Abstract
Twenty-five intertidal diatom species were isolated from the Solthörn tidal flat (Lower Saxony, southern North Sea) and grown in semi-continuous cultures under standardised conditions, in order to observe differences in their biochemical gross compositions (e.g. protein, lipid, carbohydrate and ash contents). Composition, expressed as % dry weight, indicated that the majority of species (52 %) contained only <15 % protein but had nearly twice the total amount of carbohydrate and two to three times higher ash content. In addition, most species contained a relatively constant percentage of lipids (19.4 to 25.6 %), whereas extraordinary high lipid contents (>30 %) were found for Amphora exigua, Gyrosigma spenceri, Pleurosigma angulatum and Gyrosigma littorale. Glucose, galactose, mannose and ribose constituted the majority of the sugars detected, although the levels of these varied between species. Lipid class composition showed high concentrations of phospholipids and galactolipids as major constituents (19–22 % and 40–43 % of total lipids). The major fatty acids in most species were 14:0, 16:0, 16:1(n-7) and 20:5(n-3). Significant differences in biochemical gross compositions were found in the temperature (10, 30 °C) and salinity tests (20, 35 PSU), suggesting special intracellular acclimatisation processes that provide possible explanations for the adaptability of the species to environmental variations and the distinct differences in the diatom assemblages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms are the major group of photoautotrophic organisms inhabiting marine intertidal fine sediments (Admiraal 1984; Underwood 1994). Diatom-dominated biofilms are important in the ecology of mudflats as they exhibit high rates of primary production (Pinckney and Zingmark 1991), influence erosion and deposition of sediment (Underwood and Paterson 1993; Paterson 1994) and affect sediment water nutrient fluxes (Sundbäck and Granéli 1988; Rysgaard et al. 1995).
Intertidal mudflats constitute a highly unstable environment due to continuously changing water levels and currents as well as temporary exposure to air and higher solar irradiation and temperatures (Reineck 1983). Most marine benthic diatom species, isolated from intertidal environments, showed only slight differences in growth responses to temperature and salinity fluctuations in laboratory trials (e.g. Admiraal 1977; Scholz and Liebezeit 2012a). Salinity influences diatom physiology directly by exerting an osmotic stress. Upon this stress, algae exhibit a wide range of adaptations at the molecular, cellular and organism level (Hare and Cress 1997; Bohnert et al. 2001). Besides free amino acids (e.g. proline), several other organic osmolytes have been detected in marine and estuarine diatoms such as cyclohexanetetrol (Fujii et al. 1995; Garza-Sánchez et al. 2009), glycerol (Dickson and Kirst 1987) and mannose (Paul 1979). In addition to their function in the alleviation of osmotic stress, some organic osmolytes also have shown cryoprotective properties during freezing (e.g. Helliot and MortainBertrand 1999; Takagi et al. 2000). These include the protection of membranes and proteins by sugars (Uemura and Steponkus 2003) and the scavenging of reactive oxygen species by proline (Hare and Cress 1997). The main lipid classes of the thylakoid membrane of diatoms are galactolipids (Harwood 1998). For polar diatoms, it has been shown that both lipid classes contained high concentrations of certain polyunsaturated fatty acids (PUFAs, Mock and Kroon 2002) such as stearidonic (18:4 (n-3)) or eicosapentaenoic acid (20:5 (n-3)). In particular, 6,9,12,15-hexadecatetraenic acid, which is a typical constituent of diatom lipids, has so far only been found in cold-acclimated cells of other algal groups (Thompson et al. 1992) and is assumed to be responsible for increase of membrane fluidity at low temperatures (Jüttner 2001).
Species dominance in intertidal flats is probably related to small intraspecific differences in, e.g. salinity and temperature tolerance (Underwood 1994). Therefore, the main objective of the present investigation is to find first indications of acclimatisation to fluctuating salinities and temperatures on the level of biochemical gross compositions. Thus, laboratory trials were conducted to determine their protein, lipid, carbohydrate and ash contents under standardised growth conditions in semi-continuous cultures as well as in relation to different salinities and temperatures. Furthermore, lipid classes as well as fatty acid and carbohydrate compositions were also considered under standardised growth conditions. To our knowledge, most of these Solthörn tidal flat isolates have so far not been considered in studies describing the biochemical compositions.
Material and methods
Diatoms were isolated by dilution series of samples from microbial biofilms on sediments in the intertidal zone of the Solthörn tidal flat, located in the eastern part of the Inner Jade near the village of Tossens in Lower Saxony, Germany (53° 34′ 2.03″ N 8° 13′ 54.66″ E, Scholz and Liebezeit 2012b, c). Samples were taken at low tide from sediments characterised by mixed sands at biweekly intervals from June 9 to August 22, 2008. In a first step, subsamples of approximately 2–3 g were evenly distributed on glass Petri dishes, diluted with 10 mL sterile filtered sea water from the sampling area (spreading plating method, Hasle and Fryxell 1970) and stored until isolation at 18 ± 2 °C and an irradiance of 400 μmol photons m−2 s−1 (Phillips, Germany, Master TL-D 18 W/840). PAR irradiance (400–700 nm) was measured with an underwater quantum sensor LI-192 (Q 17079) connected to a Licor Data Logger LI-1000. In the second step, both epipsammic and epipelic species were collected with shortened glass pipettes under the microscope and distributed over agar plates, using f/2 medium (Guillard 1975). Artificial seawater salt (Tropic Marin®, GmbH Aquarientechnik, Germany) dissolved in de-ionised water was used with a salinity of 30 PSU and pH of 8.0. Diatom identification was conducted as described in Scholz and Liebezeit (2012b).
