Abstract
We tested the hypothesis that increased pH reduces the amount of structural lipids. To do this, we used three different diatoms (Phaeodactylum tricornutum CCAP strain, P. tricornutum TV strain and Amphiprora sp). We tested the effect of rapid increase from pH 7.5 to 10 by adding NaOH. The total lipid content was reduced by 13, 36 and 47 % in the P. tricornutum CCAP strain, TV strain and Amphiprora sp., respectively, 1 h after increasing the pH. The P. tricornutum CCAP strain was used for further testing the effect of pH on the lipid content during active growth. This strain was cultivated at pH 7.5 and 10, and the pH was regulated by the CO2 inflow. The growth rate was similar (0.3 day−1) in both pH treatments, but the lipid content in the pH 10 treatment was on average 28 % lower than in the pH 7.5 treatment. Our data support the hypothesis that structural lipids are reduced when pH increases to high levels. The results suggest that regulating the pH during algae cultivation could be used to refine the lipid composition in the harvested algal biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Algal biomass has received considerable interest over the last decades as a source of lipids for both feed and fuel purposes. There is a good overview of how different algal species accumulate lipids depending on environmental conditions such as light, temperature and nutrient concentration (Guschina and Harwood 2006; Williams and Laurens 2010). However, a factor that has not received much attention is pH, which can be of importance, in particular, in dense cultures. At high biomass concentrations, the uptake of inorganic carbon during photosynthesis can rapidly increase pH and thus influence the growing conditions and physiology of the algal cells.
Guckert and Cooksey (1990) showed that high pH can increase triacylglycerol (TAG) accumulation, and several recent studies have pointed to the same direction, at least for green algae (Gardner et al. 2011; Santos et al. 2012). However, the trend has been hypothesised to be opposite for structural lipids (Guschina and Harwood 2009), but very little work has been carried out on this topic (Guckert and Cooksey 1990).
Structural lipids, which are mainly lipids bound to membranes, have overall not received the same attention as TAGs because they are less suited for biofuel purposes. However, from a nutritional point of view, many of the interesting fatty acids, e.g. polyunsaturated fatty acids (PUFAs), are associated with membranes inside the algal cells. These are, for example, the membranes of the photosynthetic pigments, and these structural lipids have clearly a potential as a source of fatty acids in feed (Brown et al. 1997).
In this study, we investigated the effect of pH on the structural lipids of two diatom species, under conditions when the TAG content was expected to be minimal. Our aim was to test the hypothesis that increased pH decreases the amount of these lipids.
Materials and method
Two strains of Phaeodactylum tricornutum and one strain of Amphiprora sp. were used in this study. The two P. tricornutum strains used were CCAP 1055/1, originating from the Culture Collection of Algae and Protozoa, Scottish Marine Institute, UK; and TV335, from the Tvärminne Zoological Station, University of Helsinki, Finland. The CCAP strain is marine, whereas the TV strain has been isolated from the brackish Baltic Sea. The Amphiprora sp. culture originated from a single cell isolated in an estuary on the west coast of Iceland and is maintained at Blue Lagoon Ltd., Iceland.
All cultures were maintained in sterile, artificial seawater with 0.1 g L−1 dissolved Cell-Hi WP (Varicon Aqua Solutions) nutrient mixture. The salinity for P. tricornutum CCAP strain and Amphiprora sp. was 25 practical salinity units (PSU), and the P. tricornutum TV strain’s salinity was 6 PSU.
Effect of rapid pH increase
The culture volume was 2 L, and the culture flasks were aerated with pre-filtered air (0.2 μm) and allowed to grow until stationary growth phase was reached. The cultivation bottles were placed inside a water bath kept at constant 20 °C. Light was provided by daylight spectrum, fluorescent tubes (Philips TLD-95), providing ~50 μmol photons m−2 s−1, 16:8-h light/dark cycle. The dense cultures and low light ensured light limitation and prevented accumulation of triacylglycerols. The pH was kept at 7.5, which was the pH at the time of the first harvesting.
During harvesting, subsamples were taken (~100 mL) for determining dry weight (DW) and fatty acid (FA) compositions. DW was determined by filtration onto pre-weighted filters that were dried over night at 60 °C. DW was calculated by subtracting the filter weight from the weight of the dried filter with algae. The biomass that was used for determining the FA composition was concentrated by centrifugation and stored at −80 °C until freeze dried (Steris, Lyovac GT2). The dried biomass was kept at −80 °C until the FAs were determined.
