The average stem dry weight of seedlings treated with all the extracts was not significantly different except for E1060 and E1180, which were significantly different compared with the control (water) (p < 0.05, Fig. 4).
Effects of extracts of M. pyrifera at a concentration of 1 % (v/v) on the length of shoots and roots and fresh and dry weights of tomato seedlings. Water control C; T1-E840; T2-E860; T3-E880; T4-E940; T5-E960; T6-E980; T7-E1040; T8-E1060; T9-E1080; T10-E1180, and T12-E1280. Different letters indicate significant differences between treatments (p < 0.05) according to ANOVA test followed by Tukey mean comparison
Most of the extracts in the present study increased fresh plant weight but not dry weight. This suggests that algal extracts affected water absorption in the plants because of the presence of osmoprotectant agents in algal extracts. Osmotic adjustment maintains the cellular water content when there is a reduction in the osmotic potential due to the accumulation of organic solutes in the cytoplasm and in the vacuole in stress situations arising due to soil salinity (Serraj and Sinclair 2002). Compatible solutes also known as osmolytes are hydrophilic metabolites such as sugars (sucrose and invert sugars), amino acids (proline and betaine), polyols (glycerol, mannitol), and other low molecular weight metabolites (Chen and Murata 2002). They have a primary role in the maintenance of the osmotic potential in the cytosol and are involved in protein stability and cell structures (Cushman 2001; Zhu 2003; Bartels and Ramanjulu 2005).
The osmoprotectants present in seaweeds and commercial seaweed extracts are betaines and mannitol. Betaines are an important group of osmoprotectors with glycine betaine as the most common (Blunden et al. 1993; Whapham et al. 1993; Mackinnon et al. 2010). The polyhydric alcohol mannitol is widely distributed in plants and brown algae. It is an early product functioning as an energy reserve in the short term and in osmoprotection when the algae are subjected to salinity changes (Lobban and Harrisson 1997). Mannitol content in M. pyrifera shows a seasonal variation, increasing from 2 to 15 % in winter and decreasing in autumn (Rodríguez-Montesinos and Hernández-Carmona 1991). Temperature and nutrients are most probably the factors affecting the seasonal variation of the chemical composition of M. pyrifera in the Pacific area of Baja California (Hernández-Carmona et al 2000; Hernández-Carmona et al. 2001), including mannitol.
The activity of the seaweed extracts as plant growth promoters is not only due to low molecular weight components, such as plant hormones, but is also attributed to major components such as polysaccharides (alginate, fucoidan and laminaran) and polyphenols (Craigie 2011). Oligosaccharides formed by the alkaline hydrolysis process can be recognized in the plant cell wall and act as signaling molecules, inducing the response to produce some phytochemical compounds (Klarzynski et al. 2003; Chandía et al. 2004; Pardee et al. 2004). Viscosity profiles of seaweed extracts showed a possible degradation of polysaccharides, and effects in plants were favored as the temperature and pH were increased. This suggests that these polysaccharides have different depolymerization degrees, resulting in different effects in plants. Iwasaki and Matsubara (2000) compared the activity of alginate and its oligomers formed by enzymatic digestion to increase root length of lettuce seedlings and found increased activity in the tri-, tetra-, penta-, and hexamers. In another study, Xu et al. (2003) demonstrated how the degree of depolymerization of the guluronic fractions of alginate favored the root growth of carrot and rice plants.
Conclusions
The information from this research has led to the development of a general method to produce a seaweed liquid extract with high polysaccharide content with the possibility of scaling up to an industrial production level. The process involves the following steps: (1) algae hydration, (2) alkaline hydrolysis for at least 2 h, (3) dilution in a ratio 2:1, (4) removing the algal residue by centrifugation, (5) neutralizing the extract, and (6) adding a preservative. As the pH and temperature of the extraction process were increased, the activity of the extracts on the experimental plants increased. The most active extract was produced at pH 12 and 80 °C. Under these conditions the seaweed liquid extract enhanced adventitious root formation in the mung bean cuttings assay, similar to the effect of IBA, and increased seedling growth in tomato.
