Abstract
The influence of artificial illumination on upstream and downstream operations for biomass production of Tolypothrix tenuis as a basic component of a powdered cyanobacterial biofertilizer was studied. Cultures were operated semi-continuously for 18 months at harvesting frequencies of 4, 7, 10, and 14 days in two vertical plate photobioreactors of 1.5 and 5 cm of light path and illuminated at two different light intensities: high (290 μmol photons m−2 s−1) and normal (60 μmol photons m−2 s−1). Biomass was separated by self-flocculation and finally processed as a dried powder. The cellular concentration and volumetric productivity were superior in photobioreactors of short light path at high light intensity, while the overall areal productivity was higher in the photobioreactor of 5 cm at normal light intensity with weekly harvest frequency. The viability preservation of the dried and milled biomass was greatly enhanced by the use of halogen lamps and subsequent ionic flocculation with 10 mM MgSO4 plus 10 mM CaCl2. An optimum value of the retained viability index (RVI10) was maintained for 24 months, while a sharp viability declination and cellular death were produced after 12 months with fluorescent tubes, which represents a relevant aspect in the commercialization step of this type of biofertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial fertilizers can play an important role in eco-friendly and sustainable agricultural practices by increasing the efficiency of plant nutrient acquisition and enhancing soil quality (Singh et al. 2011). Recently, its use in consortia with plant growth-promoting rhizobacteria has been proposed, reaching more consistency in the fertilization of rice and wheat crops (Rana et al. 2012; Prasanna et al. 2012). While cyanobacteria have been widely used in many Asian countries (Mishra and Pabbi 2004; Saadatnia and Riahi 2009) and its presence confirmed in rice fields of Spain and South America (Irisarri et al. 2001; Pereira et al. 2009), worldwide application has been limited by the lack of a consistent production technology like that of chemical fertilizers (Hashem 2001). Tolypothrix tenuis Kützing is one of many diazotrophic species extensively used for the inoculation of tropical rice fields (Irisarri 2006). Particularly, in Argentina, it has been used in combination with Nostoc muscorum for the production of a cyanobacterial biofertilizer since the late 1990s (Brenzoni 1999).
The commercial production of phototrophic microbial biomass is currently limited to a few species cultivated in open ponds in selective environments or with high growth rate. However, most microalgal cultures cannot be sustained in this system mainly due to contamination with other biological agents, competition with other microalgae, and availability of light (Tredici 2004; Brennan and Owende 2010). Conversely, in a closed system, the degree of control is very high and it is possible to handle crucial parameters that influence the culture (Carvalho et al. 2006). Photobioreactors can be operated under indoor and outdoor conditions; however, the indoor results cannot be extrapolated to outdoor conditions because the physiological behavior and culture performance of microalgae subjected to natural light irradiance, light cycles, and temperature vary continuously (Fuentes-Grünewald et al. 2013). Artificial light, considered too expensive in terms of investment and running costs, can be utilized for culture development and scaling up in locations with rigorous winters, cloudy days, and lingering rain periods (Schwarz et al. 1995; Muller-Feuga et al. 1998).
Separation of microorganisms is a critical point in many biotechnological and environmental applications. In microalgal biomass production, it is estimated that cell recovery accounts for at least 20–30 % of the total production cost (Molina Grima et al. 2003). The selection of the harvesting technique is dependent on culture broth density, size and charge of cells, and the value of the products. Flocculation is a preparatory step prior to other harvesting methods such as filtration, flotation, or gravity sedimentation, aimed at reducing the negative charge that prevents the natural aggregation of cells in suspension (Brennan and Owende 2010). For low-value products, gravity sedimentation, enhanced by flocculation, may be the method of choice (Molina Grima et al. 2003).
