Abstract
The culture of abalone is a growth industry in Australia that primarily utilises terrestrial crops to produce formulated pellet feeds. The use of cultivated macroalgae in place of such feeds could provide for better environmental, nutritional and/or economic outcomes for this industry. However, direct comparison trials using macroalgae and formulated crop feeds are rare, and it is therefore difficult to ascertain the benefits and costs of each feed type. This study compares the benefits to growth and performance of the cultivated hybrid abalone cross (Haliotis rubra 1814 Leach and Haliotis laevigata 1808 Donovan) which was fed one of eight dietary treatments, including two commercially formulated pellet feeds and six mixed macroalgae dietary treatments. Macroalgae dietary treatments comprised the three macroalgae species Grateloupia turuturu Yamada, Ulva australis Areschoug and/or Ulva laetevirens Areschoug. Four replicate tubs, each containing 40 juvenile abalone (10–15 mm), were used to test each dietary treatment over a 12-week period. Macroalgae dietary treatments provided for significantly higher specific growth rates of abalone compared to formulated feeds, by orders of magnitude, for both length (>0.2 % compared to <0.1 % day−1, F 7, 31 = 22.3, p < 0.0001) and weight (from <0.4 to >0.8 % day−1, F 7, 31 = 24.4, p < 0.0001). In addition, abalone health and condition increased, and the proximate composition of abalone tissue had a higher carbohydrate/protein ratio, higher ash content and lower lipid content. These findings suggest that the juvenile abalone may benefit from macroalgae diets in comparison to two formulated feeds as a result of optimal proximate composition of the algae biomass and improved condition of the abalone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abalone are increasingly being farmed in aquaculture systems in response to their high value, high demand and declining wild harvest (Shpigel et al. 1999; Gordon and Cook 2001; Cook and Gordon 2010). For example, in NSW, Australia, wild harvest production has declined steadily from an annual production of 320 t in 1999 to 110 t in less than a decade (NSW Fisheries data 2010). A challenge in the transition to abalone cultivation in Australia, however, is the availability, cost and nutritional value of feeds that can deliver suitable growth rates and condition of abalone. In other parts of the world where abalone farming has been established, the availability of wild harvested macroalgae, the natural food of abalone, has been practical and cost-effective as an aquaculture feed. In contrast, Australia has neither the abundance of macroalgae, regulatory condition or cost-effective solutions that facilitate the use of wild harvest macroalgae as a feed in aquaculture. Therefore, formulated feeds were developed as the preferred feed source for abalone aquaculture in Australia.
The benefits of formulated feed include controlled and consistent nutritional profiles and ease of purchase and handling, including longer storage periods. However, as many formulated feeds are not based on the natural diet of macroalgae and are manufactured with land-sourced crops and protein instead, it is unclear how formulated feeds compare to a natural macroalgae diet nutritionally. For economic reasons, it is important that abalone feeds can deliver commercially produced abalone that reach a marketable size within the shortest possible time (Qi et al. 2010) as abalone have a long grow-out period of 3–4 years (Shpigel et al. 1999; Demetropoulos and Langdon 2004b). In addition, and not unrelated to growth rates, feed can contribute to improved abalone condition and survival, reduced waste and reduced energy requirements through improved water quality. Thus, feed can contribute to a more sustainable industry with both environmental and economic gains (Bolton et al. 2009).
Abalone feeds are currently formulated using terrestrial cereal crops (such as wheat and wheat by-products) as well as some animal proteins including fish oil and fishmeal (Kirkendale et al. 2010). These ingredients are sourced from terrestrial, freshwater crops and from fisheries resources that are in high demand (Vandepeer 2002). Therefore, there is a potential to investigate further biomass sources from macroalgae, the natural feed of abalone. Macroalgae could deliver more environmentally sustainable abalone feed, by reducing demands on terrestrial and freshwater resources, as well as provide a more nutritionally suitable feed based on the natural diet. Further gains might be made if on-site macroalgae cultivation systems are developed to facilitate concurrent recapture of nutrients and bioremediation of the farm waste water (Robertson-Anderson et al. 2008). There are costs and benefits to such systems (Bolton et al. 2009), but the integration of macroalgae culture into abalone production systems has been shown to produce sufficient biomass of macroalgae to supplement abalone feed and potentially reduce the cost of production (Bolton et al. 2009; Neori et al. 1998). The cultivation of macroalgae has not been developed in Australia, and as such, the costs of production for macroalgae in Australia are currently unknown. Demonstrating the nutritional benefits and quantifying the biomass requirements for production, however, are a precursor to costing a transition to or inclusion of macroalgae feed for abalone in Australia.