Determination of growth rates measurement of cell sizes
The counting of samples was performed microscopically, every second day, using a Neubauer Improved counting chamber with 0.1 mm depth (LO Laboroptik GmbH, Germany). Only cells which exhibited red fluorescence under UV light were counted. At least 1,000 cells were counted in each sample at × 400 magnification. The specific growth rate (μ) was calculated according to μ = ln c1 − ln c0/t 1 − t 0 where c 1 and c 0 denote cell counts at times t 1 and t 0, respectively, within the exponential phase. Data are presented in Scholz and Liebezeit (2012a).
In addition, the diatoms were embedded in Naphrax (refractive index 1.74, Northern Biological Supplies Ltd., UK), and dimensions were measured under a light microscope at × 1,000 magnification. Cell volumes were calculated according to the method described by Snoeijs et al. (2002).
Cultivation of monocultures prior to experiments
Non-axenic cultures of individual species were grown in 2 L Erlenmeyer flasks (approximately 600 mL culture volume) using f/2 medium as a base medium (pH 8.04, salinity = 30 PSU). To reflect Solthörn tidal flat conditions (Scholz and Liebezeit 2012c), the medium was modified: (1) we used two nitrogen sources, 500 μM NaNO3 and 150 μM NH4Cl; (2) the N/P ratio was changed to 17:1 and (3) NaHCO3 was added after autoclaving to a final concentration of 2 mMol/L−1. The irradiance was 600 μmol photons m−2 s−1 (Phillips, Germany, Master TL-D 18 W/840) at a light/dark regime of 16:8 h; cultures were maintained at 18 ± 2 °C. Glass beads (Sartorius, Ø 300–700 μm) were placed on the bottom of culture vessels with a layer of approximately 3 mm thickness and used as substratum. Salinity, pH and conductivity were measured using handheld probes (YK-31SA, YK-2001PH SI Model 33, Engineered Systems and Designs-Model 600, Philips W9424). Cultures were transferred to new medium every 7 days to maintain optimal growth. After biofilm development (21 days after inoculation), the culture broths were removed with a sterile sleeve until only a thin liquid film remained over the biofilms. There was only minimal loss of cells (0.5–0.8 %) during the removal of the culture broth. Subsequently, the experiments were started by addition of fresh culture medium.
Experimental setup
Besides the standardised conditions described above, two different test series (one-factor-at-one-time) were conducted in addition, with variations in salinities (20, 35 PSU) and temperatures (10, 30 °C). Different salinities were obtained by dissolving defined quantities of artificial sea salt in de-ionised water, whereas temperature experiments were carried out in an incubator (Certomat T plus, Sartorius, Germany). Once per day, 60 mL of culture suspension was replaced by fresh medium (semi-continuous culture) which gave a dilution rate of 0.6 d−1. The experiments were started with eight replicate flasks per treatment and species. The position of replicate flasks was randomly changed every other day to eliminate any location effects due to minor changes in external conditions.
Harvesting and sample treatment
Generally, biofilms were allowed to grow without physical disturbance on the glass beads, from which diatoms could be easily removed for sampling by shaking on a rotating shaking device (350 rpm), thus dislodging the biofilm from the substrate and homogenising the culture. Microscopic observation of the beads after shaking confirmed the complete release of the cells. Biomass was harvested by centrifugation at 680 rpm for 10 min. The eight replicates with the diatom biomass were subdivided each into nine aliquots and used for different extraction procedures as described below.
Analyses
A spectrophotometric method adapted from Parsons et al. (1984) using Lorenzen’s equations (Lorenzen 1967) was used to determine chlorophyll a (chl a) contents in the samples. Measurements were conducted following the method described by Brito et al. (2009). Tests were performed in triplicate. Chl a concentrations were calculated in μg × μm−3 × 10−10.
Total protein, lipid, carbohydrate and ash contents in the diatom species were determined at exponential phase (16–26 days after inoculation, for details see Scholz and Liebezeit 2012a), growing under standardised (e.g. 20 °C, 30 PSU) and different salinity (20, 35 PSU) and temperature (10, 30 °C) conditions. The compositions of lipid classes, fatty acids and carbohydrates were only determined for the standardised assays. All tests were performed in triplicate. To rupture diatom cells, all samples were homogenised with a mortar and pestle prior to assay.
Protein was determined spectrophotometrically using a Pierce test kit (BCA 23225) based on the procedure of Lowry et al. (1951) and standardised bovine serum albumin. In brief, filtered diatoms were prepared for analysis in four steps, with cooling intervals in an ice bath. Firstly, the filters were suspended in 4 mL 0.1 M NaOH in a 15-mL polypropylene test tube. Then the filter was ground in a tissue homogeniser for 1 min and sonicated for 20 s. Samples were diluted to 8 mL with 0.1 M NaOH, heated to 60 °C, sonicated for 10 s and centrifuged (4 °C, 800 × g, 10 min). A total of 10 μL of each supernatant was transferred to each triplicate well in a 96-well microplate; 200 μL of BCA reagent was added to each well and incubated for 30 min at 37 °C. Finally, the microplate was analysed at 526 nm with a microplate reader.