In the present study, detailed information is provided on biomass production of T. tenuis as a basic component of a dry and powder cyanobacterial biofertilizer suitable for aerial application on land-extensive rice cultivation. Biomass productivities in terms of several indexes were determined in vertical plate photobioreactors illuminated with artificial light sources and operated semi-continuously. The biomass was separated by self-flocculation enhanced by the addition of metal cations and processed as a dried powder with shelf-life analysis.
Materials and methods
The species used in this work, Tolypothrix tenuis, a filamentous nitrogen-fixing cyanobacterium, was kindly donated by Rizobacter Argentina S.A. Cultures were grown in two plate glass photobioreactors (PBRs) 5 mm thick with a constant surface of 0.1 m2 (height, 40 cm; width, 25 cm), 1.5 and 5 cm of light path, and height of 32 cm, providing culture volumes of 1.2 and 4 L, respectively. Illumination was provided by fluorescent tubes and halogen lamps. The condition of normal light intensity (NLI) of 60 μmol photons m−2 s−1 was obtained using a panel of four 40-W fluorescent tubes placed between two PBRs at a distance of 5 cm, while the high light intensity condition (HLI) of 290 μmol photons m−2 s−1 was obtained using two halogen lamps (70–150 W) placed at 50 cm on both sides of one PBR unit (Fig. 1). The cultures were incubated at 30 °C in a constant temperature room and inoculated at 10 % (v/v) with a 2-week-old inoculum. Cultures were mixed by bubbling air through a sparger located at the base of the PBRs at flow rates sufficient to reach appropriate cellular suspension. T. tenuis was grown in optimized Watanabe culture medium (Silva and Silva 2007). The PBRs were operated for 18 months as semi-continuous cultures by replacing half the volume with fresh medium at harvesting frequencies of 4, 7, 10, and 14 days.
Schematic of plate glass photobioreactors with a surface of 0.1 m2 illuminated at 60 μmol photons m−2 s−1 (a) and at 290 μmol photons m−2 s−1 (b). Two light paths of 1.5 and 5 cm were used to provide cultures of 1.2 and 4 L at each light regime. Halogenated mercury lamps (1), fluorescent tubes (2), air supply (3), and sparger (4)
Growth and photosynthetic pigment determination
T. tenuis growth was estimated by dry weight determinations in centrifuged samples at 1,200×g for 20 min, washed twice with distilled water, and dried at 96 °C until constant weight. Chlorophyll a and carotenoids were determined in 80 % (v/v) acetone extracts of cells incubated for 30 min at 30 °C and then for 16 h at 4 °C. Chlorophyll a and total carotenoid concentrations were calculated according to McKinney (1941) in the supernatant at 663 and 460 nm, respectively, after the acetone extracts were centrifuged at 1,200×g for 5 min.
For the extraction of phycobiliproteins, the residual biomass was suspended in 30 mM Na-phosphate buffer (pH 7). The water-soluble proteins were released by two freezing and thawing steps followed by incubation at 4 °C for 96 h in the dark. After centrifugation at 1,200×g for 5 min, phycobiliproteins levels were estimated spectrophotometrically at 562, 615, and 652 nm for C-phycoerythrin, C-phycocyanin, and allo-phycocyanin, respectively, using Bennett and Bogorad’s (1973) equations.
Dry weight and photosynthetic pigment determinations were carried out in samples withdrawn every 48–72 h at all harvest frequencies of the semi-continuous cultures. Each sampling event was made in duplicate and the results are the average of three independent experiments.
Cellular flocculation and viability
For the flocculation assay, previously homogenized cell suspensions coming from cultures obtained in both PBRs at the two light intensities (V C, 250 mL) were left standing still in a cylinder and the volume of sedimented cells (V S) recorded until it remained constant. In some experiments, solutions of NaCl 100 mM, CaCl2, and/or MgSO4 1–10 mM (Watanabe et al. 1998) were added to the cellular suspension prior to flocculation. The flocculation activity (F) was calculated with the expression F = 1 − V S/V C. After the addition of Na+, Ca2+, and Mg2+, cultures were subjected to agitation by air bubbling for a period of 24 h.