Although numerous studies have established and compared the growth rates of abalone using formulated feeds or macroalgae, there have been no directly comparable trials of the Australian hybrid abalone (blacklip Haliotis rubra and greenlip Haliotis laevigata) directly comparing the effects of macroalgae and formulated feeds, under consistent and controlled experimental conditions (Kirkendale et al. 2010). A few studies elsewhere have shown higher growth rates in abalone fed with formulated diets in relation to macroalgae diets (Viana et al. 1996; Coranzi and Illanes 1998). However, these findings are not conclusive as other studies contradict this and indicate that macroalgae feeds can potentially deliver equal or superior growth rates compared to formulated feeds (Mai et al. 1994, 1995, 1996; Rosen et al. 2000; Demetropoulos and Langdon 2004a, b; Naidoo et al. 2006). The outcomes of dietary trials will depend on the feed formulations as well as the species and condition of the macroalgae. For example, the literature demonstrates consistently that growth is improved when abalone are fed with a combination of macroalgae species in preference to feeding a single species of macroalgae (Owen et al. 1984; Day and Fleming 1992; Stuart and Brown 1994; Fleming 1995b; Simpson and Cook 1998; Gordon et al. 2006; Qi et al. 2010).
Other studies have also identified the difficulty in comparing feed trials across species of abalone, the condition and selection of macroalgae used, feed formulations and experimental systems (Fleming et al. 1996). A review of 130 diets and growth rates from the scientific literature showed that directly comparable macroalgae and formulated feed trials have rarely been undertaken (Kirkendale et al. 2010). Across trials of growth rates using formulated or macroalgae feed, there are some patterns of nutritionally suitable macroalgae and feeds that emerge from the review of the literature (Fig. 1), but there are also contradictions, inconclusive findings and outcomes that are only relevant in the local context. Therefore, the extrapolation of results from the literature to other conditions is not valid, and there are many contradictions to the suitability of macroalgae as a feed source without further directly comparable trials within the same experimental conditions. Thus, there is a need to assess abalone growth rates in directly comparable feeding trials within the same experimental conditions beyond the Australian context as well.
Specific growth rates of the length of abalone as identified in over 130 diets from the peer-reviewed literature and adapted from Kirkendale et al. (2010). Diets are categorised across artificially formulated feeds (A) and macroalgal diets including species of Phaeophyta (B), mixed macroalgae (M), Rhodophyta (R) and Chlorophyta (G)
In testing macroalgae feeds, the life stage of the abalone is important to consider as nutritional requirements and behaviour shift over the 3–4-year cultivation period. One particular life stage of abalone in a farming system is the weaning stage following a 6- to 9-month nursery stage on a diet of microalgal biofilm. Typically for Australian cultivation systems, abalone at about 10 mm in length is transitioned to small-particle formulated diets towards a grow-out stage for between 2 and 4 years (industry information). There are a number of reasons why including macroalgae in the diet at the weaning stage of the abalone farming system might be of benefit. This includes textural, sensory and nutritional similarity to the nursery diet in comparison to formulated pellet feeds that are based terrestial and fishmeal sources. In addition, there may be benefits to farm management as the small-particle formulated feeds incur water quality and labour maintenance costs, while live macroalgae improve water condition and do not leach or degrade as rapidly.
The objective of this study was to assess the performance of abalone fed with mixed, cultivated macroalgae dietary treatments, in direct comparison to commercially formulated feeds. Macroalgae dietary treatments comprised three species of macroalgae, Grateloupia turuturu Yamada (Rhodophyceae), and the two species of Ulva, Ulva australis Areschoug and Ulva laetevirens Areschoug (Chlorophyceae), and were compared against two commercially available formulated pellet feeds. The abalone were a typical Australian hybrid (H. rubra × H. laevigata), which were being weaned from nursery microalgal diets at the beginning of their grow-out phase.
Materials and methods
Juveniles of the hybrid abalone species Haliotis rubra × H. laevigata were selected at the abalone farm, Abtas Seafoods©, in Clarence Point (41.12 S, 146.81 E), Tasmania, Australia, between June and September 2010. This hybrid abalone is now commonly grown in Australia, and details of the selective breeding lines are usually specific in the commercial property of the individual farms. Although the genetic predisposition will always affect performance, the purpose of this study is a direct comparison across feeds for a typical cohort of randomly selected abalone from a farm situation, and details of the hybrid context are beyond the scope of this manuscript.
Juvenile abalone were from the same cohort and were 9 months old at a late-nursery stage (average length 11.73 ± 1.16 mm and weight 0.196 ± 0.0704 g). Abalone (n = 1,280) between 10 and 15 mm in length were randomly selected from one nursery tank in which they had been feeding on microalgae (Ulvella lens and diatoms). Tissue samples from an additional 40 abalone from the cohort were kept for determination of the proximate composition of the starting abalone tissue.