Total soluble carbohydrates were determined using the phenol sulphuric acid method of Kochert (1978) and Ben-Amotz et al. (1985) incorporating the modifications of Mercz (1994) and Buttery (2000). The samples were homogenised in 1 M H2SO4, and after heating at 100 °C for 60 min, 0.3 mL of the supernatant was transferred into a fresh vial and made up to 2 mL with deionized water. Sets of glucose standards were prepared and 1 mL of 5 % (w/v) phenol solution was added to all samples, after which 5 mL concentrated H2SO4 was added. Absorbance was read at 485 nm. For carbohydrate composition, the extraction followed the method of Karsten et al. (1991), using 70 % EtOH. Saccharides were identified and quantified by thin layer chromatography (TLC Silica Gel 60 F254; Merck) according to the method described by Zhang et al. (2009). As standards arabinose, galactose, glucose, fucose, mannose, rhamnose, ribose and xylose (Merck) were used.
Lipids
The total lipid determination was based on the method of Bligh and Dyer (1959) as modified by Kates and Volcani (1966) and adapted by Mercz (1994) and Buttery (2000) using methanol/chloroform/de-ionised water (2:1:0.8, v/v/v). The extracts were dried under a stream of ultrapure N2 gas and placed in a vacuum desiccator over silica gel overnight and then weighed. The lipids were resuspended in 100 μL 2:1 (v/v) chloroform/methanol, sealed, flushed with N2 and then stored at −80 °C until lipid classes analysis. Lipid class were quantified by thin layer chromatography–flame ionisation detection using an Iactroscan Analyzer with methods described by Parrish (1987) and modified by Kreeger et al. (1997). Free fatty acids (FA), diglycerides (DG), hydrocarbons, triglycerides (TG), sterols, galactolipids and phospholipids were quantified using standards of nonedecane, triolein, oleic acid, dipalmitin, cholesterol, digalactocyl diglyceride and phosphatidyl choline, respectively.
Fatty acid and PUFA compositions
Lipid fractions were transesterified by methanolysis (1 % H2SO4 in methanol) for 3 h at 70 °C (Blank et al. 2002). After cooling, the resulting fatty acid methyl esters (FAMEs) were extracted with n-heptane and dried over anhydrous sodium sulphate. The FAMEs were analysed with argentation chromatography according to the method of Wilson and Sargent (1992). TLC plates were sprayed with a solution of 5 % silver nitrate in acetonitrile and activated by heating at 110 °C for 30 min. Plates were developed in a TLC chamber saturated with toluene/acetonitrile (96:4, v/v). C14:0, C16:0, C16:1, C16:2ω4, C16:3, C16:4, C18:0, C18:4ω3, C20:5ω3, C24:0 and C22:6ω3 markers were used as standards. Samples and standards were visualised by spraying the plates with a solution of 3 % Cu(C2H2O2)2H2O and 8 % H3PO4, followed by heating at 180 °C for 20 min. Plates were scanned from the origin to the solvent front, using a Shimadzu CS-9 10 scanning densitometer, and the integrated areas were computed by a Shimadzu CRl A data processor (Shimadzu Scientific Instruments). Plates were scanned at 350 nm (tungsten source) in the reflectance mode. The beam dimensions were 4.0 × 0.2 mm and the scan speed was 24 mm/min−1. Each lane was scanned three times and the average values for the three scans were determined. The ratio of the density (pV-sec) of each marker to the density (pV-sec) of the internal standard was calculated for both the standard and sample lanes on each plate. The density ratios for the different standard amounts (1.0, 3.0, 5.0 and 10.0 pg) were used to obtain standard curves for each marker. The ratios of the density of each marker in the sample lane to the density of the internal standard in the same lane were used to determine the absolute amounts of each marker from their respective standard curves.
Total ash weights were determined by following the methods of Mercz (1994) and Buttery (2000). The glass fibre filters were placed within crucibles before combustion at 475 °C for 24 h. After combustion, the samples were weighed to five decimal places and total ash was calculated by subtracting the initial weight of the filter.
Statistical analyses
To assess variability of abundances and chemical compositions, analyses of variance (ANOVA) were performed on logarithmically transformed data to adhere to normality. In particular, abiotic parameters (temperatures, salinities) were used as source of the variance, while biochemical compositions of lipids, proteins, carbohydrates and ash contents were defined as dependent variables. All tests were performed with the programme XLSTAT 2011, Version 2011.2.08 Addinsoft.
Results
Cell volumes and chl a contents
The mean cell volume varied between 72 μm3 for Navicula perminuta and 15.6 × 103 μm3 for Pleurosigma angulatum (Table 1). Of the 25 isolates, 36 % had a cell volume <500 μm3 (classified as small diatoms) and 32 % had a cell volume >5,000 μm3 (classified as large diatoms). In addition, 28 % showed cell volumes >1,000 μm3 and were classified as medium-sized diatoms in the following. Only 4 % of the diatom species were found in the cutoff between the groupings <500 and >1,000 μm3 (Pinnularia ambigua [23]). The chl a contents varied as a function of cell volume, being the highest for P. angulatum [24].
