Abstract
Higher lipid production and nutrient removal rates are the pursuing goals for synchronous biodiesel production and wastewater treatment technology. An oleaginous alga Chlorella sp. HQ was tested in five different synthetic water, and it was found to achieve the maximum biomass (0.27 g L−1) and lipid yield (41.3 mg L−1) in the synthetic secondary effluent. Next, the effects of the stationary phase elongation and initial nitrogen (N) and phosphorus (P) concentrations were investigated. The results show that the algal characteristics were affected apparently under different N concentrations but not P, which were verified by Logistic and Monod models. At the early stationary phase, the algal biomass, lipid and triacylglycerols (TAGs) yields, and P removal efficiency increased and reached up to 0.19 g L−1, 46.7 mg L−1, 14.3 mg L−1, and 94.3 %, respectively, but N removal efficiency decreased from 86.2 to 26.8 % under different N concentrations. And the largest TAGs yield was only 6.4 mg L−1 and N removal efficiency was above 71.1 % under different P concentrations. At the late stationary phase, the maximal biomass, lipid and TAGs yields, and P removal efficiencies primarily increased as the initial N and P concentrations increase and climbed up to 0.49 g L−1, 99.2 mg L−1, 54.0 mg L−1, and 100.0 %, respectively. It is concluded that stationary phase elongation is of great importance and the optimal initial N/P ratio should be controlled between 8/1 and 20/1 to serve Chlorella sp. HQ for better biodiesel production and secondary effluent purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid depletion of fossil fuel reserves and the global awareness of climate change have promoted the exploration of renewable and sustainable energy sources, especially biofuels, which is superior in keeping the environmental and economic sustainability (Balat 2008; Patil et al. 2008). Recently, microalgae have emerged as a promising alternative to substitute for traditional biofuel feedstock, such as oil crops, waste cooking oils, and animal fats, owing to their low land requirements (Deng et al. 2009; Schenk et al. 2008), high photosynthetic efficiency (Deng et al. 2009), and being non-toxic and biodegradable products (Schenk et al. 2008).
Cultivating microalgae in wastewater to produce lipid and purify water synchronously has been widely discussed because of the cultivation-cost-reduction ability in that the algae can utilize valuable inorganic nutrients from wastewater and simultaneously save significant freshwater resources (Aslan and Kapdan 2006; Shi et al. 2007). So far, microalgae have been applied in many types of wastewater treatment, i.e., in domestic secondary effluent, Chlorella ellipsoidea YJ1 was observed to achieve 11.4 mg L−1 biomass and remove more than 99 % nitrogen (N) and 90 % phosphorus (P) (Yang et al. 2011); in aquaculture wastewater, 96.3 % nitrite and 98.8 % P were removed by Scenedesmus sp. LX1 with 31.6 % lipid content (Ma et al. 2012); and in waste discharges, Scenedesmus obliquus was found to accumulate as high as 1 g L−1 lipid up to stationary phase followed with an optimized stage for 8 days of cultivation (Mandal and Mallic 2011).
However, to improve the economic feasibility of the synchronous technology for lipid production and wastewater treatment, screening for suitable algae species is still crucial. Previous studies have found several algae species with high lipid content, i.e., Botryococcus braunii (15–75 %) (Chisti 2007), Schizochytrium sp. (50–77 %) (Chisti 2007), Nannochloropsis sp. (31–68 %) (Chisti 2007), and Chlorella protothecoides (up to 55 %) (Xiong et al. 2008). Apart from that, some algal strains’ superior abilities of nutrient removal were also verified, for instance, Spirulina could remove 84–96 % NH4–N and 72–87 % P from pig wastewater (Olguín et al. 2003), 88.8 % N and 70.3 % P were removed by Chlorella pyrenoidosa in soybean processing wastewater (Su et al. 2011), and C. ellipsoidea YJ1 was found to remove more than 99 % N and 90 % P from secondary effluent (Yang et al. 2011). However, the problem of finding more algae strains with high lipid production and strong abilities of removing N and P to compensate for the limited number of species and strains has not been solved yet.