Experimental design
Groups of 40 abalone were kept in each one of 32 rectangular, 15-L tubs with a 6-mm polyethylene oyster mesh basket lining for ease of maintenance with minimal disturbance to the abalone. Two corrugated plastic plates (30 × 8 cm each) were provided as hides, and the water was aerated from perforated pipes along the tub base. The system delivered 0.3 L min−1 of UV-filtered seawater to each tub. The system was set up indoors with fluorescent cool white lights at a distance of 1.5 m as the only light source, with a photoperiod of 12:12 h dark/light. The temperature was maintained at 13.71 °C (±0.31) using a heat exchanger. Dissolved oxygen content, salinity and pH were recorded each day in each tub to ensure that tub water quality was not compromised.
For a relative comparison of experimental system conditions with extant farm system conditions, an additional population of abalone was moved into a farm weaning tank at low density and fed with a diet of commercially formulated feed (FF1) at normal farm feeding rates for the duration of the experimental period. In addition, another population of abalone was left at 50 % of the normal farm stocking density in the nursery tanks on farm and fed on plates of the microalgae for the duration of the experiment.
One of eight dietary treatments was provided to four replicate tubs of abalone; thus, four of 40 abalone received one of eight unique dietary treatments throughout the experiment (Table 1). Two dietary treatments comprised two commercially formulated feeds (FF) sourced from Australian independent feed suppliers. These feeds were stored in dry, laboratory conditions. FF1 was a formulated feed specifically designed with a small grain size (<1 mm) for juvenile abalone being weaned onto the formulated diets used on farm. FF2 was a formulated feed developed for larger abalone but was crushed to produce pellets of a similar size as FF1. A sample of each dietary treatment was stored for analysis of proximate composition.
Macroalgal dietary treatments included one formulated feed supplemented with fresh macroalgae and five combinations of the three species of macroalgae G. turuturu Yamada (Rhodophyceae) and the two species of Ulva, U. australis 1854 Areschoug and U. laetevirens Areschoug (Chlorophyceae). The macroalgae culture stocks were collected from self-seeding populations in the nursery tanks (U. australis) and the outflow drains (U. laetevirens and G. turuturu) of the abalone farm in north-eastern Tasmania. Samples of each of the macroalgae were identified using molecular barcodes by Gary Saunders at the University of New Brunswick, Canada (G. turuturu), and Kirkendale et al. (2012) (Ulva spp.). The macroalgae were on grown in 600-L tanks with aerated tumble culture, shaded natural sunlight supplemented with a 12-h photoperiod of artificial light and weekly seawater exchange. A tank of U. laetevirens was also cultivated in elevated ammonia nitrogen conditions at weekly starting concentrations of 0.5 mg L−1 to enhance the protein content, by the addition of ‘Abasol©’ fertiliser at 0.8 g L−1 a week. Macroalgae samples were taken weekly from each of these four tubs over a 12-week period and combined for each species for proximate composition analysis.
All dietary treatments were provided to the abalone tubs at consistent dry weight equivalents, although in dried and fresh forms, respectively, for each of the formulated and macroalgal dietary treatments. The macroalgae dry weight equivalents were determined prior to the experiment by spinning to a constant wet weight and then drying at 70 °C to consistently dry weight equivalents (G. turuturu 23.72 %, U. australis 23.40 %, U. laetevirens 19.11 % of wet weight). The abalone were fed fresh macroalgae to excess dry weight equivalent at a rate of 10 % of initial wet body weight per day (0.787 g dw day−1). These feeding rates were suitably in excess throughout the experiment, and feed was accessible at all times. As the macroalgae were live feeds, which did not degrade, macroalgae dietary treatments were replaced weekly, at the same starting amount, while the commercially formulated feeds (which are subject to degradation over time) were replaced daily. Feed remaining in the tubs at the end of each feeding period was removed and collected using a siphon and sieve to separate it from the abalone faeces.
Comparison across dietary treatments
All abalone were measured for weight and length every 4 weeks within a 12-week period. Abalone were anaesthetised for 15 min using 2-phenoxyethanol, removed from the tub and blotted dry. Abalone shell lengths (longest axis) were measured using digital callipers to the nearest 0.01 mm, and then abalone were weighed to the nearest 0.001 g. The specific growth rates (SGR) for both length and weight gains and the condition index (CI) and muscle (g) to shell ratios (mm) were calculated to compare the abalone growth and condition using the following formulae:
where l f and l i were the final and initial mean lengths (mm), w f and w i were the final and initial mean wet weights (g) and t was time in days. Mortality rates were also monitored throughout the trial.
