Abstract
Although adhesion of bacteria and yeast have been extensively studied by a wide range of experimental and theoretical approaches, significantly less attention has been focused on microalgae adhesion to solid materials. This work is focused on physicochemical aspects of microalgae adhesion. The results are based on experimental characterization of surface properties of both microalgae and solids by contact angle and zeta potential measurements. These data are used in modeling the surface interactions (thermodynamic and colloidal models) resulting in quantitative prediction of the interaction intensities. Finally, the model predictions are compared with experimental adhesion tests of microalgae onto model solids in order to identify the physicochemical forces governing the microalgae–solid interaction. The model solids were prepared in order to cover a wide range of properties (hydrophobicity and surface charge). The results revealed that, in low ionic strength environment, the adhesion was influenced mostly by electrostatic attraction/repulsion between surfaces, while with increasing ionic strength grew the importance of apolar (hydrophobic) interactions. The impact of solid surface properties on the degree of colonization by microlagae was statistically more significant than the influence of medium composition on cell surface of Chlorella vulgaris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the wide range of biotechnological applications of microalgae (Pulz and Gross 2004; Raja et al. 2008), the methods of their culturing and processing are receiving particular attention. The design of microalgae bioreactors is particular, since the light penetration into submerged cultures is one of the crucial parameters influencing the overall productivity of the algae cultivation systems (Carvalho et al. 2011). Prevention of the fouling of inner walls of the closed photobioreactors by adhering microalgae is therefore an opportunity to improve the process productivity and decrease the production cost (Carvalho et al. 2006). Algal biofilms are also responsible for the biodeterioration of a variety of man-made structures in both the aerial and aquatic environments, which result in severe economic penalties (Callow 2000; Sekar et al. 2004; Goers et al. 2007). In a specific case, however, the algal adhesion to solid surfaces can offer savings, for instance in the step of harvesting the microalgae biomass from diluted cell suspensions (Chen et al. 2011; Xu et al. 2011) or it can be exploited as an immobilized growth system (Johnson and Wen 2010).
The adhesion of microbial cells to solid materials is often considered to have a prevailing physicochemical character (Bos et al. 1999). Several theoretical models are able to quantitatively predict the cell–cell and cell–solid interaction based on physicochemical properties of interacting surfaces, such as the thermodynamic balance of interaction energies, classical Derjaguin–Landau–Verwey–Overbeek (DLVO), and extended DLVO (XDLVO) theory (Bos et al. 1999; Sharma and Hanumantha Rao 2002; van Oss 2003). These theoretical approaches enable to evaluate the contribution of each force, probability of adhesion, and, eventually, predict its intensity.
The objective of this work was to study the mechanism of microalgae adhesion to model surfaces by identifying the most important forces playing role in this interaction. The experimental campaign was carried out with an algae strain (Chlorella) previously used for bioremediation of flue gas carbon dioxide (Doušková et al. 2009) and starch production (Brányiková et al. 2011). The adhesion properties of algae grown under different medium compositions were demonstrated on their interaction with glass slides with/without surface modifications. The glass modifications were chosen in order to cover a wide range of properties from hydrophilic to hydrophobic and negatively to positively charged surfaces. The practical importance of this study lies in the development of experimental tools and verification of predictive theoretical models for selecting the proper growth conditions and solid surfaces (or their modification) suitable for either preventing or inducing adhesion of microalgae to solid surfaces.
Materials and methods
The microalgae strain Chlorella vulgaris Beijerinck (CCALA 924) was isolated in Southern Greece, further selected in the Laboratory of Cell Cycles of Algae, Institute of Microbiology of ASCR, and deposited in the Culture Collection of Autotrophic Organisms in Třeboň, Czech Republic. In the collection, the strain has been maintained on agar slants under irradiance of about 23 μmol photons m−2 s−1), 12/12 h (light/dark) regime and at temperature of 12–15 °C (http://www.butbn.cas.cz/ccala/index.php). The strain is also deposited as strain P12 in the Laboratory of Cell Cycles of Algae, Institute of Microbiology of ASCR, Czech Republic.