Biochemical compositions under standardised conditions
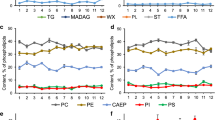
The gross compositions of each species (protein, lipid, carbohydrate and ash contents) expressed in % of dry weight (DW) showed partly significant differences between the individual species (Table 1). In detail, 24 % of the 25 species were rich in protein, ranging from 25.9 % (Navicula gregaria [17]) to 31.2 % (N. salinicola [20]) of DW and contained 7.5 to 9.6 % carbohydrate and 9.1 to 18.7 % ash. The majority of species (52 %) contained only <15 % protein but had nearly twice the total carbohydrate (20.3 [1] to 31.5 % [10]) and two to three times the ash (21.3 [5] to 32.5 % [9]) of the other species. In addition, 24 % of the isolated species showed protein contents between 15 and 25 %, with nearly twice the total carbohydrate (20.8 to 37.1 %) but significantly lower ash contents (8.7 to 18.6 %, ANOVA F 1,24 = 24.8, P < 0.0001) as compared to the majority. Similar to the dry weight, the comparison of the ash-free dry weight (AFDW) showed a high level of differences between the taxa analysed. Here, protein ranged from 40.7 to 61.8 %, whereas carbohydrates varied between 21.3 and 47.0 % (Table 1). Glucose, galactose, mannose and ribose constituted the majority of the sugars detected, although the levels of these varied between species (Fig. 1). From this glucose showed the highest proportions in each species, varying between 0.4 and 1.8 μg μm3 × 10−8. In much lower concentrations, the isolates contained arabinose, xylose, rhamnose, fucose and ribose in addition to the major neutral sugars detected in all cultures.
Most species had similar relative lipid content (19.4–25.6 % DW; 11.2–19.7 % AFDW). Extraordinary high lipid contents (>30 % DW) were found for Amphora exigua [6], Gyrosigma spenceri [13], P. angulatum [24] and Gyrosigma littorale [12]. Lipid class composition showed high contents of phospholipids and galactolipids as major constituents in the diatom species (19–22 % and 40–43 % of the total lipids, respectively, Fig. 2). Within the individual species, fatty acids varied between 6 and 12 % of the total lipids, being the highest for Achnanthes brevipes [1]. The major fatty acids in most species were 14:0, 16:0, 16:1(n-7) and 20:5(n-3) (Fig. 3). The polyunsaturated fatty acids 16:2(n-4), 16:3(n-4), 16:4(n-1), 18:4(n-3) and 22:6 (n-3) also comprised a significant proportion (>4 % each) of the total fatty acids in the isolates. Seven species contained significant proportions of arachidonic acid, 20:4(n-6) (3.9–6 %, [3, 7, 9, 13, 14, 18, 23]), but most species examined contained very low proportions of (n-6) polyunsaturated fatty acids.
Influence of salinity and temperature variations on the biochemical gross composition
The effect of variations in salinity and temperature on the 25 isolates is shown in Figs. 4 and 5. In comparison to values obtained from the standardised treatments (Table 1), relative differences in chl a concentrations of the 25 isolates varied only in a narrow range in the salinity and temperature variations (−0.5 to 1.2 %, Figs. 4a and 5a). Significantly higher levels of protein and lipids were found in most of the species tested in the high salinity experiment (35 PSU, up to 10.9 %, Fig. 5b, c; ANOVA F 1,48 = 29.1, P < 0.0001) and an increase in carbohydrates at both low and high salinities (Fig. 5d; ANOVA F 2,23 = 25.7, P < 0.0001). On the contrary, significantly lower levels of protein, lipids and carbohydrates were found in most of the tested species in the high temperature treatment (30 °C, Fig. 4b–d; ANOVA F 1,48 = 34.5, P < 0.0001). The ash content of all species increased with lower salinity, with the greatest increase in Amphora graeffii [7] (4.5 % dry weight, Fig. 5e, ANOVA F 1,24 = 24.8, P < 0.0001). In contrast, the ash content in the high salinity test series varied only in a narrow range (−0.8 to 2.0 % dry weight, Fig. 5e, ANOVA F 1,24 = 3.0, P = 0.076), similar to the high temperature test series (Fig. 4e). Moreover, similarities between lipid and carbohydrate levels in the low salinity and low temperature test series are noteworthy (Figs. 4c, d and 5c, d).