After the first harvesting, the pH was increased to pH 10.0 in the remaining culture by adding NaOH (5 M). The culture was left for 1 h before the next subsampling took place. Subsequent harvesting took place 6, 24, and 48 h after increasing the pH. The lipid data before pH increase was compared with the concentration after 1 h at elevated pH with a two-tailed, paired Student’s t test (n = 3).
Exponential growth at different pH levels
The P. tricornutum CCAP strain was grown at two different pH treatments with three replicates. The culture was grown in 2-L flasks connected to a gas control system, enabling different mixtures of air and CO2, and a pH control unit suspended in each of the culture bottles. Cultures were continuously bubbled with pre-filtered air (0.2 μm). Two pH levels used were pH ~ 7.5 and ~10. The trigger for CO2 addition was set at pH 7.6 and 10.1 for the low and high pH levels, respectively. When the pH increased above this trigger, CO2 was added to the inflowing gas (5 % v/v CO2). The CO2 addition was automatically shut off once the pH had decreased to pH 7.3 and 9.8 for the low and high pH levels, respectively.
The cultivation bottles were placed inside a water bath kept at constant 20 °C. The irradiance was ~100 μmol photons m−2 s−1 with a 16:8-h light/dark cycle. Biomass was monitored daily by measuring absorption at 620 nm as a proxy for biomass. The growth rate was calculated by linear regression from the natural log-transformed absorption data. Harvesting was done as described above, and results from the two treatments were compared with a two-tailed, Student’s t test (n = 3).
Determination of fatty acids
We added 500 μL of chilled (−20 °C) methanol with 0.1 % butylated hydroxytoluene to 5 mg DW algae sample in order to break the cells. The resulting solution was incubated at −20 °C for 10 min in reaction vials. Algae samples were further disrupted with two 4-mm stainless steel balls in a mixer mill (Retsch MM 301) for 3 min at 25 Hz. Temperature for these first steps was kept at <0 °C by pre-chilling all equipment.
The following steps were performed at room temperature: 1,000 μL chloroform and 150 μL internal standard (1,549 mg L−1 triheptadecanoin, Sigma-Aldrich; 1,029.6 mg L−1 heptadecanoic acid in chloroform/methanol, 2:1, Fluka, Sigma-Aldrich) were added to each sample and mixed for 10 min. After centrifugation, the supernatant was acidified with 300 μL, 20 mM acetic acid, mixed for 5 to 10 min and centrifuged again. The organic phase was extracted two times using 500 μL chloroform, mixed 10 min and centrifuged. Organic phases were pooled, and a subsample of 750 μL was dried in a glass tube under nitrogen flow. The residue was dissolved in 300 μL isopropanol. Each sample was extracted, and the FAs were determined in duplicates. The average of these measurements was used in data analysis.
The FAs were determined using transmethylation and gas chromatography (GC) with flame ionisation detector (FID). The samples were dried again under nitrogen flow, taken up in 700 μL petroleum ether, and 125 μM sodium methoxide (97 %, dissolved in methanol) was added before boiling the solution at 45 °C for 5 min. After cooling, 500 μL NaHSO4 (15 % m/v) and 200 μL petroleum ether were added, and the samples were mixed. After separation of the two phases by centrifugation, the petroleum ether phase was transferred to a GC vial. The solvent was evaporated, and residue was dissolved in 1,000 μL hexane. Subsequently, 1 μL of this solution was used for further gas chromatography analysis. This transmethylation procedure does not methylate the free fatty acids (FFAs), making it possible to distinguish between FFA and structural FAs in the following GC step.
FAs were separated and evaluated quantitatively using a capillary GC (7890A with sampler CTC Analytics GC-PAL system, Agilent Technologies), equipped with a BP-21 column (25 m × 0.2 × 0.3 mm; HP-FFAP Polyethylene Glycol TP, Agilent), and connected to a FID. The injector temperature was 260 °C. For every sample, 1 μL was injected and transferred splitless to the column. The oven temperature was programmed to increase from 70 °C (1.5 min) to 240 °C at the rate of 7 °C min−1. The carrier gas was helium with a pressure of 114.94 kPa. Peaks were allocated to substances via multicapillary column gas chromatography and via comparison to reference substances [fatty acids methyl esters (FAME) Mix, no. 1891, Supelco, Sigma-Aldrich]. Altogether, 30 FAs or FA methyl esters were identified and quantified.