Algae cultivation
A set of glass cylinders (inner diameter, 36 mm; height, 500 mm) were placed in a thermostatic bath (30 °C) and continuously illuminated by a panel of dimmable fluorescent lamps (Osram Dulux L55 W/950 Daylight, Italy) with an incident light intensity of 780 μmol photons m−2 s−1). The incident and transmitted light intensities were measured using a quantum radiometer–photometer (LI-COR, Inc. USA). The cylinders were bubbled using a mixture of air and CO2 (2 % v/v). The volume of the algal suspension in each cylinder was 300 mL, and each cylinder was supplied with gas mixture at a flow rate of 15 L h−1. The pH of cultures was maintained in the range of 6.0–7.0 by the addition of 1 M NaOH. The experiments were carried out in a batch culture regime for 220 h. After this time, the cells were harvested by centrifugation and used for cell surface characterization and adhesion tests. The complete mineral medium (CMM) used for algal growth was based on the mean content of P, N, K, Mg, and S in algal biomass (Doucha and Lívanský 2006) and had the following initial composition of macroelements (mg L−1): 1,100 (NH2)2CO, 237 KH2PO4, 204 MgSO4⋅7H2O; and microelements (mg L-1): 40 FeNa-EDTA, 88 CaCl2, 0.83 H3BO3, 0.95 CuSO4⋅5H2O, 3.3 MnCl2⋅4H2O, 0.17 (NH4)6Mo7O24⋅4H2O, 2.7 ZnSO4⋅7H2O, 0.6 CoSO4⋅7H2O, and 0.014 NH4VO3 in distilled water.
In experiments where limitations were tested, the original CMM was removed from the cell suspension by centrifuging after 135 h of cultivation, and the cells were resuspended in one of the following modified mineral media, in which they were allowed to grow for 85 h (Fig. 1). For the microelement-limiting medium (MLM), FeNa EDTA, CaCl2, H3BO3, CuSO4⋅5H2O, MnCl2⋅4H2O, (NH4)6Mo7O24⋅4H2O, ZnSO4⋅7H2O, CoSO4⋅7H2O, and NH4VO3 were omitted from the original mineral medium.
For the sulfur-limiting medium (SLM), MgSO4⋅7H2O from the original mineral medium was replaced by 168 mg L−1 MgCl2⋅6H2O to keep the concentration of Mg2+ ions the same as in the original mineral medium.
For the nitrogen limiting medium (NLM), the (NH2)2CO was omitted from the original mineral medium.
Contact angle measurement
The surface properties of cells, in the form of algal layers on membrane filters, and glass slides with different surface treatments were characterized by contact angles (CAs). In order to create flat layers, C. vulgaris cells were deposited on a filter (nitrate cellulose membrane, 0.45 μm pore size, 47 m diameter, Whatman) under negative pressure. In the case of C. vulgaris, the suspension was highly concentrated (cell concentration determined with a Bürker chamber) in order to gain 7 × 106 cells mm−2 on the filter. The obtained microbial lawns were then deposited on agar plates to stabilize moisture content (Dengis et al. 1995), fixed to a microscopic glass slide, allowed to dry for 50 min to reach the plateau region (Sharma and Hanumantha Rao 2002). The CA measurements of both algal lawns and glass slides were carried out by the sessile drop technique (volume of ∼3 μL) using the CAM 200 goniometer (KSV Instruments, Finland). Measurements were performed at 25 °C with three test liquids (water, formamide, and 1-bromnaphtalene); readings were taken after 0.5 s of deposition, and each sample was tested ten times. The total surface tension and its components and the values of the free energy of interaction between cells and carrier in water were calculated according to van Oss (1995).
Preparation of solid carriers
The surfaces tested for adhesion experiments were microscopic glass slides, which either passed a cleaning procedure or functionalizing by self-assembled monolayers. The model materials were prepared by the following procedures.
Glass
The microscopic glass slides (Knittel-Glass, 26 × 76 mm) were washed with detergent, rinsed with deionized water, and treated by ultrasound (Sono Swiss, SW3H, 280W) in water bath for 10 min. Then, the slides were treated with Piranha solution (conc. H2SO4 and 30 % H2O2 in the ratio of 7:3 v/v) for 24 h at 25 °C. After removal from Piranha solution, the glass slides were rinsed with deionized water and dried at 95 °C for 1 h.