Cell viability of T. tenuis was estimated using the retained viability index (RVI10) specifically designed (Silva and Silva 2003) using biomass powder obtained by drying biomass in an oven at 30 °C and then milling the flakes with a commercial grinder, producing particles of approximately 90 μm. RVI10 was calculated as (B 10 − B 0)/B 0, where B 0 is the initial biomass dry weight and B 10 the biomass dry weight after 10 days of incubation.
Positive values of RVI10 indicate net yields of actively growing biomass, while negative values correspond to cases with partial survival or total death of cyanobacterial biomass. Estimations were made in duplicate and the results were the average of three independent experiments.
Kinetic and productivity parameters
The kinetic and productivity parameters evaluated included the specific growth rate, final biomass, volumetric productivity, illuminated surface productivity, reactor productivity, and overall areal productivity (Qiang et al. 1998; Tredici 2004).
Specific growth rate (μ) was calculated by the expression lnx/x o⋅t −1 in the exponential growth phase, where x o is the initial biomass concentration (g dry weight L−1) and x is the biomass concentration at time t. Volumetric productivity (VP), defined as the quantity of biomass obtained per unit of cultivation volume and time, was given as VP (g L−1 day−1) = (x 2 − x 1)/(t 2 − t 1). The illuminated surface productivity (ISP) of the reactor was estimated as ISP (g m−2 day−1) = VSI(x 2 − x 1)/(t 2 − t 1), where VSI is the culture volume per square meter of illuminated surface of the PBR unit.
The reactor productivity (RP), meaning the productivity per cultivation unit, was calculated as RP (g day−1) = VP(x 2 − x 1)/(t 2 − t 1), where VP is the PBR volume. The overall areal productivity (OAP) that expresses the PBR productivity considering the whole area required to settle the PBR, including empty spaces, was estimated as OAP (g m−2 day−1) = VLA(x 2 − x 1)/(t 2 − t 1), where VLA is the PBR volume per unit area needed to locate the PBR and the illumination system.
Data were statistically analyzed (Student’s t test) and the results were the average of three independent experiments.
Results
Semi-continuous culture of T. tenuis
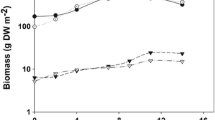
The results obtained in cultures operated semi-continuously at normal and high light intensities are shown in Table 1. At HLI, the reduction of the light path (1.5 cm) produced an increase of 88.64 % in the final biomass, while at NLI the increase was 29.72 %. VP showed a similar tendency of data. However, when RP was calculated, the highest level was obtained in the PBR of 5 cm light path at HLI. The ISP and OAP productivities gave the highest values in the PBR of 5-cm light path at NLI. In the last condition, T. tenuis cultures exhibited adequate rheological properties induced by bubble turbulence at an air flow of 1.2 vvm, with an even distribution of cell aggregates that easily flocculated when aeration was stopped. Conversely, the cultures developed in the PBR of 1.5-cm light path at NLI showed a deposit of cells at the bottom with no dispersion even at an air flow of 3.12 vvm.
The pigment composition of cyanobacterial biomass varied markedly with light intensity in each PBR. Chlorophyll a decreased 21 and 6 times and the total carotenoids 3 and 1.78 times in each PBR by effect of HLI, respectively. Of the phycobiliproteins only C-phycoerythrin remained with a content reduction of 1.53 and 3.89 times, while C-phycocyanin and allo-phycocyanin were not detected at HLI in any of the PBRs (Table 1).