References
Avery GS Jr, Berger J, Shalucha B (1941) The total extraction of free auxin and auxin precursor from plant tissue. Am J Bot 28:596–607
Avery GS Jr, Berger J, Shalucha B (1942) Total auxin extraction from wheat. Am J Bot 29:612–616
Bartel B (1997) Auxin biosynthesis. Annu Rev Plant Physiol 48:51–66
Bartels D, Ramanjulu S (2005) Drought and salt tolerance in plants. Plant Sci 24:23–58
Blunden G (1991) Agricultural uses of seaweeds and seaweed extracts. In: Guiry MD, Blunden G (eds) Seaweed resources in Europe: uses and potential. John Wiley & Sons, London, pp 61–81
Blunden G, Smith BE, Irons MW, Yang MH, Roch OG, Patel AV (1993) Betaines and tertiary sulphonium compounds from 62 species of marine algae. Biochem Syst Ecol 20:373–388
Blunden G, Jenkins T, Liu Y (1997) Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol 8:535–543
Booth E (1969) The manufacture and properties of liquid seaweed extracts. Proc Int Seaweed Symp 6:655–662
Chandía NP, Matsuhiro B, Mejías E, Moenne A (2004) Alginic acids in Lessonia vadosa: partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J Appl Phycol 16:127–133
Chen T, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Plant Biol 5:250–257
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Craigie JS, MacKinnon SL, Walter JA (2007) Liquid seaweed extracts identified using 1HNMR profiles. J Appl Phycol 22:489–494
Crouch IJ, van Staden J (1992) Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J Appl Phycol 4:291–296
Crouch IJ, van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Crouch IJ, Smith MT, van Staden J, Lewis MJ, Hoad GV (1992) Identification of auxins in a commercial seaweed concentrate. J Plant Physiol 13:590–594
Cushman JC (2001) Osmoregulation in plants: implications for agriculture. Amer Zool 41:758–769
Gribble GW (2003) The diversity of naturally produced organohalogens. Chemosphere 52:289–297
Haug A, Larsen B, Smidsröd O (1963) The degradation of alginates at different pH values. Act Chem Scan 17:1466–1468
Haug A, Larsen B, Smidsröd O (1967) Alkaline degradation of alginate. Act Chem Scan 21:2859–2870
Hernández-Carmona G, Arvizu-Higuera DL, Rodríguez-Montesinos YE (1996) Efecto de la temperatura y tiempo de extracción en el proceso de obtención de alginato de sodio a partir de Macrocystis pyrifera. Cienc Mar 22:511–521
Hernández-Carmona G, McHugh DJ, Arvizu-Higuera DL, Rodríguez-Montesinos YE (1999) Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. J Appl Phycol 10:507–513
Hernández-Carmona G, Garcia O, Robledo D, Foster M (2000) Restoration techniques for Macrocystis pyrifera populations at the southern limit of their distribution in México. Bot Mar 43:273–284
Hernández-Carmona G, Robledo D, Serviere-Zaragoza E (2001) Effect of nutrient availability on Macrocystis pyrifera recruitment survival near its southern limit of Baja California. Bot Mar 44:221–229
Holme HK, Davidsen L, Kristiansen A, Smidsrød O (2008) Kinetics and mechanisms of depolymerization of alginate and chitosan in aqueous solution. Carbohydr Polym 73:656–664
Iwasaki I, Matsubara Y (2000) Purification of pectate oligosaccharides showing root-growth-promoting activity in lettuce using ultrafiltration and nanofiltration membranes. Biosci Bioeng 89:495–497
Khan W, Rayirath UP, Subramanian S, Mundaya N, Jithesh MN, Rayirath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Kingman AR, Moore J (1982) Isolation, purification and quantitation of several growth regulating substances in Ascophyllum nodosum (Phaeophyta). Bot Mar 25:149–154
Klarzynski O, Descamps V, Plesse B, Yvin JC, Kloareg B, Friting B (2003) Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol Plant Microbe Interact 16:115–122