3-Aminopropyltriethoxysilane
The glass slides were prepared for surface modification with 3-aminopropyltriethoxysilane (APTES) by washing with detergent, rinsing with deionized water, and treating by ultrasound (Sono Swiss, SW3H, 280W) in water bath for 10 min. The glass slides were then immersed into Piranha solution (conc. H2SO4 and 30 % H2O2 in the ratio of 7:3 v/v) for 1 h at 25 °C. Subsequently, the slides were successively washed in deionized water, ethanol, and acetone and dried at 110 °C for 1 h. After drying, the slides were immersed for 1 h into a mixture of APTES (5 % v/v) and toluene. Finally, the modified glass slides were successively rinsed in toluene and acetone and dried again for 1 h at 110 °C.
Propyltriethoxysilane
The functionalization of glass slides with propyltriethoxysilane (PTES) was identical with that of APTES with the difference that the surface modification was carried out with a mixture of PTES (5 % v/v) and toluene.
Zeta potential measurement
The zeta potential (ZP) of microalgal cells was measured by Zetasizer Nano-ZS (Malvern, UK) in 10 or 100 mM KCl at pH 6.5. The surface charge of glass slides with/without surface modification was determined by an electrokinetic analysis. All samples were studied in adjustable gap cell on SurPASS (Anton Paar, Austria) in contact with electrolytes (10 or 100 mM KCl at pH 6.5). For zeta potential determination, a streaming current approach and Helmholtz–Smoluchowski equation was used (Kolská et al. 2012). All samples were measured twice with experimental error ±10 %.
Adhesion tests
The initial adhesion of algae on various surfaces was compared using the solid carriers (glass slides) with/without surface modifications. Cell suspension (30 mL, cell conc. 5 ± 0.2 g L−1) in 5 mM KCl at pH 6.5 was poured into a Petri dish (9.5 cm diameter) with one glass slide (26 × 76 mm) and was laid on a flat desk for 120 min (daylight, 25 °C) without agitation. Subsequently, the slides were transferred into a clean Petri dish and were washed twice with 30 mL of 10 mM KCl (pH 6.5) under shaking (70 rpm, GFL 3005, Germany) for 3 min. The evaluation of algal adhesion intensity was based on the autofluorescence of algae cells. The percentage of surface colonized by algae was determined by image analysis (Cellavista Analyzer, Roche) after taking 45 pictures from a total evaluated area of 6.325 ± 0.02 mm2 using red fluorescent channel (620 nm). The surface colonization results were analyzed by two-way analysis of variance. A post hoc Scheffe's test was used to assess significant differences between different materials. All statements of significance were based on probability of p < 0.05. Statistical analyses were performed using MS Excel software.
Other analytical methods
Starch analysis was carried out as described in Brányiková et al. (2011) in triplicate. The size of microalgae C. vulgaris (minimum of 50 readings) was determined microscopically (Nikon Eclipse E400) by using an image analysis software (NIS-Elements, Laboratory Imaging s.r.o., Czech Republic).
Results
Growth of algae in different media
In order to evaluate the influence of medium composition on adhesiveness of C. vulgaris to solid surfaces, these microalgae were grown in different media. The media were chosen to simulate a process, where nutrient limitation is used to provoke intracellular starch accumulation (Brányiková et al. 2011). The growth curve of microalgae in CMM shows a typical linear kinetics (Fig. 1). This can be ascribed to limitation by light since the increasing number of cells in bubble column is illuminated constantly with 780 μmol photons m−2 s−1. In this type of cultivation, the mean light intensity in the course of algal growth was found to decline exponentially (Brányiková et al. 2011). The algae harvested at a dry biomass concentration of about 7.5 g L−1 from CMM had a starch content of 6.5 % dry weight and an average cell diameter of 6.8 μm (Table 1).