Effect of harvesting frequency
The effect of harvesting frequency on T. tenuis semi-continuous cultures grown in a 5-cm light path PBR illuminated with high and normal light intensities is shown in Table 2. The specific growth rate increased with the harvesting frequency at normal and high light intensities, while the cell concentration at HLI was two times higher than that obtained at NLI at every frequency. The short crop cycle (4 days) produced the highest productivities (VP, ISP, and OAP) under both conditions of illumination; however, these productivities could not be sustained in time because the dilution rate was higher than the growth rate, leading to cell washout. The highest VP was obtained with HLI at all the harvesting frequencies assayed (p < 0.05). The ISP did not show significant differences when comparing both light intensities (p > 0.05), and the values of OAP with NLI were superior (p < 0.05) at the three crop frequencies evaluated, with an increment of 79 and 91 % for harvesting cycles of 10 and 4 days, respectively (Table 2).
Effect of Na+, Ca2+, and Mg2+ on T. tenuis cell flocculation
The effect of light intensity on the self-flocculation of T. tenuis cultures grown in the 5-cm light path PBR is shown in Fig. 2a. The cultures raised at NLI formed cell aggregates evenly distributed while agitation was in operation, which self-flocculated when aeration was stopped. On the other hand, at HLI, the cultures showed a detrimental alteration in this behavior with a practical loss of self-flocculation. The maximum F was reached after 1 h in cultures grown at NLI, while the F at HLI was 11 times lower for the same period of time (Fig. 2a).
Flocculation activity of T. tenuis cultures. a Without ions addition at NLI (filled circle) and HLI (empty circle). b With addition of 10 mM MgSO4 (filled square), 10 mM CaCl2 (empty square), and 100 mM NaCl (gray square) at HLI. c Added with 10 mM MgSO4 plus 1 mM CaCl2 (empty triangle), 10 mM MgSO4 plus 5 mM CaCl2 (gray triangle), and 10 mM MgSO4 plus 10 mM CaCl2 (filled triangle) at HLI. After ion addition, the cultures were agitated by air bubbling for a period of 24 h and left standing still until the sedimented cell volumes remained constant. Data are the mean of three independent experiments performed in duplicate samples with a standard error of <10 %
With the exception of Na+, the addition of Ca2+ and Mg2+ showed a positive effect on the flocculation activity of cultures grown at HLI (Fig. 2b). The F evolution with Mg2+ was similar to that observed with normal irradiance, while with Ca2+ the effect was less marked. The effect of 10 mM MgSO4 was further enhanced by the addition of CaCl2 at concentrations of 1, 5, and 10 mM (Fig. 2c). The rate of self-flocculation with 10 mM CaCl2 was 27 times higher than that obtained without ions. A clear compaction of cells in the floc was observed at all CaCl2 concentrations, allowing efficient separation of the biomass. The highest flocculation activity was reached after 2 min with the addition of Mg2+ plus Ca2+ at 10 mM; in this case, the solids content of the wet floc increased from 2.47 % without ions to 4.66 %. The positive effect of ions in T. tenuis sedimentation occurred only in suspensions previously submitted to a step of air bubble agitation for 24 h. This agitation step was critical since flocculation was poor in its absence, regardless of the presence of cationic ions.
Cell viability of powder biomass
The cell viability of T. tenuis biomass, grown at two irradiances, flocculated with Mg2+ plus 10 mM Ca2+ in the case of HLI and produced in a dry and powder state was analyzed in terms of the retained viability index (RVI10).
The viability of biomass obtained at NLI showed a sharp declining trend with storage time, with a net reduction of 2.4 units in the RVI10 after 16 months, even though the initial index was 0.34 unit higher (Fig. 3). Instead, the index evolution for biomass produced at HLI remained always at positive values, with a decline of only 0.5 unit after a storage time of 24 months.
Cell viability of powdered T. tenuis biomass expressed by the RVI10 from cultures developed at NLI (empty circle) and HLI (filled circle). The powder was obtained by drying the biomass at 30 °C and milling the flakes to give particles of approximately 90 μm. Data are the mean of three independent experiments performed in duplicate samples with a standard error of <10 %
Discussion
Biomass production and cell recovery are important factors that influence the cost and quality of the products (Lee et al. 1998). Consequently, analysis of upstream and downstream operations in the production of a cyanobacterial fertilizer as an integrated process can be considered relevant for agricultural applications.