Not only algal species selection but also environmental factors and growth conditions should be taken into consideration when applying the coupled system above. Those factors include pH (Abu-Rezq et al. 1999), temperature (Csavina et al. 2011), light source and intensity (Tang et al. 2011), trace elements (Lin et al. 2012), nutrient concentration (Cakmak et al. 2012; Khozin-Goldberg and Cohen 2006; Merzlyak et al. 2007), as well as growth phase (Liang and Mai 2005), etc. Among these factors, N and P as the essential nutrient elements for algal growth are believed to exert great influence on algal lipid accumulation and nutrient removal ability. Converti et al. (2009) showed that an N concentration decrease to 25 % significantly increased the lipid content of Nannochloropsis oculata from 7.9 to 15.3 % and from 5.9 to 16.4 % in Chlorella vulgaris. Li et al. (2010a) investigated the different concentrations of N and P on the lipid accumulation in Scenedesmus sp. LX1 and found that the lipid content was enhanced up to 30 % under 2.5 mg L−1 N and 53 % under 0.1 mg L−1 P. In fact, triacylglycerols (TAGs) in algal lipids are the best feedstock to produce biodiesel through transesterification (Sharma et al. 2012). Although a host of recent studies have discussed the effects of N and P concentrations on algal lipid accumulation so far, little attention has been paid to their effects on TAGs accumulation.
Additionally, the growth phase of algae also has been found to have a significant impact on lipid accumulation. Su et al. (2013) discovered that there existed significant differences in the intercellular lipid metabolites among exponential, early stationary, and late stationary phases. Liang and Mai (2005) found that the fatty acid composition of four species of marine diatoms (Chaetoceros gracilis, Cylindrotheca fusiformis, Phaeodactylum tricornutum, and Nitzschia closterium) were changed when the algae were cultivated at the exponential growth phase, the early stationary phase, or the late stationary phase. Especially the eicosapentaenoic acid (EPA) and polyunsaturated fatty acids were found to decrease with increasing culture age. Additionally, the lipid content of C. ellipsoidea YJ1 in secondary effluents was reported to be dramatically higher at the stationary phase (up to 40 %) than that at the log phase (up to 15 %) (Yang et al. 2011). However, studies of when in the stationary phase was the best time to harvest microalgae for lipid production and nutrient removal are few, especially for TAGs accumulation.
Based on the above, the effects of stationary phase elongation and initial N and P concentrations on lipid (TAGs) production and nutrient removal of the oleaginous alga Chlorella sp. HQ were examined in this paper, the findings of which will help determine the optimal algae growth conditions for simultaneous lipid production and nutrient removal and provide theoretical and technical support for future application.
Materials and methods
Algal growth assay
The Chlorella sp. HQ (collection no. GCMCC7601 in China General Microbiological Culture Collection Center) was unexpectedly isolated from a culture of aging B. braunii exposed to air in a previous study. In this study, Chlorella HQ was cultivated at initial inoculation density of 2 × 105 cells mL−1 in 300 mL autoclaved culture medium in 500-mL conical flasks in an artificial climate chamber (HPG-280H, HDL, China) at 25 °C and an irradiance of 60 μmol photons m−2 s−1 and light/dark ratio of 14:10. All tests were carried out in triplicate.
The culture media used in this study were SE medium, modified BG11 (50 % BG11) medium, and five different synthetic water samples. The SE medium was composed of (in mg L−1) 250 NaNO3, 75 K2HPO4·3H2O, 75 MgSO4·7H2O, 25 CaCl2·2H2O, 175 KH2PO4, 25 NaCl, 5 FeCl3·6H2O, 0.81 FeCl3, 10 Na2EDTA, 2.86 H3BO3, 1.81 MnCl2·4H2O, 0.22 ZnSO4· H2O, 0.079 CuSO4·5H2O, and 0.039 (NH4)6Mo7O24·4H2O. The mBG11 contained 15 mg L−1 N and 1.5 mg L−1 P simulating the secondary effluent of municipal wastewater treatment plants. NaNO3 and K2HPO4·3H2O were used as the N and P resource. Apart from N and P, there were (in mg L−1) 37.5 MgSO4·7H2O, 18 CaCl2·2H2O, 3 citric acid, 3 ferric ammonium citrate, 0.5 EDTA, 10 Na2CO3, and 1 A5 + Co. The A5 + Co solution contained 2.86 g L−1 H3BO3, 1.81 g L−1 MnCl2 H2O, 222 mg L−1 ZnSO4·7H2O, 79 mg L−1 CuSO4·5H2O, 390 mg L−1 Na2MoO4·2H2O, 49 mg L−1 Co (NO3)2·6H2O. Five synthetic water samples (A, B, C, D, and E) were generated by adding different amounts of nitrate and phosphate into N- and P-free SE medium according to the water quality standards: “A” stands for the surface water with 1 mg L−1 N and 0.05 mg L−1 P that can attain the third level surface water quality standards (GB 3838-2002 China), “B” stands for the municipal wastewater with 15 mg L−1 N and 0.5 mg L−1 P that can attain the first level of municipal wastewater discharge standards (GB 18918-2002), “C” stands for N-deprivation with 0.05 mg L−1 N, 50.1 mg L−1 P, “D” stands for P-deprivation with 41.2 mg L−1 N, 0.002 mg L−1 P, and “E” stands for SE medium with 41.2 mg L−1 N, 50.1 mg L−1 P.