In order to induce the accumulation of reserve polysaccharides, after about 135 h of cultivation in CMM, the C. vulgaris was harvested and resuspended in one of the modified media, in which they were allowed to grow for 85 h (Fig. 1). The results show that upon nutrient deprivation, the cell growth and division are either slowed or ceased completely (Fig. 1). Both sulfur and nitrogen limitation induced an accumulation of intracellular starch, while the limitation by microelements did not result in significant starch production (Table 1). The modified media influenced also the average cell size, which increased in the case of starch accumulation (SLM and NLM) and remained almost unchanged for MLM (Table 1). The cell size is one of the input parameters for the colloidal adhesion models (XDLVO) used in this work. Algae harvested from different media were further characterized in terms of their surface properties and used in adhesion tests.
Physicochemical properties of interacting surfaces
The ionic strength (I) 10 mM KCl of the electrolyte and its pH (6.5) are comparable with that of the media (CMM and limiting media) used throughout the algal cultivation. The higher I (100 mM KCl) used in thermodynamic and XDLVO models and adhesion tests were simulating the situation when NaNO3 is used instead of urea as a source of nitrogen. The average ZP measurements of algae grown in different media and solid model substrates are shown in Table 2. The electrophoretic mobility data indicate that C. vulgaris was independent of the medium composition dominantly electronegative both at I = 10 and 100 mM and pH 6.5, as previously already reported (Hadjoudja et al. 2010). A slightly lower electronegativity was found in the case of cells grown in CMM as compared to nutrient-limiting media. The surface charge of microalgae grown in CMM was somewhat lower in the electrolyte with higher ionic strength (Table 2).
Measurements of the CA revealed a complex behaviour of algal surfaces (Table 2). More predicative than CA are the values of total surface tensions (γ TOT), their apolar (γ LW) and polar (γ AB) components, as well as the electron donor (γ −) and electron acceptor (γ +) components of the γ AB, calculated from CA values according to van Oss (1995) (Table 3). These show that algae grown in CMM are characterized by the lowest willingness to provide polar interaction (γ AB = 2.2 mJ m−2, γ − = 70.1 mJ m−2, γ + = 0.02 mJ m−2) followed by algae-SLM < algae-MLM < algae-NLM. Simultaneously, the apolar components (γ LW) of the cell surface tensions from different media show lower variability (Table 3). The total surface tension (γ TOT) and its components clearly place the algae C. vulgaris among cells with hydrophilic surfaces. These are characterized with γ LW ≅ 40 mJ m−2, γ + ≅ 0 mJ m−2, and γ − > 28 mJ m−2 (van Oss 1995), which is in accordance with surface tension components of the algae grown under different medium compositions. A slight deviation can be observed only in the case of algae grown under nitrogen limitation (NLM) resulting in higher polar components of surface tension (γ AB = 21.3 mJ m−2, γ + = 1.35 mJ m−2, γ − = 83.85 mJ m−2). The higher values obtained for as compared to γ + is not surprising, since the zeta potential measurements also showed a negative surface charge of the algae cells (Table 2).
The ZP values of solid materials are in line with expectations based on the performed surface modification. The surface charge of hydrolyzed and cleaned borosilicate glass was negative both in 10 mM (−35 mV) and 100 mM (−26 mV). The same surface property was found to be less negative for propyl-modified PTES glass (−28 mV in 10 mM, −18 mV in 100 mM electrolyte), where part of the OH groups were replaced by a less polarized CH3 terminated monolayers. Simultaneously, the APTES glass showed positive surface charge in 10 mM KCl (48 mV) thanks to the modification with NH2-terminated monolayers. However, the same surface modification possessed at higher I (100 mM) a slightly negative charge (Table 2).
The CA values of model solids (Table 2) were determined and subsequently used to calculate their surface tensions (Table 3). As expected, PTES is the most hydrophobic surface (highest water CA) with low tendency to provide polar interactions (γ AB). The surface modification of glass with APTES led to less hydrophobic surface (lower water CA and higher γ TOT) as compared to PTES glass. Conversely, the hydrolyzed glass slides had a hydrophilic surface as it can be seen from both CA and total surface tension values (Tables 2 and 3).
Adhesion tests
An adhesion test designed to swiftly evaluate the colonization of solid surfaces by microalgae was applied in this work. The percentage of colonized surface was evaluated after 120 min of contact between cells and solids. The contact time was chosen based on previous tests (data not shown). These revealed that at contact times from 90 to 220 min, the results (percentage of colonized surface) are little affected by the duration of interaction between algae and abiotic surfaces.