Differences in chlorophyll a contents (a) and biochemical gross compositions (protein (b), lipids (c), carbohydrates (d) and ash contents (e)) of 25 diatom species as percent of dry weight (n = 8). The differences were calculated for each species, based on the data in Table 1, for low (10 °C) and high temperatures (30 °C)
Differences in chlorophyll a contents (a) and biochemical gross compositions (protein (b), lipids (c), carbohydrates (d) and ash contents (e)) of 25 diatom species as percent of dry weight (n = 8). The differences were calculated for each species, based on the data in Table 1, for low (20 PSU) and high salinities (35 PSU)
Discussion
Biochemical compositions and fatty acid profiles under standardised conditions
Proteins, carbohydrates and lipids are the major constituents of algal cells, and their relative portions vary with algal species (Brown et al. 1997). According to Parsons et al. (1961), the biochemical composition of diatoms is similar to that of other microalgae. Rapidly growing cells during exponential growth phase contain 30 to 60 % protein, 10 to 50 % carbohydrate and 5 to 20 % lipid (ash-free dry weight) (Brown et al. 1997). In particular, planktonic diatoms contain 1.9–6.92 μg μm−3 × 10−8 protein, 3.5–9.02 μg μm−3 × 10−8 carbohydrate and 3.0–15.6 μg μm−3 × 10−8 lipid (e.g. Lynn et al. 2000). In general, the results in the present study are within this range (Table 1, Figs. 1 and 2), although three classes of combinations in protein, carbohydrates and ash contents could be distinguished under standardised growth conditions, whereas, with the exception of four species, lipid contents did not vary considerably. The major lipid class in the isolates was polar lipids (48–66 %), although in some species, TG (0.3–25 %) and free fatty acids (0.6–15 %) were also abundant in the exponential phase cultures. In addition, Fig. 2 shows that the percentage of TG in general is lower compared to glycolipids and phospholipids. TGs have been reported to increase from 5 % in unstressed cultures to 70 % of total cell volume in nitrogen-stressed cultures of the planktonic diatom Chaetoceros gracilis Schütt (= Chaetoceros neogracile S.L.VanLandingham) (Lombardi and Wangersky 1995). Although the occurrence and the extent to which TG is produced appear to be species/strain-specific and are ultimately controlled by the genetic design of individual organisms, oleaginous algae produce only small quantities of TG under optimal growth or favourable environmental conditions (Hu 2004). Synthesis and accumulation of large amounts of TG accompanied by considerable alterations in lipid and fatty acid composition occur in the cell when species are placed under stress conditions imposed by chemical or physical environmental stimuli, either acting individually or in combination. The major chemical stimuli are nutrient starvation, salinity and growth medium pH. The major physical stimuli are temperature and light intensity (Hu et al. 2008). In the present investigation, the initial nitrogen concentrations as well as the semi-continuous cultivation were sufficient to avoid nutrient-limiting circumstances and thus, nitrogen assimilation was not controlled during the course of the experiments. However, differences in the quality of the compounds accumulated during the cultivation processes, such as high TG concentrations or carbohydrates, support the assumption of nutrient limitation in some of the isolates.
The fatty acid profiles of the five diatom taxa were characteristic of most Bacillariophyceae: high proportions of 16:1(n-7) and 16:0 with variable, but usually high, proportions of 20:5(n-3) (Kates and Volcani 1966; Ackman et al. 1968; Dunstan et al. 1994; Renaud et al. 1994; Brown et al. 1997). In contrast, most species in the present study contained unusually high proportions of myristic acid (14:0, up to 13.9 %). For instance, values of 14:0 of up to 7.8 % of total fatty acids has been reported in Gomphonema parvulum (Kützing) Kützing (Jüttner 2001) and 8.8 % in the polar diatom Fragilariopsis cylindrus (Grunow in Cleve & Möller) W. Krieger in Helmcke & Krieger (Krell 2006) obtained by GC-MS analysis. In the present study, argentation TLC was applied, which can separate fatty acids based on the total number of ethylenic bonds. Although GC remains the most valuable and most efficient method of resolution and quantisation of lipids, including the non-polar higher molecular mass components, argentation TLC has been extensively utilised for analytical and semi-preparative resolution of methyl and glyceryl esters of saturated and unsaturated fatty acids (Arab and Akbar 2002). According to Özcimder and Hammer (1980), the recovery of highly unsaturated esters (n-5, n-6) was about 80 % and concluded that the decrease was a result of oxidation. Due to this observation, it is high likely that some of the unsaturated FA esters were underestimated and therefore the relative proportions were shifted for the benefit of the short-chain FA esters.
Finally, although glucose has been referred to be the major monosaccharide by most investigations including the present data, other monosaccharides (e.g. fucose) are dominant in some planktonic diatom cultures (Myklestad 1995). It may be concluded that the biochemical composition of the exponential phase cells of different species of marine benthic diatoms grown under identical conditions differs markedly. Similar observations were made by Myklestad (1974) for several planktonic species.
Effects of temperature and salinity on the biochemical gross composition
The specific growth rates of the 25 benthic marine diatoms in a preceding investigation did not vary considerably in a broad salinity (15–35 PSU) and temperature range (10–35 °C; Scholz and Liebezeit 2012a), whereas in the present study, significant differences in biochemical compositions were found in the temperature (10, 30 °C) and salinity tests (20, 35 PSU). In general, protein is the major cellular component in rapidly growing cells, while carbohydrate is the dominating substance among the extracellular products, especially polysaccharides (Myklestad 1995). Little is known about the ecological significance of the increased production of extracellular carbohydrates for the enhancement of the growth and survival of the producing species, especially under fluctuating physical conditions. The composition of extracellular polysaccharides has been observed to be different than that of the intracellular reserve (Allan et al. 1972; Myklestad et al. 1972) and more similar to that of the mucilaginous sheath surrounding the diatom (Allan et al. 1972). Myklestad (1974) observed marked distinctions between different planktonic diatom taxa in the accumulation of the storage product glucan and extracellular polysaccharide. The author recorded for stationary phase cultures (12 days) that the ratio of extracellular polysaccharide to cellular carbohydrate varied from 0.01 in Skeletonema costatum (Greville) Cleve to 1.25 in Chaetoceros curvisetus Cleve and the content of glucan was 81.0 and 24.8 % of the organic dry matter, respectively (Myklestad 1974). Although the present study did not differentiate between extracellular products and intracellular pools, an increase in relative carbohydrate contents at both low and high salinities was recorded in the present investigation, suggesting a direct relation in the acclimatisation processes. In contrast, lower levels of carbohydrates were found in most of the tested taxa in the high temperature treatment, which is similar to data presented by Renaud et al. (2002). The authors observed that carbohydrate contents were significantly higher between 25 and 30 °C decreasing at lower and higher temperatures. Here, carbohydrates seemed not to be involved in the extreme temperature acclimatisation process.