Algal density (N, cells mL−1) was determined by counting cell numbers using a hemocytometer every 48 h. Finally, the growth curves were obtained and cultures were harvested at two different points of the growth curve: the early stationary phase (15 days) and the late stationary phase (30 days).
Algal growth can be described by the Logistic model, as is shown in Eq. (1), and the transformed form is shown in Eq. (2):
where N is the algal density at time t (days), K (cells mL−1) is the maximum algal density in the culture, a is a constant, and r (day−1) is the specific growth rate. The values of a and r can be obtained by logistic fit with the data series of N and t.
When N equals half of K, the population growth rate reaches its maximal value R max [mg (L·day)−1], which is presented in Eq. (3):
Biomass evaluation
Triplicates of 40 mL samples were filtered through pre-weighed 0.45-μm membraneswhich were then dried at 110 °C for 24 h, and the dry weight of the algal biomass was determined gravimetrically.
Algal total lipid and TAGs determination
Triplicates of 40 mL cultures transferred from flasks were concentrated to about 0.8 mL by centrifugation (CR22G, Hitachi, Japan) for 10 min at 12,000 rpm and 4 °C. The total lipid was extracted by adding 2 mL chloroform, 2 mL methanol, and 1 mL distilled water and mixing. After centrifugation at 4,000 rpm for 10 min, the mixture separated into three layers. The chloroform layer was transferred into a pre-weighed glass tube and evaporated by a nitrogen evaporator (DC-12, Anpel, China) to obtain the dry lipid. Finally, the total lipid was weighed (Bligh and Dyer 1959). After the determination of total lipid, the dried lipid was dissolved in 0.4 mL isopropanol. The TAGs were determined with an enzymatic colorimetric method (Li et al. 2010b).
Nitrogen and phosphorus concentration assay
The algae culture was filtered through 0.45-μm membranes, and the filtrate transferred into another clean tube. Subsequently, an 8 mL sample was used for the determination of N concentration by using a total organic carbon analyzer (TOC-VCPH, Shimadzu, Japan). Samples of 1 mL were digested by adding into 3 mL distilled water and 1 mL potassium persulfate (5 %) in a digestion apparatus (DRB 200, Hach, USA) at 130 °C for 30 min and the assay of P concentration was by the ammonium molybdate spectrophotometric method (State Environmental Protection Administration 2002) using a spectrophotometer (DR 5000, Hach, USA) at 700 nm.
Experimental design
-
1.
Experimental design in synthetic water. After 18 days of cultivation, the algal biomass (dry weight), lipid content per algal biomass (%, dry weight) and TAGs content per lipid (%, dry weight) were determined and compared among the five synthetic water samples.
-
2.
Experimental design of stationary phase elongation and initial nitrogen and phosphorus influence. When initial P was maintained at 1.5 mg L−1, the initial N concentration gradient was designed as 3.0, 6.0, 12.0, 18.0, and 30.0 mg L−1; when initial N was maintained at 15 mg L−1, the initial P concentration gradient was 0.3, 0.5, 0.8, 1.3, and 1.9 mg L−1. Algal densities were tested every 48 h. At the early and late stationary phases, the algal biomass (dry weight), lipid content per algal biomass (%, dry weight), and TAGs content per lipid (%, dry weight) were determined. Additionally, the concentrations of N and P from algal cultures filtered through 0.45-μm membranes were determined every other day.