Feed intake (g week−1) was calculated for each of the dietary treatments in terms of dry weight by subtracting the amount of feed remaining from the amount of feed provided. The remaining formulated feed was oven dried at <80 °C to a constant weight before being weighed. The remaining macroalgae were collected, spun dried and weighed, and macroalgae weights were converted to dry weight equivalents. Control experiments, without abalone, were run concurrently to determine the water stability of each of the diets, so that the intake rate of consumption could be adjusted accordingly. The feed conversion (FCR) was calculated as the ratio between the growth of the abalone and the amount of food ingested, in terms of weight.
A short stress test was also undertaken at completion of the dietary treatment trials. Twenty randomly selected abalone were left in each of the dietary treatment tubs. These were then subject to a period of high stress conditions, which included 5 days of heat stress (where the temperature was raised from 13.87 ± 0.31 to 23.51 ± 0.43 °C) and 4 days of salinity stress (where the salinity was lowered from 32.73 ± 1.18 to 20.11 ± 1.55 ppt). The mortality rates in each dietary treatment were used as a measure of stress.
Proximate composition analysis
At the end of the trial, the dietary treatments and the abalone tissue samples were freeze dried, homogenised and pooled for each tub. Standard methods were used to determine the dry weight (drying at 135 °C to a constant weight; AOAC 1990), nitrogen content (Kjeldahl using a Cu/Se catalyst), total lipid (chloroform methanol extraction; Bligh and Dyer 1959) and ash content (combustion at 600 °C for 2 h; AOAC 1990). Crude protein was calculated as nitrogen × 6.25, although there may be more biomass-specific conversion factors that are determined in the future (Sriperm 2011). Carbohydrate content was calculated by the mass difference post-combustion. Gross energy was calculated as the sum of the energy within each of the macronutrients based on energy values of 23.6 MJ kg−1 for protein, 36.2 MJ kg−1 for lipids and 17.2 MJ kg−1 for carbohydrate (Brafield 1985).
Statistical analyses
Analysis of variance and comparisons of means were performed using JMP software for specific growth rates, condition indices and productive energy value. Heterogeneity of variance was visualised using box plots, and normal distribution was confirmed a priori. Tukey–Kramer HSD (p = 0.05) analyses of the abalone growth was used a posteriori to determine which dietary treatments differed for all of the parameters. Proximate composition profiles of both dietary treatment and the initial and final abalone tissue was analysed visually as multivariate data in multidimensional scaling plots (MDS) (PRIMER-e software; Plymouth Marine Labs; Clarke and Gorley 2006) to identify patterns of differences across dietary treatments. Euclidean distance resemblance matrix data were used to create MDS plots with both untransformed and fourth root transformations to assess the relative influence of differences in relation to highly abundant compositional categories of abalone tissue. Any differences were tested using a one-way analysis of similarity (ANOSIM application in PRIMER-e software), and similarity percentage analysis (SIMPER application in PRIMER-e software) was used to identify which parameters contributed most to the differences between dietary treatments.
Results
Abalone cultures were successfully maintained for all dietary treatments for 12 weeks with minimal variation in water quality over the experimental period: dissolved oxygen between 92.4 and 97.3 % (SD = 1.1), salinity between 26.1 and 34.3 ppt (SD = 1.2) and the pH between 7.28 and 8.39 (SD = 0.28). There was an overall survival rate of over 95 % and an increasing trend of specific growth rates for abalone length throughout the experiment, while it decreased for weight; however, this was minimal and consistent across all of the dietary treatments and is not reported on further here.
Juvenile abalone fed any of the macroalgae treatments had significantly improved performance compared to the formulated feeds. Macroalgae dietary treatments provided for significantly higher SGR of abalone, at 0.32 mm day−1 and 1.05 g day−1, compared to 0.06 mm day−1 and 0.39 g day−1 for the best-performing formulated feed dietary treatments (SGRl F 7, 31 = 22.3, p < 0.0001, and SGRw F 7, 31 = 24.4, p < 0.0001) (Fig. 2). There was no significant difference between the growth rates of abalone across the macroalgae dietary treatments, although there was a trend for both greater length and weight SGRs for the macroalgal combination of G. turuturu and U. laetevirens and U. laetevirens that had been protein enhanced.