Several authors reported changes in cellular lipid characteristics in planktonic species from variations in salinity (e.g. Renaud and Parry 1994; Chelf 1990) and temperatures (James et al. 1989). In the present study, higher levels of relative protein and lipid contents were found in most benthic taxa in the high salinity assays. In contrast, Araújo and Garcia (2005) found for the planktonic diatom Chaetoceros wighamii Brightwell lowered protein content at a salinity of 35 PSU, while other constituents increased. In addition, Fabregas et al. (1985) observed a decrease in protein content with a salinity increase. Since lipids are a heterogeneous group of compounds of varying structures and functions, it is likely that the measurement of total lipids may not be sufficient to indicate different trends occurring among the individual lipid classes. For example, temperature has a major effect on the types of fatty acids produced by microalgae (Ackman et al. 1968; Sato and Murata 1980; Thompson et al. 1992). Many microalgal species respond to decreased growth temperatures by increasing the ratio of unsaturated to saturated fatty acids (Ackman et al. 1968; Mortensen et al. 1988; James et al. 1989; Thompson et al. 1992; Renaud et al. 1995). Thus, further detailed investigations are needed in which the amounts of individual fatty acids and the composition of carbohydrates in relation to different salinities and temperatures have to be analysed.
Applications in applied phycology
The biochemical composition of diatoms is of considerable interest and has been dealt with in applied phycology studies in areas such as aquaculture, the nutritional quality of foods fed to cultured animals (e.g. Wikfors et al. 1984) and biofuel research (Griffiths and Harrison 2009). Planktonic diatoms, in particular, are regarded as useful neutral lipid sources, both as liquid fuel precursors and as foods for marine culture of zooplankters (Ahlgren et al. 1990), larval and postlarval shrimp (Chu 1989), copepods (Bourdier and Amblard 1989) and juvenile oysters (Tsitsa-Tzardis et al. 1993). In contrast, benthic diatoms are very unlikely to have high potential for aquaculture or biodiesel production as they tend to settle in cultures. Furthermore, as the fatty acid profiles showed, the lipid contents of most benthic species in the present investigation were rather low, ranging from 19.4 to 25.6 % DW. On the other hand, the results of the acclimatisation potential gave evidence of the ability to manipulate diatom lipid quantity, which might be necessary for an effective use of the species as fuel or food resource (McGinnis et al. 1997). Finally, mutual changes among lipids, polysaccharides and proteins can help explain basic adaptations in algal physiological functions and are important for the food quality in aquatic food webs with subsequent trophic level effects (Volkman et al. 1989; Lynn et al. 2000).
Conclusions
The biochemical compositions of the 25 benthic diatoms, isolated from an intertidal environment, showed partial pronounced species-specific characteristics. Furthermore, all the taxa in the current study seemed to alter their relative protein, carbohydrate and lipid contents in response to variations in temperatures and salinities, but not all responses were equal. Generally, the intracellular concentrations of these compounds depend on environmental conditions and on the physiological potential of each diatom species. In summary, some benthic diatoms are able to react in a more efficient way, e.g. producing more thermo- and osmoprotectants, and are therefore able to grow over wider environmental ranges. These different acclimation potentials indicate niche separation in intertidal flats.