Kumari R, Kaur I, Bhatnagar AK (2011) Effect of aqueous extract of Sargassum johnstonii Setchell Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633
Lobban CS, Harrisson PJ (1997) Seaweed ecology and physiology. Cambridge University Press, London
MacKinnon SA, Craft CA, Hiltz D, Ugarte R (2010) Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Pardee KI, Ellis P, Bouthillier M, Towers GHN, French CJ (2004) Plant virus inhibitors from marine algae. Can J Bot 82:304–309
Reinecke DM (1999) 4-Chloroindole-3-acetic acid and plant growth. Plant Growth Regul 27:3–13
Rodríguez-Montesinos YE, Hernández-Carmona G (1991) Variación estacional y geográfica de la composición química de Macrocystis pyrifera en la Costa Occidental de Baja California. Cienc Mar 17:91–107
Sanderson KJ, Jameson PE, Zabkiewicz JA (1987) Auxin in a seaweed extract: Identification and quantification of indole-3-acetic acid by gas chromatography–mass spectrometry. J Plant Physiol 129:363–367
Serraj R, Sinclair T (2002) Osmolyte accumulation: can it really help increase in crop yield under drought conditions? Plant Cell Environ 25:333–341
Stephenson WA (1974) Seaweed in agriculture and horticulture. Rateavers Press, USA
Stirk WA, van Staden J (1997) Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J Appl Phycol 8:503–508
Stirk WA, van Staden J (2006) Seaweed products as biostimulants in agriculture. In: Critchley AT, Ohno M, Largo DB (eds) World seaweed resources: an authoritative reference system. ETI Bioinformatics, Amsterdam
Stirk WA, Ördög V, van Staden J, Jäger K (2002) Cytokinin- and auxin-like activity in Cyanophyta and microalgae. J Appl Phycol 14:215–221
Stirk WA, Arthur GD, Lourens AF, Novak O, Strnad M, van Staden J (2004) Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J Appl Phycol 16:31–39
Tarakhovskaya ER, Maslov Yu I, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physl 54:163–170
Turan M, Köse C (2004) Seaweed extracts improve copper uptake of grapevine. Acta Agric Scand B 54:213–220
Ueda M, Bandurski RS (1969) A quantitative estimation of alkali-labile indole-3-acetic acid compounds in dormant and germinating maize kernels. Plant Physiol 44:1175–1181
Whapham CA, Blunden G, Jenkins T, Hankins SD (1993) Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J Appl Phycol 5:231–234
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 95:707–735
Xu X, Iwamoto Y, Kitamura Y, Oda T, Muramatsu T (2003) Root-growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci Biotechnol Biochem 67:2022–2025
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Plant Biol 6:441–445
Acknowledgments
The University of KwaZulu-Natal, Consejo Nacional de Ciencia y Tecnología (Fellowship # 37647), Fondo mixto CONACYT-QUINTANA ROO Project # QROO-2011-C01-174594, Programa Institucional de Formación de Investigadores (PIFI), Programa de Becas de Exclusividad (COFAA-IPN), Estimulo al Desempeño de los Investigadores (EDI-IPN), and Secretaria de Investigación y Postgrado of IPN (Project #s 20090563, 20100889, and 20111212) are thanked for financial support. We wish to thank MCs Ivonne Cruz-Santander and MCs Renato Borras-Chavez for suggestions to the manuscript, Mr. Kim Siewers for English editing, and two anonymous reviewers that significantly improved this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Briceño-Domínguez, D., Hernández-Carmona, G., Moyo, M. et al. Plant growth promoting activity of seaweed liquid extracts produced from Macrocystis pyrifera under different pH and temperature conditions. J Appl Phycol 26, 2203–2210 (2014). https://doi.org/10.1007/s10811-014-0237-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0237-2