Unlike in similar adhesion tests (Sekar et al. 2004), the surface colonization was not quantified by staining with fluorescent dyes, but using the autofluorescence of the green microalgae. This alternative evaluation method was selected in order not to influence the algal adhesion by the staining procedure.
The adhesion tests carried out in the frame of this work revealed differences in the disposition of cells to attach to the studied solids. During the adhesion experiments carried out in low I electrolyte (10 mM KCl), the most extensive adhesion was recorded in the case of APTES-modified glass, followed by a significantly less colonized PTES glass and finally unmodified glass (Table 4). The results also show that the adhesion in low I was influenced most significantly by the character of the solid surfaces [p calculated (4.3 × 10−6) < 0.05], while the impact of medium composition on the adhesiveness of algal surfaces was statistically not significant [p calculated (0.33) > 0.05].
In the case of high I electrolyte (100 mM), there were different average surface colonizations of the model solids decreasing the order APTES > glass > PTES (Table 4). However, given the high standard deviation of the average percentage of colonized surface, the influence of the material surface properties on microalgae adhesion can be considered at high I as statistically not significant [p calculated (0.38) > 0.05].
Comparison of model prediction with experimental algal adhesion to solid surfaces
In the thermodynamic approach to microbial adhesion, the values of the total free energy of adhesion between solid surfaces in water (ΔG TOT) prompt about the stability of the surface interaction (energetically favourable when ΔG TOT < 0, unfavorable when ΔG TOT > 0). However, the thermodynamic approach does not include the role of long range electrostatic interactions, and therefore, it is valid only at close contact (Bos et al. 1999).
A favorable (negative) adhesion energy balance was obtained only in the case of algae grown in MLM and SLM adhering to PTES glass. The calculations concerning the remaining combinations of algae and surfaces resulted in energetically unfavorable balances (Table 5). Comparing the total free adhesion energies (Table 5) with the real microalgal adhesion experiments (Table 4), it can be seen that the thermodynamic approach was not able to predict the significant colonization of the APTES glass by algae. Simultaneously, the prediction of the thermodynamic model concerning energetically unfavorable interaction of algae with hydrolyzed glass (Table 5) was confirmed by the least extensive adhesion of algae to this material (Table 4).
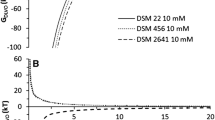
Since the thermodynamic model was not able to provide a generalized description of algal microbial adhesion to solid surfaces, the XDLVO theory was used subsequently. It combines the classical noncovalent Liftshitz–van der Waals (LW) and electrostatic (EL) interactions with the Lewis acid–base (AB) interactions (van Oss 2003). The simulations according to the XDLVO theory were made for sphere–flat plate interactions, I of either 10 or 100 mM, and the Hamaker constant was estimated from the ΔG LW (Table 5) of the given combination of algae support (van Oss 2006). The extended version of the XDLVO theory also used the characteristic decay length (0.6 nm) of AB interactions in water (Bos et al. 1999). The profile of total interaction free energy (ΔG TOT) vs. the separation distance predicted the interaction between algae (sphere) and solid surfaces (flat plate) in 10 and 100 mM KCl, as they can be seen on Figs. 2 and 3, respectively. The presence of potential energy barriers preventing the collision (close contact) of cell with solids can be seen for all algal cells in contact with glass and PTES glass. However, the energy barrier is lower in the case of algae grown in MLM (about 2,600 kT) adhering to PTES glass (Fig. 2). This energy barrier is surmountable with the kinetic energy of algal cells (about 3,500 kT) travelling at a speed of approximately 1 cm s−1 (cell density, 1,080 kg m−3). These values are certainly achievable in photobioreactors (Carvalho et al. 2006) as well as at the beginning of the adhesion tests, when the cell suspension is poured into Petri dish. Therefore, the adhesion of algae grown in MLM to PTES glass was the highest (2.3 % of colonized surface) among glass and PTES glass combinations in 10 mM electrolyte, which is in accordance with the XDLVO model prediction.