Specific growth rate (% day−1), for length and weight, in juveniles of the hybrid abalone species (H. rubra × H. laevigata) fed a range of commercially formulated and mixed macroalgae dietary treatments over 12 weeks (SE bars shown, n = 160). Content of feeds detailed in Table 1. Note feeds sharing the same letter were not significantly different (Tukey’s test, p < 0.05)
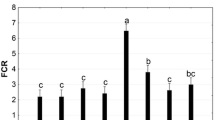
The food conversion ratios differed across some of the dietary treatments (F 7, 31 = 4.36, p = 0.033); however, overall, they were comparable on a dry-weight basis with no trends in relation to macroalgae and formulated feeds dietary treatments (Table 2). Feed stability tests indicate some instability of dietary treatment feeds, namely, some sporulation of Ulva and degradation of formulated feeds. However, formulated feeds demonstrated an overall lower stability (FF1 27.8 %, FF2 76.2 % of feed remaining after a week) compared to macroalgae dietary treatments (G. turuturu 99.7 %, U. australis 99.3 %, U. laetevirens 94.5 %, U. laetevirens (E) 95.3 %). This may have contributed to some of the variation in FCR results but is considered here as part of the natural variability of feed quality and is accounted for a feed cost (i.e. part of the feed requirement and FCR in each dietary treatment).
Both condition indices of the abalone, tissue weight to shell length (CI) and muscle weight to shell weight (MW/SW) ratios, were higher for macroalgae dietary treatments by between 13–43 and 18–32 %, respectively, compared to formulated feeds dietary treatments, but this was only significant for some of the macroalgae dietary treatments (Table 2; CI F 7, 31 = 7.07, p = 0.0001; MW/SW F 7, 31 = 2.98, p = 0.02). There was >95 % survival for all dietary treatments and no significant difference between them, while further stress tests demonstrated an increased tolerance to stress for macroalgae dietary treatments (Table 2); however, this was not significant in the time frame of 9 days of the test (F 31 = 2.21, p = 0.07).
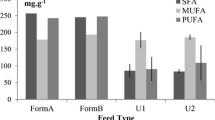
The proximate composition of abalone tissue across the dietary treatments differed significantly between the formulated feeds and all of the dietary treatments containing macroalgae (ANOSIM global R = 0.455, p = 0.001) (Fig. 3, Table 3). This relative difference in proximate composition of abalone tissue was due to an increase in the carbohydrate/protein ratio and higher ash content in the macroalgae fed abalone tissue (Table 4). In addition, the formulated feed dietary treatments produced tissue with a higher lipid content as well reduced dry matter recovery. This reflected a significant difference in the proximate composition of macroalgae and formulated dietary treatments (ANOSIM Global R = 1, p = 0.036); however, the difference in the proximate composition of dietary treatments mostly reflected up to a tenfold higher lipid content in formulated feeds as well as up to 70 % more protein, while macroalgae dietary treatments had up to a fivefold higher ash content. The productive energy value of the dietary treatments was significantly higher for the macroalgae compared to the formulated feeds (F 7, 31 = 5.21, p = 0.001).
Discussion
Mixed macroalgae dietary treatments, as well as a macroalgae-enhanced formulated feed dietary treatment, delivered significantly greater specific growth rates of juvenile abalone in comparison to formulated feeds. Abalone SGR for macroalgae dietary treatments was higher by more than twofold for both length and weight. This supports the contention that mixed macroalgae diets can provide beneficial nutritional value and improved growth rates of farmed abalone during weaning from microalgal diets, compared to current commercial formulated feeds. This is consistent with and supports the few studies with a similar contention (Daume et al. 2007) and feeding trials that directly compare formulated feeds and macroalgae (Capinpin and Corre 1996; Naidoo et al. 2006).
While this trial demonstrates good growth rates for abalone fed macroalgae in the late nursery growth stage, other studies (Capinpin and Corre 1996; Dlaza 2006) have shown that macroalgae can provide for better long-term growth than artificial feeds even at later stages of abalone life history; however, this is also dependent on the macroalgae species. In contrast to many of these studies, the current trial could not prioritise types of macroalgae or any of the six diverse compositions of macroalgae dietary treatments as there was no significant difference to abalone growth or condition across these treatments. Specifically, the red macroalgae G. turuturu provided for good and comparable, but not higher, growth rates compared to the other macroalgae, as other studies have generalised in favour of Rhodophycean macroalgae. The most evident, yet non-significant trend in this way was for improved growth in the protein-enhanced U. laetevirens mixed dietary treatments. Protein-enhanced Ulva has been shown to provide good growth rates for Haliotis tuberculata (Neori et al. 1998; Shpigel et al. 1999), Haliotis discus hannai (Corazani and Illanes 1998; Shpigel et al. 1999) and Haliotis roei (Boarder and Shpigel 2001).