Results
Comparison of growth and lipid accumulation in different types of synthetic water
Figure 1 shows the algal biomass (dry weight), lipid content per algal biomass (%), TAGs content per lipid, and lipid and TAGs yields of Chlorella sp. HQ after 18 days cultivation in five different types of synthetic water. The algal biomass in the synthetic secondary effluent reached the highest level of 0.27 g L−1, which was nearly two times that in the SE medium while the N and P concentrations in the synthetic secondary effluent were only 2.7 and 1.0 % of those in SE medium.
Effect of N and P concentrations on algal growth
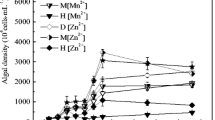
Figure 2 shows the growth curves of Chlorella sp. HQ after 30 days of cultivation under different initial N and P concentrations. It is clear that the algal cells could adapt to the environments reached log phase quickly after inoculation and the stationary phase from days 15 to 30. Thus, days 15 and 30 were considered as the early and late stationary phases, respectively.
Through the Logistic model (Eqs. (2) and (3)), the maximal algal density (K, cells mL−1) and population growth rate [R max, cells (L·day)−1] were obtained, describing the carrying capacity and maximal biomass yield rate, respectively. As is shown in Figs. 2 and 3, the algal growth was enhanced significantly as N concentration increased, but there was no change with the P concentration increase.
To explore the relationship between K N(P), R max,N(P), and initial N or P concentration, the Monod model was applied, as is exemplified in Eqs. (4) and (5):
where S N(P) (mg L−1) stands for the initial N or P concentration in the culture medium; K S,N(P) (mg L−1) for the half-saturation constant for maximal algal density; K′ S,N(P) (mg L−1) for the half-saturation constant for the population growth rate; K′max,N(P) (cells mL−1) for the maximal value of K max,N(P) at the saturated N or P concentration, and R′max,N(P) (cells mL−1 day−1) for the maximal value of R max,N(P) at the saturated N or P concentration.
In the study, the initial N or P concentration and K max,N(P), R max,N(P) complied with the Monod model and could be calculated as Eqs. (6) and (7):
If both N and P are identified as growth-limiting substrates, the Monod model will be transformed to Eqs. (8) and (9):
The Monod parameters in Eqs. (8) and (9) were obtained through “Datafit version 7.1.44,” and the integrated forms of Monod model for both N and P as growth-limiting substrates of Chlorella sp. HQ are shown in Eqs. (10) and (11).
As shown in Eqs. (10) and (11), the K max and R max of Chlorella sp. HQ indicated that both N and P can be considered as limiting substrates. Equations (10) and (11) are used to evaluate and predict the biomass production of Chlorella sp. HQ, as well as estimate N and P demands for biodiesel production of this alga.
Effect of N and P concentrations on algal lipid accumulation with stationary phase elongation
The algal biomass, lipid content, TAGs content per lipid, and lipid and TAGs yields of Chlorella sp. HQ at the early and late stationary phases under different N concentrations are shown in Fig. 4. Normally, the algal biomass should increase as nutrient concentrations rise. In Fig. 4a, after 15 days of cultivation, the highest biomass 0.19 g L−1 was obtained at the initial N of 12.0 mg L−1 while not at 30.0 mg L−1, which may indicate that the initial N/P ratio of 8/1 was more appropriate for algal growth.
As N concentration increased, the algal lipid content increased from 20.0 to 30.6 %. As a result, the maximal lipid yield (46.7 mg L−1) was obtained at 12.0 mg L−1 N. Additionally, TAGs content and yield in algal total lipids ranged from 10.5 to 55.7 % and from 4.2 to 14.3 mg L−1, respectively.
After 30 days of cultivation, the algal biomass increased dramatically compared with that at the early stationary phase Fig. 4b. Moreover, the algal biomass increased linearly with increasing initial N concentration (R 2 = 0.96). The highest lipid content (33.2 %) was obtained under N-deprivation (3.0 mg L−1), while the lipid yield was the lowest (28.8 mg L−1) due to the low algal biomass. The highest lipid yield (99.2 mg L−1) occurred at the initial N of 30.0 mg L−1 as the algal biomass was highest. Furthermore, TAGs content and yield were also found to increase significantly with stationary phase elongation from 15 to 30 days, i.e., the TAGs content rose more than four times, from 14.2 to 59.6 % and the TAGs yield increased more than 12 times, from 4.2 to 54.0 mg L−1 at initial N of 18.0 mg L−1.