Total interaction free energy (ΔG TOT) as a function of the separation distance between algae grown in different media (CMM complete mineral medium, MLM microelement-limiting medium, SLM sulfur-limiting medium, NLM nitrogen-limiting medium) and flat surfaces of solids (glass, APTES glass, and PTES glass) calculated according to the XDLVO theory in 10 mM KCl at pH 6.5
It is known that, in spite of the potential energy barriers, the cells may be retained in the so-called secondary potential minimum at a certain distance from the solid surface (Redman et al. 2004). The XDLVO model indeed predicted the existence of interaction energy valleys for algal adhesion in both low (10 mM) and high (100 mM) I electrolytes at distances 3–7 nm from the surface of APTES-modified glass (Figs. 2 and 3). Although the increasing depth of the secondary minima in 10 mM KCl (NLM < MLM < SLM < CMM) does not correspond with the increasing intensity of the APTES surface colonization, the XDLVO model was able to clearly confirm the experimental finding that APTES glass is the most prone surface to algal colonization (Table 4). Significantly less pronounced secondary minima were predicted in 100 mM KCl (Fig. 3). The deepest valley foreseen for the interaction of algae-CMM with APTES glass was experimentally verified with the highest degree of surface colonization (Table 4). The predicted difference between glass and PTES glass (Fig. 3) was experimentally not proved by statistically different adhesion intensity (Table 4).
Among the interactions involved in the XDLVO theory, the attractive electrostatic (EL) interactions proved to be the most important of all at low I (10 mM) and separation distances larger than 3 nm. For instance, in the case of interaction between algae-CMM and APTES-glass at secondary minimum (3 nm), the contribution of noncovalent LW, EL, and AB interactions was −4.24 × 102, −6.88 × 103, and 1.61 × 103 kT, respectively. The prevailing EL character of the algae–solid interactions is enhanced by the low I (10 mM) since it increases the distance (Debye length), over which significant charge interaction can occur (Boonaert et al. 1999). Given the opposite charge of algae and APTES-modified glass, it is no surprise that the APTES glass has already been found to be the most attractive surface for green microalgae (Barberousse et al. 2007). At higher I of the electrolyte (100 mM), the importance of the EL interaction in the XDLVO model decreased, and the formation of the secondary minimum was driven by attractive LW (hydrophobic) interactions. For instance, in the case of interaction between algae-CMM and APTES glass at secondary minimum (6 nm), the contribution of noncovalent LW, EL, and AB interactions was −212, 2.15, and 10.8 kT, respectively. Both the prediction based on the depth of the secondary minima (Fig. 3) and the degree of surface colonization (Table 4) correlates with the apolar (γ LW) components of the model materials surface tension (Table 3). The deeper is the secondary minimum, the larger is the surface colonization and the higher is the γ LW of the solid surface.
Discussion
In general, adhesion of microalgae to solid substrates is a case-dependent interaction covering, from practical point of view, wide ranges of examples from industrially applicable (desired) to deleterious (unwanted) ones. This diversity of interactions between algae and solid surfaces stems from the wide variety of algae and their surface properties, accompanying microorganisms and solid (biotic or abiotic) surfaces, which can be involved. However, little is known on the adhesion mechanism, its driving force, and possibilities of its prevention/control for industrially important microalgae grown in axenic cultures in the environment of photobioreactors (Borowitzka 1999). Studying the adhesion mechanism of commercially and industrially important microalgae is therefore of high relevance both for process productivity and hygiene.
Light penetration into submerged culture is one of the crucial parameters influencing overall productivity of the cultivation system. Unwanted microalgae adhesion on the photobioreactor surfaces can negatively influence the whole process yield, especially at higher culture densities. One of the key factors influencing the growth yield of algae in photobioreactors is thus the supply and distribution of light in the whole working volume of reactors (Carvalho et al. 2011). However, the penetration depth of the electromagnetic radiation is often negatively influenced by the phenomenon of algal adhesion to the inner side of the photobioreactor walls.
However, algal biofilms are also responsible for the biodeterioration of a variety of man-made structures in both the aerial and aquatic environments, which result in severe economic penalties (Ludyansky 1991; Barberousse et al. 2007). At the same time, microalgae were identified as the major components in the biofilms causing damage to water distribution (pipes and storage tanks) and cooling systems. Therefore, adhesion of microalgae onto solid substrates plays a crucial role in a whole range of deleterious biofouling side effect (Callow 2000).