The ingestion rates of the commercially formulated dietary treatments were very low in comparison to that of the macroalgae and could also account for the slow growth rates of abalone fed these dietary treatments. This suggests that the pellets are either less familiar or palatable to the abalone coming from a diet of microalgae in the nursery stage. This is an important consideration when weaning abalone onto new feed types, and macroalgae can potentially provide a suitable feed to extend or transition from the nursery phase of production.
The feed conversion ratios did not differ significantly across the dietary treatments on a dry-weight basis. For economic comparisons across feed types to be made, dry weight equivalents must be used as, anecdotally, industry have shunned seaweed feeds based on high wet weight FCR equivalents. To avoid confusion over such comparisons across wet weight and dry weight feed sources, it is suggested that cost conversion ratios (dollar cost × kg feed) (kg growth)−1) rather than feed conversion ratios need to be determined to establish the economic viability of using macroalgae as a feed source. However, the production costs of a reliable cultivated source of macroalgae in Australia have not been established and require further attention; stand alone large-scale macroalgae cultivation would incur substantial costs, but the business case for this is not known and has proven to be viable in other nations such as Japan where macroalgae as a human food fetches a high price [e.g. the Porphyra cultivation industry produced an estimated biomass value of US $1.25 billion in 2009 (FAO data 2010)]. In addition, factors such as the water stability of the feed must be considered in cost–benefit analyses, and data in this study demonstrate an 18–72 % higher water stability of macroalgae compared to formulated feeds. Furthermore, tank maintenance, feeding regimes and labour costs need to be considered and are expected to be lower for macroalgae diets.
The overall differences in the proximate composition of the tissue of the abalone fed macroalgae dietary treatments compared to formulated feeds suggest that they provide distinct nutritional value as a seafood product. The macroalgae dietary treatments provided for increased carbohydrate/protein ratios and ash content (5–10 %) in abalone tissue in comparison to abalone fed formulated feeds. In contrast, the formulated feed dietary treatments provided for up to a tenfold higher lipid content in abalone tissue. The fact that carbohydrates are primarily used for energy storage within abalone suggests that there were higher carbohydrate levels within the muscle tissue from well-balanced feeds that allowed for the storage of energy (Dunstan 2010). Carbohydrate storage of glycogen is potentially a good indicator of health and taste (Brown et al. 2008).
The macroalgae dietary treatments in this study had close to 33 % lower protein content than formulated feeds, yet still supported higher growth rates in the abalone. The macroalgae dietary treatments provided for higher productive energy value, in terms of converting dietary proteins and lipids into muscle tissue. This implies that although the macroalgae contain lower levels of protein, they have higher digestible protein content (Fleming 1995a; Shpigel et al. 1999; Boarder and Shpigel 2001) and a more suitable balance of amino acids (Britz and Hecht 1997; Dunstan 2010) than the two commercially formulated feeds. This is consistent with other studies that identify that the amino acid balance is more important than protein content (Sales and Britz 2001; Gómez-Montes et al. 2003; Dunstan 2010). The lower growth rates for abalone fed the formulated feeds are supported by previous research where growth rates were lower in abalone fed high-protein diets due to displacement of other essential nutrients (Mercer et al. 1993) and a reduction in protein utilisation (with excess protein being catabolised) (Britz 1996; Gómez-Montes et al. 2003). Feeds that are formulated for smaller abalone (e.g. commercial feed FF1) could be produced to have lower protein content as juvenile abalone have lower overall protein requirements than larger abalone (Britz and Hecht 1997).
In addition to the basic elements of nutrition, our findings support the concept of functional foods for cultivated species where the complexity of feeds is as important to abalone as the basic macronutrients. As abalone have evolved on a diet of macroalgae, there are numerous complex nutritional, immunomodulatory, trace element and chemical processes that are important to their growth and that are difficult to replicate in processed and formulated diets, especially from terrestrial sources. For example, carbohydrates in macroalgae are different to those of land crops, and abalone digestive enzymes are specific to just those types of polysaccharides (Vandepeer 2002). This needs to be taken into account when developing feeds for abalone culture.
This study, in synthesis, indicates that locally cultivated mixed macroalgae dietary treatments provided for higher growth rates and improved abalone condition, compared to two independently sourced and widely used formulated abalone feeds, for juvenile Australian hybrid abalone (H. rubra × H. laevigata). It reveals the potential of three local macroalgae species, including the invasive G. turuturu, which could be utilised further or used in conjunction with other readily available macroalgae species. The positive trend of increasing growth with a nutrient-enriched Ulva species also demonstrates the whole farm improvements that could potentially be made by the integration of macroalgae feed production on farm through bioremediation and integrated multi-trophic aquaculture technologies that can provide a protein-enhanced macroalgae feed supply for abalone (Neori et al. 1998; Bolton et al. 2009). The study also determined that the experimental conditions and findings were not directly comparable to farm scale conditions. Replicated farm scale trials will be an important future study to confirm the commercial relevance of these findings and to determine if macroalgae can provide for improved environmental standards of abalone farming, operational efficiency and potentially economic gains. Australian culture systems are predominately designed for the use of formulated feeds and would need to be adapted to incorporate macroalgae feeds.