References
Ackman RG, Tocher CS, McLachlan J (1968) Marine phytoplankton fatty acids. J Fish Res Board of Can 25:1603–1620
Admiraal W (1977) Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar Biol 39:1–9
Admiraal W (1984) The ecology of estuarine sediment inhabiting diatoms. In: Round FE, Chapmann DJ (eds) Progress in phycological research. Biopress, Bristol, pp 269–322
Ahlgren G, Lundstedt L, Brett M, Forsberg C (1990) Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters. J Plankton Res 12:809–818
Allan GG, Lewin J, Johnson PG (1972) Marine polymers. IV. Diatom polysaccharides. Bot Mar 15:102–108
Arab L, Akbar J (2002) Biomarkers and the measurement of fatty acids. Public Health Nutr 5:865–871
Araújo SC, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii Brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246:405–412
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Blank C, Neumann MA, Makrides M, Gibson RA (2002) Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J Lipid Res 43:1537–1543
Bligh EG, Dyer WJ (1959) Arapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bohnert HJ, Ayoubi P, Borchert C, Bressan RA, Burnap RL, Cushman JC, Cushman MA, Deyholos M, Fischer R, Galbraith DW (2001) A genomics approach towards salt stress tolerance. Plant Physiol Biochem 39:295–311
Bourdier GG, Amblard CA (1989) Lipids in Acanthodiaptomus denticornis during starvation and fed on three different algae. J Plankton Res 11:1201–1212
Brito A, Newton A, Tett P, Fernandes TF (2009) Temporal and spatial variability of microphytobenthos in a shallow lagoon: Ria Formosa (Portugal). Estuar Coast Shelf Sci 83:67–76
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Buttery MJ (2000) Culture studies of two toxic dinoflagellate species, Alexandrium minutum and Gymnodinium catenatum. PhD Dissertation, Murdoch University, Perth, Western Australia
Chelf P (1990) Environmental control of lipid and biomass production in two diatom species. J Appl Phycol 2:121–129
Chu KH (1989) Chaetoceros gracilis as the exclusive feed for the larvae and postlarvae of the shrimp Metapenaeus ensis. Aquaculture 83:281–287
Dickson DMJ, Kirst GO (1987) Osmotic adjustment in marine eukaryotic algae: the role of inorganic ions, quaternary ammonium, tertiary sulfonium and carbohydrate solutes: 1. diatoms and a rhodophyte. New Phytol 106:645–655
Dunstan GA, Volkman JK, Barrett SM, Leroi JM, Jeffrey SW (1994) Essential polyunsaturated fatty acids from fourteen species of diatom (Bacillariophyceae). Phytochemistry 35:155–161
Fabregas J, Herrero C, Cabezas B, Abalde J (1985) Mass culture and biochemical variability of the marine microalgae Tetraselmis suecica Kylin (Butch) with high nutrient concentrations. Aquaculture 49:231–244
Fujii S, Nishimoto N, Notoya N, Hellebust JA (1995) Growth and osmoregulation of Chaetoceros muelleri in relation to salinity. Plant Cell Physiol 36:759–764
Garza-Sánchez F, Chapman DJ, Cooper JB (2009) Nitzschia ovalis (Bacillariophyceae) mono lake strain accumulates 1,4/2,5 cyclohexanetetrol in response to increased salinity. J Phycol 45:395–403
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RR (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 26–60
Hare PD, Cress WA (1997) Metabolic implications of stress-induced accumulation in plants. Plant Growth Reg 21:79–102
Harwood JL (1998) Membrane lipids in algae. In: Siegenthaler PA, Murata N (eds) Lipids in photosynthesis: structure, function and genetics. Kluwer Academic, Dordrecht, pp 53–64
Hasle GR, Fryxell GA (1970) Diatoms: cleaning and mounting for light and electron microscopy. Trans Am Microscop Soc 89:469–474
Helliot B, MortainBertrand A (1999) Accumulation of proline in Dunaliella salina (Chlorophyceae) in response to light transition and cold adaptation. Effect on cryopreservation. Cryobiol Lett 20:287–296
Hu Q (2004) Environmental effects on cell composition. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science Ltd, Oxford, pp 83–93
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacyglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
James CM, Al-Hinty S, Salman AE (1989) Growth and ω3 fatty acid and amino acid composition of microalgae under different temperature regimes. Aquaculture 77:337–357
Jüttner F (2001) Liberation of 5,8,11,14,17-eicosapentaenoic acid and other polyunsaturated fatty acids from lipids as a grazer defense reaction in epilithic diatom biofilms. J Phycol 37:744–755
Karsten U, Wiencke C, Kirst GO (1991) Growth pattern and β-dimethylsulphonio-propionate (DMSP) content of green macroalgae at different irradiance. Mar Biol 108:151–155
Kates M, Volcani BE (1966) Lipid composition of diatoms. Biochim Biophys Acta 116:264–278
Kochert G (1978) Carbohydrate determined by the phenol-sulfuric acid method. In: Hellebust JA, Craigie JJ (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 95–97
Kreeger DA, Goulden CE, Kilham SS, Lynn SG, Datta S, Interlandi SJ (1997) Seasonal changes in the biochemistry of lake seston. Freshw Biol 38:539–554
Krell A (2006) Salt stress tolerance in the psychrophilic diatom Fragilariopsis cylindrus. Dissertation, University of Bremen, Germany
Lombardi AT, Wangersky PJ (1995) Particulate lipid class composition of three marine phytoplankters Chaetoceros gracilis, Isochrysis galbana (Tahiti) and Dunaliella tertiolecta grown in batch culture. Hydrobiologia 306:1–6
Lorenzen CJ (1967) Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lynn SG, Kilham SS, Kreeger DA, Interlandi SJ (2000) Effect of nutrient availability on the biochemical and elemental stoichiometry in the freshwater diatom Stephanodiscus minutulus (Bacilliariophyceae). J Phycol 36:510–522
McGinnis KM, Dempster TA, Sommerfeld MR (1997) Characterization of the growth and lipid content of the diatom Chaetoceros muelleri. J Appl Phycol 9:19–24