Vertical flat plate photoreactors have been used for the growth of photosynthetic microorganisms (Richmond and Cheng-Wu 2001; Ugwu et al. 2008); however, there are no reports for T. tenuis growth in this type of PBR.
The biomass and volumetric productivity of T. tenuis semi-continuous cultures grown in the vertical PBR showed a clear response to light path and intensity. The higher values were obtained at HLI with short light path (Table 1). The light regime of the cells was enhanced at this condition due to a higher cell fluctuation between the photic and dark volume of the reactor, with a more efficient light utilization (Grobbelaar et al. 1996; Richmond 2004). However, this does not necessarily describe the best condition in order to achieve a set quantity of biomass and the area required using artificial light. More meaningful results were obtained when the growth parameters were analyzed in terms of different productivities that take into account the volume of the reactor, the area occupied by the reactor, and the light source (Richmond 1999). In this sense, the highest RP resulted in the PBR with long light path because the increased biomass with the short light path was counterbalanced by the volumetric reduction of the PBRs. On the other hand, the best results of ISP were obtained in the PBR with long light path at NLI, showing the behavior described by Richmond (2004), where the ISP increases with the light path at a given illumination condition until a maximum is reached.
The OAP and ISP data showed similar tendency in the PBRs with long light path at both light intensities. The higher OAP value obtained at NLI results from the lower area required to set the fluorescent tube panels in relation to the halogen lamps.
With fluorescent panels, two PBR units can be arranged in an area a quarter part of which requires setting one PBR illuminated with two halogen lamps. Halogen lamp setting needs a long distance to cast light over the whole area of the PBR and avoid overheating (Tredici 2004). The results highlight the importance of evaluating cell productivity in terms of different indexes, each one indicating which factor has more relevance in a given production system.
Variations in environmental conditions including nutrients and light intensity can produce pronounced changes in the pigmentation of cyanobacterial cells (Grossman 2003). An important reduction in the content of chlorophyll a, total carotenoids, and phycobiliproteins of T. tenuis was observed in PBRs at HLI (Table 1). T. tenuis is a benthic species able to accumulate phycoerythrin depending on their light color environment (Kehoe and Gutu 2006). It has recently been reported that changes in pigment contents is a strain-dependent process for the epibenthic cyanobacterium Synechococcus. Decreased amounts of chlorophyll a and total carotenoids were found in phycoerythrin-rich strains, while the phycocyanin-rich strains showed constant levels of chlorophyll a and enhanced total carotenoids under high light conditions in the laboratory (Lohscheider et al. 2011). Another common response of cyanobacteria to environmental stress includes an almost complete loss of phycobilisomes (Grossman et al. 2001). The degradation of these compounds can reduce the absorption excitation energy, making cells less susceptible to photodamage; the phycobiliproteins of T. tenuis decreased similarly according to this fact.
Semi-continuous culture is currently used for microalgal and cyanobacterial biomass production (Richmond and Cheng-Wu 2001; Travieso et al. 2001). Comparative studies have shown that it is better than batch and continuous cultures in terms of simplicity, stability, and productivity (Otero et al. 1998; Reichert et al. 2006). The growth and specific productivity parameters of T. tenuis cultures operated semi-continuously for 18 months at a fixed 50 % renewal rate varied with the harvesting time and light intensity (Table 2). The specific growth rate increased with the harvesting frequency at both light intensities as a result of a more frequent fresh nutrient replacement and a decreased cellular concentration of the cultures. Since the specific growth rate variations were similar at these conditions, no light stress occurred and more biomass was produced at HLI, which could lead to a stronger shelf-shading effect. However, this fact was counteracted by the higher light availability in the PBR resulting from the different setting of the light source (Fig. 1).