The algal biomass, lipid content, TAGs content per lipid, and lipid and TAGs yields at the early and late stationary phases under different P concentrations are shown in Fig. 5. After 15 days of cultivation, the biomass was between 0.12 and 0.16 g L−1 (Fig. 5a), the TAGs content per lipid was from 6.5 to 20.9 % and TAGs yield was in the range of 3.3 to 6.4 mg L−1 under different initial P concentrations (0.3 to 1.9 mg L−1). The peak content and yield of lipid occurred at the initial P of 1.9 mg L−1, reaching 39.8 % and 55.8 mg L−1, respectively.
As shown in Fig. 5b, the algal biomass at the late stationary phase ranged from 0.31 to 0.44 g L−1, which was generally higher than that at the early stationary phase. An increase in the initial P concentration had not significant effect on the lipid content (14.7–17.1 %) and lipid yield (50.8–63.8 mg L−1), while the P-deprivation (0.3 mg L−1) caused an inhibition of the lipid yield and TAGs content and yield to a certain degree. Furthermore, TAGs was observed to increase dramatically in content and yield at the late stationary phase compared with the early stationary phase, i.e., the TAGs content increased more than six times, from 6.5 to 42.4 %, and the TAGs yield raised more than eight times, from 3.3 to 62.5 mg L−1 at the initial P of 1.3 mg L−1. Thus, it is suggested that the initial P concentrations should be between 0.5 and 1.9 mg L−1 for higher TAGs content and yield.
Effect of N/P ratio on algal nutrient removal with stationary phase elongation
The changes in N and P concentrations under different initial nutrient concentrations are shown in Fig. 6, and the corresponding removal efficiencies after 15 and 30 days of cultivation are further illustrated in Tables 1 and 2. At the early stationary phase, the N removal efficiency was in the range of 26.8 to 86.2 % when P was maintained at 1.5 mg L−1, and it was significantly decreased as the initial N/P ratio increased from 2/1 to 20/1, whereas the P removal efficiency increased to 94.3 %. When N was maintained at 15 mg L−1, the N removal efficiency declined from 90.7 to 72.1 % as the N/P ratio increased, but the P removal efficiency kept rising from 84.0 to 100.0 %. After stationary phase elongation to 30 days, N removal was more than 88.6 %, whereas the P removal efficiency dropped sharply to 43.2 % when the initial N/P ratio was less than 4/1 with an initial P of 1.5 mg L−1.
In summary, the N and P removal efficiencies under and initial N of 15.0 mg L−1 were not evidently affected by the initial N/P ratio (8/1–50/1), reaching above 88.0 and 95.3 %, respectively.
Discussion
There have been some studies screening for microalgae with high biomass production and lipid content in wastewater, i.e., Li et al. (2010c) compared the microalgal biomass among 12 species of microalgae after 15 days of cultivation in secondary effluent and found that the freshwater microalga Scenedesmus sp. LX1 isolated from stored tap water achieved the highest dry weight of biomass at 0.11 g L−1. Based on the results in this study, the biomass productivity of Chlorella sp. HQ is calculated as 15.0 mg L−1 day−1, which is more than double that of Scenedesmus sp. LX1 (7.3 mg L−1 day−1). This implies that Chlorella sp. HQ is a strain with good capacity to accumulate biomass in low nutrient conditions. Generally, nutrient deficiency has been regarded as one of the most efficient approach to enhance lipid content in algal cells (Rodolfi et al. 2009). In this research, the lipid content of Chlorella sp. HQ maintained at a relatively high level, ranging from 14.3 to 48.6 %. The maximal value was obtained under the N-deprivation condition and was much higher than the normaln lipid content of Chlorella sp. (28–32 %) in other studies (Chisti 2007). Hence, it can be concluded that Chlorella sp. HQ could accumulate lipid in water with a wide range of N and P concentrations, and the low nutrient environment was more profitable for enhancing its lipid level, indicating that Chlorella sp. HQ might have great potential for combined lipid production and secondary effluent purification. To further study the effect of N and P concentrations on the growth, lipid accumulation, and nutrient removal characteristics of Chlorella sp. HQ, the value of the above characteristics was analyzed at different growth phases under varied nutrient concentrations.