Mercz TI (1994) A study of high lipid yielding microalgae with potential for large-scale production of lipids and polyunsaturated fatty acids. PhD Dissertation, Murdoch University, Perth, Western Australia
Mock T, Kroon BMA (2002) Photosynthetic energy conversion under extreme conditions. I. Important role of lipids as structural modulators and energy sink under N-limited growth in Antarctic sea ice diatoms. Phytochemistry 61:41–51
Mortensen SH, Brsheim KY, Rainuzzo JR, Knutsen G (1988) Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schütt. Effects of silicate deprivation, temperature and light intensity. J Exp Mar Biol Ecol 122:173–l85
Myklestad S (1974) Production of carbohydrates by marine planktonic diatoms. II. Comparison of nine different species in culture. J Exp Mar Biol Ecol 15:261–274
Myklestad SM (1995) Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Sci Tot Environ 165:155–164
Myklestad S, Haug A, Larson B (1972) Production of carbohydrates by the marine diatoms Chaetoceros affinis var. willei (Gran) Hustedt. II. Preliminary investigation of the extracellular polysaccharide. J Exp Mar Biol Ecol 9:137–144
Özcimder M, Hammer WE (1980) Fractionation of fish oil fatty acid methylesters by means of argentation and reversed-phase high-performance liquid chromatography, and its utility in total fatty acid analysis. J Chrom 187:307–317
Parrish CC (1987) Separation of aquatic lipid classes by chromarod thin-layer chromatography with measurement by Iatroscan flame ionization detection. Can J Fish Aquat Sci 44:722–731
Parsons TR, Stephens K, Strickland JDH (1961) On the chemical composition of eleven species of marine phytoplankters. J Fish Res Board Can 18:1001–1016
Parsons T, Maita Y, Lalli M (1984) A manual of chemical and biological methods for seawater analysis. Pergamon, New York
Paterson DM (1994) Microbiological mediation of sediment structure and behaviour. In Stal LJ, Caumette P (eds) Microbial Mats. NATO ASI Series, Springer-Verlag, Berlin, vol. G35, pp 97–109
Paul JS (1979) Osmoregulation in the marine diatom Cylindrotheca fusiformis. J Phycol 15:280–284
Pinckney J, Zingmark RG (1991) Effects of tidal stage and sun angles on intertidal benthic microalgal productivity. Mar Ecol Prog Ser 76:81–89
Reineck H-E (1983) Sediment and dynamical processes. In: Wolff WJ (ed) Ecology of the Wadden Sea. A A Balkema, Rotterdam, pp 6–49
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture II: effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Renaud SM, Parry DL, Thinh LV (1994) Microalgae for use in tropical aquaculture I: gross chemical and fatty acid composition of twelve species of microalgae from the Northern Territory Australian. J Appl Phycol 6:337–345
Renaud SM, Zhou HC, Parry DL, Thinh L-V, Woo KC (1995) Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. (clone T.ISO). J Appl Phycol 7:595–602
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rysgaard S, Christensen PB, Nielsen LP (1995) Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating in fauna. Mar Ecol Prog Ser 126:111–121
Sato N, Murata N (1980) Temperature shift-induced responses in lipids in the blue-green alga, Anabaena variabilis: the central role of diacylmonogalactosyl glycerol in thermo-adaptation. Biochem Biophys Acta 619:353–366
Scholz B, Liebezeit G (2012a) Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Res 27:65–73
Scholz B, Liebezeit G (2012b) Microphytobenthic communities in the Solthörn tidal flat (southern North Sea)—part I: seasonal and spatial variations of diatoms in relation to macronutrient supply. Eur J Phycol 47:105–119
Scholz B, Liebezeit G (2012c) Microphytobenthic communities in the Solthörn tidal flat (southern North Sea)—part II: seasonal and spatial variability of non-diatom components in relation to abiotic parameters. Eur J Phycol 47:120–137
Snoeijs P, Busse S, Potapova M (2002) The importance of diatom cell size in community analysis. J Phycol 38:265–272
Sundbäck K, Granéli E (1988) Influence of microphytobenthos on the nutrient flux between sediment and water: a laboratory study. Mar Ecol Prog Ser 43:63–69
Takagi H, Sakai K, Morida K, Nakamori S (2000) Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol Lett 184:103–108
Thompson PA, Guo M-X, Harrison PJ (1992) Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J Phycol 28:481–488
Tsitsa-Tzardis E, Patterson GW, Wikfors GH, Gladu PK, Harrison D (1993) Sterols of Chaetoceros and Skeletonema. Lipids 28:465–467
Uemura M, Steponkus PL (2003) Modification of the intracellular sugar content alters the incidence of freeze induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ 26:1083–1096
Underwood GJC (1994) Seasonal and spatial variation in epipelic diatom assemblages in the Severn Estuary. Diatom Res 9:451–472
Underwood GJC, Paterson DM (1993) Seasonal changes in diatom biomass, sediment stability and biogenic stabilization in the Severn estuary. J Mar Biol Assoc U.K. 73:871–887
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Biol Ecol 128:219–240
Wikfors GH, Twarog JW, Ukeles R (1984) Influence of chemical composition of algal food sources on growth of juvenile oysters, Crassostrea uirginica. Biol Bull 167:251–263
Wilson R, Sargent JR (1992) High-resolution separation of polyunsaturated fatty acids by argentation thin-layer chromatography. J Chromatogr 623:403–407
Zhang Z, Xiao Z, Linhardt RJ (2009) Thin layer chromatography for the separation and analysis of acidic carbohydrates. J Liq Chrom Relat Tech 32:1711–1732
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholz, B., Liebezeit, G. Biochemical characterisation and fatty acid profiles of 25 benthic marine diatoms isolated from the Solthörn tidal flat (southern North Sea). J Appl Phycol 25, 453–465 (2013). https://doi.org/10.1007/s10811-012-9879-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9879-0