References
AOAC (Association of Official Analytical Chemists) (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Boarder SJ, Shpigel M (2001) Comparative growth performance of juvenile Haliotis roei fed on enriched Ulva rigida and various artificial diets. J Shellfish Res 20:653–657
Bolton J, Robertson-Andersson D, Shuuluka D, Kandjengo L (2009) Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: a SWOT analysis. J App Phycol 21:575–583
Brafield AE (1985) Laboratory studies of energy budgets. In: Tytler P, Calow P (eds) Fish energetics: new perspectives. The Johns Hopkins University Press, Baltimore, MD, pp 257–281
Britz PJ (1996) The suitability of selected protein sources for inclusion in formulated diets for the South African abalone Haliotis midae. Aquaculture 140:63–73
Britz PJ, Hecht T (1997) Effect of dietary protein and energy level on growth and body composition of South African abalone, Haliotis midae. Aquaculture 156:195–210
Brown MR, Sikes AL, Elliot NG, Tume RK (2008) Physicochemical factors of abalone quality: a review. J Shellfish Res 27:835–842
Capinpin EC, Corre KG (1996) Growth rate of the Philippine abalone, Haliotis asinina fed an artificial diet and macroalgae. Aquaculture 144:81–89
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth UK, 192 pp
Cook PA, Gordon HR (2010) World abalone supply, markets, and pricing. J Shellfish Res 29:569
Coranzi D, Illanes JE (1998) Growth of juvenile, Haliotis discus hannai Ino 1953 and Haliotis rufescens Swainson 1822, fed with different diets. J Shellfish Res 17:663–666
Daume S, Davidson M, Ryan S, Parker F (2007) Comparisons of rearing systems based on algae or formulated feed for juvenile greenlip abalone (Haliotis laevigata). J Shellfish Res 26:729–735
Day RW, Fleming AE (1992) The determinants and measurement of abalone growth. In: Shepherd SA, Tegner MJ, del Proo SA G (eds) Abalone of the world: biology, fisheries and culture. Blackwell Scientific Publications, Oxford, UK, pp 141–168
Demetropoulos C, Langdon C (2004a) Pacific dulse (Palmaria mollis) as a food and biofilter in recirculated, land-based abalone culture systems. Aquat Eng 32:57–75
Demetropoulos CL, Langdon CJ (2004b) Effects of nutrient enrichment and biochemical composition of diets of Palmaria mollis on growth and condition of Japanese abalone, Haliotis discus hannai and red abalone, Haliotis rufescens. J Exp Mar Biol Ecol 308:185–206
Dlaza TS (2006) Growth of juvenile abalone under aquaculture conditions. M.Sc. Thesis University of the Western Cape, Bellville, South Africa
Dunstan GA (2010) A simple model for the determination of the relative utilization efficiency of protein by blacklip abalone (Haliotis rubra Leach). Aquacult Nutr 16:1–12
Fleming AE (1995a) Growth, intake, feed conversion efficiency and chemosensory preference of the Australian abalone, Haliotis rubra. Aquaculture 132:297–311
Fleming AE (1995b) Digestive efficiency of the Australian abalone Haliotis rubra in relation to growth and feed preference. Aquaculture 134:279–293
Fleming AE, Van Barneveld RJ, Hone PW (1996) The development of artificial diets for abalone: a review and future directions. Aquaculture 140:5–53
Gomez-Montes L, Garcia-Esquivel Z, D'Abramo LR, Shimada A, Vasquez-Pelaez C, Viana MT (2003) Effect of dietary protein: energy ratio on intake, growth and metabolism of juvenile green abalone Haliotis fulgens. Aquaculture 220:769–780
Gordon HR, Cook PA (2001) World abalone supply, markets and pricing: historical, current and future. J Shellfish Res 20:567–570
Gordon N, Neori A, Shpigel M, Lee J, Harpaz S (2006) Effect of diatom diets on growth and survival of the abalone Haliotis discus hannai post larvae. Aquaculture 252:2–4
Kirkendale L, Robertson-Andersson D, Winberg PC (2010) Review on the use and production of algae and manufactured diets as feed for sea-based abalone aquaculture in Victoria. Report to Department of Primary Industries, Fisheries Victoria, University of Wollongong. Retrieved from http://ro.uow.edu.au/smfc/7/ on 11/08/2012
Kirkendale L, Saunders G, Winberg PC (2012) A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. J Phycol. doi:10.1111/jpy.12016