The growth responses of different algal species to N and P concentrations are varied. For example, the growth rate of Scenedesmus sp. LX1 increased with an increase in initial N and P concentrations (Li et al. 2010a). With a decrease in N concentration, N. oculata exhibited a gradual decrease in specific growth rate, but there was no evident change of the growth rate of C. vulgaris (Converti et al. 2009). In the present study, the growth of Chlorella sp. HQ positively responded to N concentration, but there was significant correlation with P concentration. Using the Monod model, the maximal values of K max,N(P) and R max,N(P) of Chlorella sp. HQ at saturated N and P concentrations were four to five orders of magnitude higher than those of C. ellipsoidea YJ1 (Yang et al. 2011) and one order of magnitude higher than those of Scenedesmus sp. LX1(Li et al. 2010a), suggesting that Chlorella sp. HQ can utilize less nutrient to achieve more algal biomass in wastewater.
During the algal growth process, N and P always play vital roles in the synthesis of proteins, nucleic acids, ATP, etc. (Shen et al. 2006). The results in this study indicate that the lipid content of Chlorella sp. HQ was greatly increased under N-deprivation, which also has been observed in other oleaginous algae. For example, the lipid contents of Scenedesmus sp. LX1 and Nannochloropsis sp. were significantly enhanced by N-deprivation, increasing from 20 to 30 % and 29 to 60 %, respectively (Li et al. 2010a; Rodolfi et al. 2009). However, although the lipid content of Chlorella sp. HQ was the highest under N-deprivation, the total lipid yield was the lowest due to growth inhibition. Hence, to obtain both high lipid content and yield, a sequential two-stage cultivation process has been proposed (Rodolfi et al. 2009), in which the first stage with sufficient nutrient is to produce high algal biomass, and then a N-deprivation phase is used to promote lipid content. Generally, cell division and products such as chlorophyll and lipid are observed be affected by P-deprivation, i.e., the TAGs content of Monodus subterraneus increased from 6.5 to 39.3 % of the total lipids in the absence of P (Khozin-Goldberg and Cohen 2006), and the lipid content of Scenedesmus sp. LX1 was increased as high as 53 % in the condition of P-deprivation (0.1 mg L−1) (Li et al. 2010a). However, in this study, the lipid content and yield of Chlorella sp. HQ were found not to be affected by P concentration. Therefore, it can be assumed that N-deprivation, but not P-deprivation, can be regarded as an effective approach to promote the lipid accumulation in Chlorella sp. HQ.
Several studies conclude that it is better to harvest the microalgae in stationary phase in contrast to the log phase. For instance, the lipid content of C. ellipsoidea YJ1 was much higher at stationary phase (35–40 %) than that at log phase (10–15 %) (Yang et al. 2011), and a similar phenomenon was observed in C. pyrenoidosa (Nigam et al. 2011). Additionally, the TAGs accumulation also conforms to these obeservation, i.e., the TAGs content of the total fatty acids in Parietochloris incisa was found to increase from 43 to 77 % when it shifted from log phase to stationary phase (Bigogno et al. 2002). However, there are only a few studies on the effect of stationary phase elongation on algal lipid accumulation. Noer et al. (2012) reported that the biomass of N. oculata in seawater with 5 % coral waste at the late stationary phase (1.92 g L−1) was significantly more than that in seawater with 15 % waste at the early stationary phase (0.59 g L−1), while the lipid content was relatively lower, reaching 29 and 39 %, respectively. Liang and Mai (2005) found that stationary phase elongation apparently affected lipid accumulation, and the fatty acid compositions of four marine diatom species were significantly different at the early and late stationary phases, i.e., the proportions of 16:0 and 16:1 n-7 fatty acids increased, while those of 16:3 n-4 and EPA decreased with increasing culture age. In the present study, the lipid yield of Chlorella sp. HQ was found to be enhanced markedly by stationary phase elongation from 15 to 30 days, and the TAGs content and yield increased by up to 6.5 and 12.8 times, respectively. These findings indicate that stationary phase