Higher biomass concentrations are expected to produce higher biomass productivities; nonetheless, with T. tenuis semi-continuous cultures, the highest values in VP, ISP, and OAP were obtained at lower biomass concentrations at both light intensities (Table 2). The operative mode utilized in this work, with constant renewal rate (50 %) and variable removal times, gave the same qualitative results on productivity as those based on variable renewal rates at a fixed removal time (Travieso et al. 2001). However, the higher productivities obtained at the lower harvesting time were not sustained over time when the sampling time was shorter than the generation time of T. tenuis.
Flocculation, a simple and cost-effective method, can be used to aggregate microalga cells, allowing easy harvesting by centrifugation, filtration, or gravity sedimentation (Chen et al. 2009). Several factors influence the flocculation potential of photosynthetic microorganisms (Avnimelech et al. 1982; Bilanovic et al. 1988; Thomas and Walsby 1985; Lee et al. 1998). Light intensity played a significant role in the flocculation capacity of T. tenuis. Cultures grown at NLI rapidly flocculated after stopping aeration, while cultures raised at HLI lost self-flocculation and the filaments remain unsettled in a state of buoyancy (Fig. 2a). Cyanobacterial cells possess a chemically reactive cellular surface with a net negative charge produced by the ionization of ionic groups, mainly of polysaccharide nature (De Philippis et al. 2001). The flocculation process involves rapid neutralization of the charged colloidal matter in suspension. Many flocculants are multivalent cations such as aluminum, iron, calcium, or magnesium, the last two being well-known microalga flocculants (Ayoub et al. 1986; Elmaleh et al. 1991). Ca2+ and Mg2+ ions increased the poor flocculation activity of T. tenuis cultures raised at HLI, with Mg2+ effectiveness higher than Ca2+ and in a magnitude similar to that obtained at NLI intensity. The flocculation activity of Mg2+ was further potentiated by the addition of Ca2+. The same degree of effectiveness has been reported using mixtures of these ions with a cyanobacterial bioflocculant (Shilo and Fattom 1990).
The illumination conditions in the growing phase of T. tenuis cultures determined the conservation characteristics of the biomass processed as dry powder. The biomass obtained at NLI displayed a declining trend in cell viability with storage time (Fig. 3). Conversely, the dry powder biomass of T. tenuis obtained at HLI and separated by Mg+2 plus Ca+2 induced flocculation and had greater viability and longer shelf-life, which is a valuable feature for its use as a rice biofertilizer. In a previous study, divalent cations increased the viability of T. tenuis cultured at NLI and preserved in a desiccated state; however, contribution to the retained viability was small in comparison to that obtained at HLI (Silva et al. 2007). The presence of residual Mg+2 and Ca+2 in the powdered T. tenuis biomass cannot be considered a drawback since they are essential nutrients for plant growth (Jan and Matsumoto 1999), and the actual amounts that might be present are well below the current content of these cations found in rice crop soils (Husnain et al. 2010)
In conclusion, artificial light intensity markedly influenced upstream and downstream operations at a bench-scale PBR for biomass production of T. tenuis intended to be a component of a dried and milled biofertilizer. While the final biomass, volumetric, and reactor productivities were higher at HLI, the productivities that take into account the whole area needed to establish a photoreactor gave better results at NLI. Additionally, under this condition, the cultures showed improved self-flocculation. The poor flocculation capacity of the cultures grown at HLI was improved by the addition of Mg2+ and Ca2+ and the conservation of the powder biomass was maintained for 24 months, while a sharp viability decline and cellular death were produced after 12 months at NLI. The results demonstrated the importance of analyzing the effect of light intensity on upstream and downstream operations in combination for defining the most suitable conditions for the process.