Mai K, Mercer JP, Donlon J (1994) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino: II. Amino acid composition of abalone and six species of macroalgae with an assessment of their nutritional value. Aquaculture 128:115–130
Mai K, Mercer JP, Donlo J (1995) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino: III. Response of abalone to various levels of dietary lipid. Aquaculture 134:65–80
Mai K, Mercer JP, Donlon J (1996) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino: V. The role of polyunsaturated fatty acids of macroalgae in abalone nutrition. Aquaculture 139:77–89
Mercer JP, Mai KS, Donlon J (1993) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata and Haliotis discus hannai Ino: I. Effects of algal diets on growth and biochemical composition. Invert Reprod Dev 23:75–88
Naidoo K, Maneveldt G, Ruck K, Bolton JJ (2006) A comparison of various seaweed-based diets and formulated feed on growth rate of abalone in a land-based aquaculture system. J App Phycol 18:437–443
Neori A, Ragg NLC, Shpigel M (1998) The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: II. Performance and nitrogen partitioning within an abalone (Haliotis tuberculata) and macroalgae culture system. Aquat Eng 17:215–239
Owen B, Disaivo LH, Ebert EE, Fonck E (1984) Culture of the California red abalone Haliotis rufescens Swanson (1822) in Chile. Veliger 27:101–105
Qi Z, Liu H, Li B, Mao Y, Jiang Z, Zhang J, Fang J (2010) Suitability of two seaweeds, Gracilaria lemaneiformis and Sargassum pallidum, as feed for the abalone Haliotis discus hannai Ino. Aquaculture 300:189–193
Robertson-Anderson D, Potgieter M, Hansen J, Bolton JJ, Troell M, Anderson RJ, Halling C, Probyn T (2008) Integrated seaweed cultivation on an abalone farm in South Africa. J Appl Phycol 20:575–595
Rosen G, Langdon CJ, Evans F (2000) The nutritional value of Palmaria mollis cultured under different light intensities and water exchange rates for juvenile red abalone Haliotis rufescens. Aquaculture 185:121–136
Sales J, Britz PJ (2001) Review: research on abalone (Haliotis midae L.) cultivation in South Africa. Aquacult Res 32:863–874
Shpigel M, Ragg NL, Lapatsch I, Neori A (1999) Protein content determines the nutritional value of the seaweed Ulva lactuca L. for the abalone Haliotis tuberculata L. and H. discus hannai Ino. J Shellfish Res 18:227–233
Simpson JA, Cook PA (1998) Rotation diets: a method of improving growth of cultured abalone using natural algal diets. J Shellfish Res 17:635–640
Sriperm N, Pesti GM, Tillman PB (2011) Evaluation of the fixed nitrogen-to-protein (N:P) conversion factor (6.25) versus ingredient specific N:P conversion factors in feedstuffs. J Sci Food Agric 91:1182–1186
Stuart MD, Brown MT (1994) Growth and diet of cultivated black-footed abalone, Haliotis iris (Martyn). Aquaculture 127:329–337
Vandepeer M (2002) Abalone aquaculture subprogram: adaptation of nutritional technologies developed for greenlip abalone for the production of suitable manufactured feeds for blacklip abalone. Fisheries Research and Development Corporation, Canberra, Australia
Viana MT, Lopez LM, Garcia-Esquivel Z, Mendez E (1996) The use of silage made from fish and abalone viscera as an ingredient in abalone feed. Aquaculture 140:87–98
Acknowledgments
The project would not have been possible without the facilities that were generously made available at Abtas Seafoods. A big thank, in particular, to Nick Savva, Bruce Hawkins and Honnie Harris from Abtas who provided a lot of support for the project. We are also grateful for the laboratory facilities made available at the Australian Maritime College, University of Tasmania. Further, we are grateful to Dr. Gary Saunders at the Centre for Environmental and Molecular Algal Research (CEMAR), Department of Biology University of New Brunswick, Canada, for genetic identification of G. Turuturu, and Dr. Lisa Kirkendale at Shoalhaven Marine and Freshwater Centre, University of Wollongong, for genetic identification of Ulva spp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulvaney, W.J., Winberg, P.C. & Adams, L. Comparison of macroalgal (Ulva and Grateloupia spp.) and formulated terrestrial feed on the growth and condition of juvenile abalone. J Appl Phycol 25, 815–824 (2013). https://doi.org/10.1007/s10811-013-9998-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-9998-2