elongation facilitates the accumulation of TAGs, indicating that this is an effective and innovative alternative to produce more lipid and TAGs in algae. When the stationary phase was extended to 30 days, the N and P removal efficiencies of Chlorella sp. HQ also were increased to a certain degree. And after 30 days of cultivation, the P removal efficiency of Chlorella sp. HQ was quite low when the initial N/P ratio was less than 4/1 with initial P of 1.5 mg L−1, while the N removal efficiency varied little. Similar results have been reported by (Yan and Yu 1997) who also found that the initial N concentration had a great influence on P removal efficiency of C. vulgaris with the optimum initial N concentration in the range of 20.0–40.0 mg L−1. The decrease in P removal efficiency of Chlorella sp. HQ mentioned above may be due to N-deprivation that was likely cause of the decline in algal growth. The optimum N/P ratio of C. vulgaris was at 8/1 (Kapdan and Aslan 2008), and Scenedesmus sp. LX1 removed N and P efficiently when the initial N/P ratio was between 5/1 and 8/1 (Li et al. 2010a). Based on the results in this study, the optimal time to harvest algal cells is at the late stationary phase in order to obtain concomitant higher N and P removal efficiencies. At the late stationary phase, the best N/P ratio for N and P removal was 4/1–20/1 when the initial P concentration was maintained, and it should be controlled between 8/1 and 50/1 at a stable initial N concentration. Comprehensive analysis indicates that the initial N/P ratio should be controlled between 8/1 and 20/1 in order to remove N and P simultaneously and efficiently when using Chlorella sp. HQ for wastewater treatment.
In conclusion, the newly isolated oleaginous alga Chlorella sp. HQ shows great lipid-producing ability when grown synthetic secondary effluent. When the alga shifted from the early stationary phase to the late stationary phase, the algal biomass, lipid and TAGs yields, and N and P removal efficiencies were significantly increased by 3.6, 2.5, 12.8, 3.4 and 1.2 times, respectively, under different N and P concentrations. Furthermore, the above parameters were mainly affected by N concentration and less by the P concentration. Logistic and Monod models were found to verify the above and demonstrated that Chlorella sp. HQ could achieve greater biomass utilizing less N and P. In addition, the stationary phase elongation is of great importance for the oil-producing alga Chlorella sp. HQ to produce more lipids (TAGs) and remove N and P more efficiently. Next, the effects of other environmental factors will be assayed for better understanding of lipid accumulation and nutrient removal characteristics of this alga. Furthermore, the energy input as well as the production costs should be taken into account for future directions to utilize microalgae for coupling lipid production and wastewater treatment.
References
Abu-Rezq TS, Al-Musallam L, Al-Shimmari J, Dias P (1999) Optimum production conditions for different high-quality marine algae. Hydrobiologia 403:97–107
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
Balat M (2008) Progress in biogas production processes. Energy Educ Sci Tech 22:15–36
Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Cakmak T, Angun P, Demiray YE, Ozkan AD, Elibol Z, Tekinay T (2012) Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol Bioeng 109:1947–1957
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Csavina JL, Stuart BJ, Riefler RG, Vis ML (2011) Growth optimization of algae for biodiesel production. J Appl Microbiol 111:312–318
Deng XD, Li YJ, Fei XW (2009) Microalgae: A promising feedstock for biodiesel. Afr J Microbiol Res 3:1008–1014
GB 18918-2002 (2002) Discharge standard of pollutants for municipal wastewater treatment plant. Beijing: State Environmental Protection Administration, (in Chinese)
GB 3838-2002 (2002) Environmental Quality Standards for Surface Water. Beijing: State Environmental Protection Administration, (in Chinese)
Kapdan IK, Aslan S (2008) Application of the Stover-Kincannon kinetic model to nitrogen removal by Chlorella vulgaris in a continuously operated immobilized photobioreactor system. J Chem Technol Biot 83:998–1005