It has been shown that cultivation of algae (C. vulgaris) under nutrient deprivation affects the cell composition (Tang et al. 2011; Brányiková et al. 2011). As it has been previously proven, the surface charge of the algae C. vulgaris, at pH values close to neutral, is negative (Hadjoudja et al. 2010). However, the zeta potential of C. vulgaris did not change significantly with the composition of culture media (Table 2). This species is known to be unable to produce extracellular polymeric substances (Irving and Allen 2011). The type of surface properties identified for C. vulgaris (dominantly electron donor properties) are very common across the whole spectrum of microbial strains, from G+ bacteria Streptococcus (van der Mei et al. 1998), G− bacteria Shewanella (Korenevsky and Beveridge 2007) to yeast Saccharomyces (Kuřec and Brányik 2011). The negative surface charge can be attributed to the microalgal cell wall composition containing sporopollenin, a biopolymer with hydroxyl and carboxyl functional groups.
The surface charge significantly determines both cell–cell and cell–solid interactions. This is the explanation why previous studies did not find correlation between solid surface hydrophobicity and degree of colonization (Irving and Allen 2011). They neglected the effect of electrostatic interactions, which is particularly important in environments with low I, as it is shown in this work. The attractive electrostatic interactions between algae and solid surfaces have already been taken advantage in harvesting microalgae by magnetite particles (Xu et al. 2011).
The model surfaces used in this study were selected in order to include the most typical construction materials of closed photobioreactors (borosilicate glass) as well as to cover the surface properties of other materials the microalgae can get in contact with. Glass modified with PTES was chosen to mimic the hydrophobic construction materials with negative surface charge such as poly(methyl methacrylate), polycarbonate, and polypropylene (Smole et al. 2009; Irving and Allen 2011). The modification with APTES was in turn introducing positive surface charge. This intended to simulate a so-called image charge developing in the conducting materials (e.g., stainless steel) surrounded by electrolyte (Mei et al. 2009) as well as possible surface conditioning with medium components, exopolysaccharides, or microbes (Lorite et al. 2011). It is known that the microbial contamination of C. vulgaris cultures shifts the growth of this microalga from planktonic to biofilm (Irving and Allen 2011).
The results showed that from the point of view of microalgae adhesion, the choice of the materials is more important than the effect of medium composition on microalgae surface properties. This applies for the model organism used in this work (C. vulgaris), and given the huge variability of microalgae and their growth conditions, this statement cannot be generalized. Simultaneously, the obtained data revealed that the environmental conditions (ionic strength) largely affect the mechanism of microalgae adhesion. In consequence of this, different adhesion mechanisms have to be distinguished. They can change from electrostatic interaction dominated adhesion in low I electrolyte to hydrophobic interaction driven adhesion in high I electrolyte. Therefore, the proper choice of the materials for photobioreactor construction in combination with the production strain and its environmental requirements (e.g., freshwater or salt water, pH) is crucial for high productivity and hygienic operation. This paper provides data and fundamental methodologies for understanding the fouling of different solid materials with microalgae, which can have practical implications in either preventing or inducing microlagae adhesion and subsequent biofilm formation.