Results and discussion
Rapid pH increase
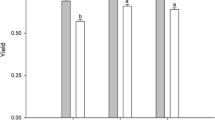
Increasing the pH with NaOH reduced the total lipid concentration clearly within an hour (Fig. 1, Electronic Supplementary Material, Table S1). The percentage lipid content at pH 7.5 was ~12.1, 6.7 and 2.9 % of the dry weight for P. tricornutum CCAP, TV strain and Amphiprora, respectively. After increasing the pH to 10, the lipid contents were reduced to 10.6, 4.3 and 1.5 %, respectively. The reduction in fatty acids content appeared as a reduction in the saturated fatty acids (SAFAs) (p = 0.007) and monounsaturated fatty acids (MUFAs) (p = 0.02), whereas no clear difference in polyunsaturated fatty acids was detected (p = 0.2). For the P. tricornutum CCAP strain, the PUFA concentration was similar in both pH treatments, and only SAFAs and MUFAs decreased (both by 13 %). For the other two diatoms, P. tricornutum TV strain and Amphiprora sp., all the fatty acids decreased by 38–50 %. Within the SAFA, MUFA and PUFA groups, there was no apparent difference between individual fatty acids (Table S1). The change in lipid concentration was apparent 1 h after the pH had been increased, and there was no further change with time, i.e. the lipid concentration did not decrease any further 6, 24 or 48 h after pH had been increased from pH 7.5 to 10.
The concentration of saturated fatty acids (SAFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) in P. tricornutum (CCAP and TV strains) and Amphiprora sp. before and after increasing the pH with NaOH. The high-pH biomass was harvested after 1 h at pH 10. The shaded area represents the fatty acid methyl esters; the remaining part is free fatty acids. Individual fatty acids are presented in Electronic Supplementary Material Table S1
Structural lipids are primarily glycolipids and phospholipids forming membranes. Inside the cell, there is an active regulation of pH, which can be regulated in individual compartments of the cell (Smith and Raven 1979). The reason for the reduction in structural lipids at elevated pH could be involved in the underlying mechanisms of internal pH regulation. However, it is neither within the scope of this paper nor do we have the data to speculate around the biochemical pathways or reasons for the rapid reduction in lipids after the pH was increased.
Individual lipids classes were not investigated in this study, and therefore, it cannot be excluded that storage lipids were present in our samples. In particular, P. tricornutum CCAP strain (with 12 % lipids) probably contained some storage lipids. However, the percentage lipids of total biomass were low. For comparison, P. tricornutum can have lipid content of >40 % after accumulating TAGs (Enss et al., in prep). Also, the P. tricornutum CCAP strain used in this study contained similar lipid percentages during exponential growth, suggesting that most of these lipids were structural lipids. Even though there might have been some storage lipids present, we are confident that it does not affect the main conclusion that increasing pH reduces the amount of structural lipids.
A decline in membrane lipids after an increase in pH has been demonstrated to take place in the green algae Chlorella sp. (Guckert and Cooksey 1990), but this is, to our knowledge, the only study of pH effect on structural lipids in algae. In their study, Guckert and Cooksey (1990) found both an absolute reduction in membrane lipids at elevated pH and also a shift in the composition towards more saturated FAs in the membranes. Our data indicate that the reduction in structural lipid content at high pH takes place also in diatoms and could possibly be a general characteristic of structural lipids in algae. However, we did not find any clear evidence for changes to the degree of saturation.
Growth at different pH
A similar result as for the rapid increase in pH was found when P. tricornutum (CCAP strain) was actively grown at different pH. The growth rate was 0.3 day−1 [±0.03 day−1 (SD) at pH 7.0 and ±0.06 day−1 (SD) at pH 10.0] in both pH treatments, but the average lipid concentration was reduced from 12 % (±0.7 %) of the DW at pH 7.5 to 9 % (±0.5 %) of DW at pH 10 (Fig 2, Electronic Supplementary Material Table S2). The amount of SAFAs and MUFAs were clearly lower at pH 10 than at pH 7.5 (p < 0.001). The overall concentration of PUFAs was less affected by the pH level, and there was no statistical support for suggesting a different PUFA concentration in the treatments (p = 0.11).