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Li X, Hu HY, Gan K, Sun YX (2010a) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Li X, Hu HY, Yang J, Wu YH (2010b) Enhancement effect of ethyl-2-methyl acetoacetate on triacylglycerols production by a freshwater microalga, Scenedesmus sp. LX1. Bioresour Technol 101:9819–9821
Li X, Hu HY, Yang J (2010c) Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol 27:59–63
Liang Y, Mai KS (2005) Effect of growth phase on the fatty acid compositions of four species of marine diatoms. J Ocean Univ China 4:157–162
Lin Q, Gu N, Lin J (2012) Effect of ferric ion on nitrogen consumption, biomass and oil accumulation of a Scenedesmus rubescens-like microalga. Bioresour Technol 112:242–247
Ma HF, Li X, Hu HY, Yu Y, Wu YH (2012) Growth, removal of nitrogen and phosphorus, and lipid accumulation property of Scenedesmus sp. LX1 in aquaculture wastewater. Huan Jing Ke Xue 33:1891–1896 (in Chinese)
Mandal S, Mallic N (2011) Waste utilization and biodiesel production by the green microalga Scenedesmus obliquus. Appl Environ Microb 77:374–377
Merzlyak MN, Chivkunova OB, Chivkunova OA, Reshetnikova IV, Solovchenko AE, Khozin-Goldberg I, Cohen Z (2007) Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). J Phycol 43:833–843
Nigam S, Rai MP, Sharma R (2011) Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Amer J Biochem Biotech 7:126–131
Noer IS, Panggabean LMG, Naderia F (2012) Influence of coral culture waste on growth, biomass and lipid production of Nannochloropsis oculata (Droop) Hibberd. Proceedings of the 9th Biomass-Asia Workshop with Asia Biomass Office Conference “Role of Biomass as Renewable Energy,” 2012 Dec 3–4, Tokyo, Japan
Olguín EJ, Galicia S, Mercado G, Pérez T (2003) Annual yield of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J Appl Phycol 15:249–257
Patil V, Tran KQ, Giselrød HR (2008) Towards sustainable production of biofuels from microalgae. Int J Mol Sci 9:1188–1195
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G et al (2009) Microalgae for oil strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res 1:20–43
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Shen SD, He LH, Pu X, Zhu JY (2006) Effect of nitrogen and phosphorus on the development and differentiation of vegetative cells of Porphyra yezoensis on solid agar medium. Bot Mar 49:372–378
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
State Environmental Protection Administration (2002) Monitoring Method of Water and Wastewater, vol 105, 4th edn. China Environmental Science Press, Beijing, p 246–248, 255–257
Su HY, Zhang YL, Zhang CM, Zhou XF, Li JP (2011) Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol 102:9884–9890
Su XL, Xu JL, Yan XJ, Zhao P, Chen JJ, Zhou CX, Zhao F, Li S (2013) Lipidomic changes during different growth stages of Nitzschia closterium f. minutissima. Metabolomics 9:300–310
Tang H, Chen M, Garcia ME, Abunasser N, Ng KY, Salley SO (2011) Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol Bioeng 108:2280–2287
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biot 78:29–36
Yan GA, Yu JY (1997) Comparison of the effects of nitrogen concentration on nutrient removal, growth and physiological characteristics of immobilized and free Chlorella vulgaris: I. growth and nutrient removal. Toxicol Env Chem 58:63–74
Yang J, Li X, Hu HY, Zhang X, Yu Y, Chen YS (2011) Growth and lipid accumulation properties of a freshwater microalga, Chlorella ellipsoidea YJ1, in domestic secondary effluent. Appl Energ 88:3295–3299
Acknowledgments
We gratefully acknowledge the assistance of Prof. Qiang Zhi-Min, Dr. Wu Yin-Hu, Prof. Yuan Yan-Wei, and Dr. Yu Yin for paper writing, and the assistance of Pro. Hu Hong-Ying for experimental facilities. This study was supported by the Fundamental Research Funds for the Central Universities (no. TD2013-2), Beijing Nova Program (no. 2010B019), and the Special Fund from the State Key Laboratory of Environmental Aquatic Chemistry (no. 11K10ESPCR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1246 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Hong, Y. Effects of stationary phase elongation and initial nitrogen and phosphorus concentrations on the growth and lipid-producing potential of Chlorella sp. HQ. J Appl Phycol 26, 141–149 (2014). https://doi.org/10.1007/s10811-013-0091-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0091-7