References
Barberousse H, Brayner R, Do Rego AMB, Castaing JC, Beurdeley-Saudou P, Colombert JF (2007) Adhesion of façade coating colonizers, as mediated by physic–chemical properties. Biofouling 23:15–24
Boonaert CJ-P, Dupont-Gillain CC, Dengis PB, Dufrene YF, Rouxhet PG (1999) Cell separation, flocculation. In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation. Wiley, New York, pp 531–548
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Bos R, van der Mei HC, Busscher HJ (1999) Physicochemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol Rev 23:179–230
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišová K, Zachleder V, Vítová M (2011) Microalgae—novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Callow ME (2000) Algal biofilms. In: Evans LV (ed) Biofilms: recent advances in their study and control. Hardwood, Amsterdam, pp 189–209
Carvalho AP, Meireles LA, Malcata XF (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Carvalho AP, Silva SO, Baptista JM, Malcata Xavier F (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89:1275–1288
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Dengis PB, Nélissen LR, Rouxhet PG (1995) Mechanism of yeast flocculation: comparison of top and bottom-fermenting strains. Appl Environ Microbiol 61:718–728
Doucha J, Lívanský K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J Appl Phycol 18:811–826
Doušková I, Doucha J, Livansky K, Machat J, Novak P, Umysova D, Zachleder V, Vitova M (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185
Goers S, Schumann R, Haeubner N, Karsten U (2007) Fungal and algal biomass in biofilms on artificial surfaces quantified by ergosterol and chlorophyll a as biomarkers. Int Biodeter Biodegr 60:50–59
Hadjoudja S, Deluchat V, Baudu M (2010) Cell surface characterisation of Microcystis aeruginosa and Chlorella vulgaris. J Colloid Interf Sci 342:293–299
Irving TE, Allen GD (2011) Species and material considerations in the formation and development of microalgal biofilms. Appl Microbiol Biotechnol 92:283–294
Johnson MB, Wen Z (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85:525–534
Kolská Z, Řezníčková A, Švorčík V (2012) Surface characterization of polymer foils. E-Polymers 083:1–13
Korenevsky A, Beveridge TJ (2007) The surface physicochemistry and adhesiveness of Shewanella are affected by their surface polysaccharides. Microbiology 153:1872–1883
Kuřec M, Brányik T (2011) The role of physicochemical interactions and FLO genes expression in the immobilization of industrially important yeasts by adhesion. Colloid Surf B-Biointerfaces 84:491–497
Lorite GS, Rodrigues CM, de Souza AA, Kranz C, Mizaikoff B, Cotta MA (2011) The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J Colloid Interf Sci 359:289–295
Ludyansky ML (1991) Algal fouling in the cooling system. Biofouling 3:13–21
Mei L, van der Mei HC, Ren Y, Norde W, Busscher HJ (2009) Poisson analysis of streptococcal bond strengthening on stainless steel with and without a salivary conditioning film. Langmuir 25:6227–6231
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34:77–88
Redman JA, Walker SL, Elimelech M (2004) Bacterial adhesion and transport in porous media: role of the secondary energy minimum. Environ Sci Technol 38:1777–1785
Sekar R, Venugopalan VP, Satpathy KK, Nair KVK, Rao VNR (2004) Laboratory studies on adhesion of microalgae to hard substrates. Hydrobiologia 512:109–116
Sharma PK, Hanumantha Rao K (2002) Analysis of different approaches for evaluation of surface energy of microbial cells by contact angle goniometry. Adv Colloid Interfac 98:341–463
Smole MS, Stakne K, Kleinschek KS, Kurecic M, Bele M, Svetec DG, Ribitsch V (2009) Electrokinetic properties of polypropylene-layered silicate nanocomposite fibers. J Appl Polym Sci 113:1276–1281
Tang HY, Chen M, Garcia MED, Abunasser N, Ng KYS, Salley SO (2011) Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol Bioeng 108:2280–2287
van der Mei HC, Bos R, Busscher HJ (1998) A reference guide to microbial cell surface hydrophobicity based on contact angles. Colloid Surf B-Biointerfaces 11:213–221
van Oss CJ (1995) Hydrophobicity of biosurfaces—origin, quantitative determination and interaction energies. Colloid Surf B-Biointerfaces 5:91–110
van Oss CJ (2003) Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific and aspecific interactions. Mol Recognit 16:177–190
van Oss CJ (2006) Interfacial forces in aqueous media. Taylor & Francis, Boca Raton, pp 22–24
Xu L, Guo C, Wang F, Zheng S, Liu Ch Z (2011) A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour Technol 102:10047–10051
Acknowledgments
The authors would like to thank the Grant Agency of the Czech Republic (project GAČR P503/10/1270) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirmerova, M., Prochazkova, G., Siristova, L. et al. Adhesion of Chlorella vulgaris to solid surfaces, as mediated by physicochemical interactions. J Appl Phycol 25, 1687–1695 (2013). https://doi.org/10.1007/s10811-013-0015-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0015-6