References
Avnimelech Y, Troeger BW, Reed LW (1982) Mutual flocculation of algae and clay: evidence and implications. Science 216:63–65
Ayoub GM, Lee Sang ILL, Koopman B (1986) Seawater induced algal flocculation. Water Res 20:1265–1271
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bilanovic D, Shelef G, Sukenik A (1988) Flocculation of microalgae with cationic polymers: effects of medium salinity. Biomass 17:65–76
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Brenzoni EO (1999) Utilización de cianobacterias como biofertilizante en el cultivo de arroz. II—Experiencias en condiciones de campo. In: Stegmayer AR, Pernasetti DS, Gómez Bello C (eds) II Reunión científico-técnica de biología del suelo del NOA y II Encuentro sobre fijación biológica del nitrógeno. Universidad Nacional de Catamarca, Catamarca, Argentina, pp 391–394
Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Chen L, Li P, Liu Z, Jiao Q (2009) The released polysaccharide of the cyanobacterium Aphanothece halophytica inhibits flocculation of the alga with ferric chloride. J Appl Phycol 21:327–331
De Philippis R, Sili C, Paperi R, Vincenzini M (2001) Exopolysaccharide-producing cyanobacteria and their possible exploitation: a review. J Appl Phycol 13:293–299
Elmaleh S, Coma J, Grasmick A, Bourgade L (1991) Magnesium induced algal flocculation in a fluidized bed. Water Sci Technol 23:1695–1702
Fuentes-Grünewald C, Garcés E, Alacid E, Rossi S, Camp J (2013) Biomass and lipid production of dinoflagellates and raphidophytes in indoor and outdoor photobioreactors. Mar Biotechnol 15:37–47
Grobbelaar JU, Nedbal L, Tichy V (1996) Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgal photoacclimated to different light intensities and implications for mass algal cultivation. J Appl Phycol 8:335–343
Grossman AR (2003) A molecular understanding of complementary chromatic adaptation. Photosynth Res 76:207–215
Grossman AR, Bhaya D, He Q (2001) Tracking the light environment by cyanobacteria and the dynamic nature of light harvesting. J Biol Chem 276:11449–11452
Hashem MA (2001) Problems and prospects of cyanobacterial biofertilizer for rice cultivation. Aust J Plant Physiol 28:881–888
Husnain H, Masunaga T, Wakatsuki T (2010) Field assessment of nutrient balance under intensive rice-farming systems, and its effects on the sustainability of rice production in Java Island, Indonesia. J Agric Food Environ Sci 4:1–11
Irisarri P (2006) Role of cyanobacteria as biofertilizers: potentials and limitations. In: Rai MK (ed) Handbook of microbial biofertilizers. Food Products Press, New York, pp 417–428
Irisarri P, Gonnet S, Monza J (2001) Cyanobacteria in Uruguayan rice fields: diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. J Biotechnol 91:95–103
Jan F, Matsumoto H (1999) Early effects of aluminium on nutrient (K, Ca, and Mg) status of different root zones of two rice cultivars. Toxicol Environ Chem 69:43–48
Kehoe DM, Gutu A (2006) Responding to color: the regulation of complementary chromatic adaptation. Annu Rev Plant Biol 57:127–150
Lee SJ, Kim SB, Kim JE, Kwon GS, Yoon BD, Oh HM (1998) Effects of harvesting method and growth stage on the flocculation of the green alga Botryococcus braunii. Lett Appl Microbiol 27:14–18
Lohscheider JN, Strittmatter M, Küpper H, Adamska I (2011) Vertical distribution of epibenthic freshwater cyanobacterial Synechococcus spp. strains depends on their ability for photoprotection. PLoS One 6/5:e20134
McKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Mishra U, Pabbi S (2004) Cyanobacteria: a potential biofertilizer for rice. Resonance 9:6–10
Molina Grima E, Belarbi EH, Acién Fernández FG, Robles Molina A, Chisti Y (2003) Recovery of microalgal biomass and metabolites. Process options and economics. Biotechnol Adv 20:491–515