The concentration of saturated fatty acids (SAFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) in P. tricornutum (CCAP strain) during exponential growth at pH 7.5 and 10.0. Error bars represent standard deviation (n = 3). The shaded area represents fatty acid methyl esters; the remaining part is free fatty acids. The growth rate was in both treatments 0.3 day−1 [±0.03 day−1 (SD) for the pH 7.5 treatment and ±0.06 day−1 (SD) for the pH 10.0 treatment] at the time of harvesting. Individual fatty acids are presented in Electronic Supplementary Material Table S2
The measured fatty acids methyl esters concentration was lower when growing at high pH. The concentration of free fatty acids was opposite to that of FAME and had a higher concentration at pH 10 compared with pH 7.5. This difference was also highest for SAFA and MUFA, where FFA was 15 and 20 % higher at the elevated pH treatment.
The pH is influenced by several factors in actively growing algal cultures but will mainly be a function of the CO2 uptake by algae, CO2 exchange with inflowing gas and respiration. There are several ways how pH might affect the growth of algae. First, the amount of dissolved CO2 decreases with increasing pH as the chemical equilibrium of inorganic carbon shifts towards the bicarbonate (HCO3 −) and carbonate (CO3 2−) forms. This, in turn, may drive the primary production into carbon limitation (Riebesell et al. 1993). However, many algae are able to enhance CO2 available for photosynthesis through CO2-concentrating mechanism, which involves active uptake of CO2 and HCO3 − or through production of the enzyme carbonic anhydrase, which speeds up the conversion rate between HCO3 − and CO2 (Badger et al. 1998). The growth rate was relatively low but similar in the two pH treatments, suggesting that light rather than carbon was governing the growth rate. However, carbon availability could play an effect for the allocation of carbon into lipids (Guschina and Harwood 2009).
In conclusion, our data support the hypothesis that structural lipids are reduced when pH increases to high levels. This reduction was primarily seen for the measured FAMEs and not for the FFAs. Our data suggest that regulating the pH during cultivation of algae could be used to refine the lipid composition in algal biomass.
References
Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot 76:1052–1071
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Gardner R, Peters P, Peyton B, Cooksey K (2011) Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the Chlorophyta. J Appl Phycol 23:1005–1016
Guckert J, Cooksey K (1990) Triglyceride accumulation and fatty acid profile changes in Chlorella (Chlorophyta) during high pH-induced cell cycle inhibition. J Phycol 26:72–79
Guschina I, Harwood J (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Guschina I, Harwood J (2009) Algal lipids and effect of the environment on their biochemistry. In: Arts M, Brett M, Kainz M (eds) Lipids in aquatic ecosystems. Springer, New York, pp 1–24
Riebesell U, Wolf-Gladrow DA, Smetacek V (1993) Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361:249–251
Santos AM, Janssen M, Lamers PP, Evers WAC, Wijffels RH (2012) Growth of oil accumulating microalga Neochloris oleoabundans under alkaline-saline conditions. Bioresource Technol 104:593–599
Smith F, Raven JA (1979) Intracellular pH and its regulation. Ann Rev Plant Physiol 30:289–311
Williams PJB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energ Environ Sci 3:554–590
Acknowledgments
This study was funded by the Academy of Finland Research Programme “Sustainable Energy” (SusEn), the Nordic Energy Research Programme “N-INNER” and the Technology Development Fund in Iceland (grant no. 101253011). Additionally, a mobility grant was given to KS from the Nordic Marine Academy. We would also like to thank Hannes Lárus Jóhannsson, Sigrún Helgadóttir, Airi Hyrkäs and Jaana Rikkinen for their excellent technical assistance. The Amphiprora sp was isolated by Sigurbjörn Einarsson.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spilling, K., Brynjólfsdóttir, Á., Enss, D. et al. The effect of high pH on structural lipids in diatoms. J Appl Phycol 25, 1435–1439 (2013). https://doi.org/10.1007/s10811-012-9971-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9971-5