Muller-Feuga A, Le Guédes R, Hervé A, Durand P (1998) Comparison of artificial light photobioreactors and other production systems using Porphyridium cruentum. J Appl Phycol 10:83–89
Otero A, Domínguez A, Lamela T, García D, Fábregas J (1998) Steady-states of semicontinuous cultures of a marine diatom: effect of saturating nutrient concentrations. J Exp Mar Biol Ecol 227:23–34
Pereira I, Ortega R, Barrientos L, Moya M, Reyes G, Kramm V (2009) Development of a biofertilizer based on filamentous nitrogen-fixing cyanobacteria for rice crops in Chile. J Appl Phycol 21:135–144
Prasanna R, Joshi M, Rana A, Shivay YS, Nain L (2012) Influence of co-inoculation of bacteria–cyanobacteria on crop yield and C–N sequestration in soil under rice crop. World J Microbiol Biotechnol 28:1223–1235
Qiang H, Zarmi Y, Richmond A (1998) Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). Eur J Phycol 33:165–171
Rana A, Joshi M, Prasanna R, Shivay YS, Nain L (2012) Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur J Soil Biol 50:118–126
Reichert CC, Reinehr CO, Costa JAV (2006) Semicontinuous cultivation of the cyanobacterium Spirulina platensis in a closed photobioreactor. Braz J Chem Eng 23:23–28
Richmond A (1999) Physiological principles and modes of cultivation in mass production of photoautotrophic microalgae. In: Cohen Z (ed) Chemicals from microalgae. Taylor & Francis, London, pp 353–386
Richmond A (2004) Principles for attaining maximal microalgal productivity in photobioreactors: an overview. Hydrobiologia 512:33–37
Richmond A, Cheng-Wu Z (2001) Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J Biotechnol 85:259–269
Saadatnia H, Riahi H (2009) Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ 55:207–212
Schwarz T, Bartholmes P, Kaufmann M (1995) Large-scale production of algal biomass for protein purification: tryptophan synthase from Euglena gracilis. Biotechnol Appl Biochem 22:179–190
Shilo M, Fattom A (1990) Process for clarifying a liquid using a polymeric substance. United States Patent 4,894,161, January 16
Silva PG, Silva HJ (2003) A retained viability index (RVI10) for evaluation of a cyanobacterial powder fertilizer. World J Microbiol Biotechnol 19:891–895
Silva PG, Silva HJ (2007) Effect of mineral nutrients on cell growth and self-flocculation of Tolypothrix tenuis for the production of a biofertilizer. Bioresour Technol 98:607–611
Silva PG, Ferrari SG, Silva HJ (2007) Preservation methods of Tolypothrix tenuis for use as a cyanobacterial fertilizer. J Appl Phycol 19:239–246
Singh JS, Pandey VC, Singh DP (2011) Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agr Ecosyst Environ 140:339–353
Thomas RH, Walsby AE (1985) Buoyancy regulation in a strain of Microcystis. J Gen Microbiol 131:799–809
Travieso L, Hall DO, Rao KK, Benítez F, Sánchez E, Borja R (2001) A helical tubular photobioreactor producing Spirulina in a semicontinuous mode. Int Biodeter Biodegr 47:151–155
Tredici MR (2004) Mass production of microalgae: photobioreactors. In: Richmond A (ed) Handbook of microalgal cultures: biotechnology and applied phycology. Blackwell, Oxford, pp 178–214
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Watanabe M, Shiba H, Sasaki K, Nakashimada Y, Nishio N (1998) Promotion of growth and flocculation of a marine photosynthetic bacterium, Rhodovulum sp. by metal cations. Biotechnol Lett 20:1113–1117
Acknowledgments
This work was supported by the Secretaría de Ciencia y Técnica, Universidad Nacional de San Luis Rizobacter Argentina, and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, P.G., Silva, H. Biomass production of Tolypothrix tenuis as a basic component of a cyanobacterial biofertilizer. J Appl Phycol 25, 1729–1736 (2013). https://doi.org/10.1007/s10811-013-0035-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0035-2