Abstract
Composition of lipids, sterols, fatty acids (FA), and phospholipids in the edible Rhodophyta Grateloupia turuturu from Britanny, France, was investigated over four seasons in order to identify compounds with potential benefits in health and nutrition. The lipid content was found to vary from 3.3 to 4.1 % dry weight. No marked variations were observed for glycolipids accounting for 42.3–46.8 %, whereas neutral lipids and phospholipids fluctuated from 20.1 % (summer) to 41.8 % (winter), and 11.2 % (winter) to 33.4 % (summer), respectively. Polyunsaturated FA of the total lipids were found from 20.4 % (winter) to 31.1 % (summer), including 20:5 ω3 acid as the major one (up to 16.3 % in summer). Phosphatidylcholine (20.0–43.7 %) and phosphatidylserine (24.6–37.5 %) were the dominant phospholipids in all seasons. Compounds of interest were identified in minor amounts such as squalene, α-tocopherol, phytonadione (vitamin K1), cholesteryl formate, cholest-4-en-3-one, and cholesta-4,6-dien-3-one. Cholesterol was the major sterol with a lower content in spring and summer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine algae are mainly used as human food sources, and they are traditionally used in Chinese, Japanese, and Korean diet since ancient times. The species used as sea vegetables are mainly red and brown algae. The edible marine algae are known for their richness in polysaccharides, proteins, minerals, and vitamins and their low lipid content (1–3 % algal dry weight) (Dawczynski et al. 2007). Moreover, seaweeds are interesting because of their high concentrations of particular polyunsaturated fatty acids (PUFA) (Bocanegra et al. 2009) and of their high content in dietary fiber (33–75 %). They are used by pharmaceutical, cosmetics, and food industries as source of phycocolloids, thickening, and gelling agents (production of agar, alginate, and carrageenan) and for direct use in human nutrition. In the 1970s, by means of importation of oyster broods (Crassostrea gigas) from Japan, the red seaweed Grateloupia turuturu was introduced in Europe (Gavio and Fredericq 2002) and rapidly expanded on the French Atlantic coast. It now constitutes an important resource but one which has not been significantly exploited to date. In Japan, this seaweed is commonly used as a sea vegetable (Pang et al. 2006).
Thus, it would be of interest to find out value-added substances in the field of health and nutrition from this abundant algal biomass in order to compensate for cost of the eradication. G. turuturu is used in the fields of foods and cosmetics, and as source of fibers, proteins, and pigments (Denis et al. 2010). The biological activities of various extracts from G. turuturu have been investigated in some studies. Thus, the most efficient extract of G. turuturu as antimicrofouling agent was the dichloromethane fraction (Plouguerné et al. 2008). Antiviral activity was observed for the methanolic extracts of this alga (Hudson et al. 1999).
A previous paper from our laboratory dealt with the general chemical composition of G. turuturu from the coasts of Brittany (France) including a survey on lipids and fatty acids (FA) (Denis et al. 2010). The present study aims at extending knowledge on lipids of G. turuturu and to identify particular components giving some biological and economical value to the lipid content. The material used in the present investigation was collected during four seasons of 2009 in the same geographical areas than in our previous work.
Materials and methods
The specimens of macroalgae G. turuturu were collected manually during winter (January), spring (April), summer (July), and autumn (October) 2009 from the intertidal zone at Piriac-sur-Mer (17°22′ N 2°33′ E; Atlantic coast, France). The algae were sorted and then thoroughly cleaned to remove epiphytes. Samples were successively rinsed with seawater, tap water, and distilled water. The rinsed thalli were frozen immediately at −20 °C, and they were thawed at the time of lipid analyses. Measurements were made in triplicate for each season except for the unsaponifiable fraction analyses which were carried out in only one sample. Kruskal–Wallis test was used to test significant differences within the year (p < 0.05). Dunn’s multiple comparison test was used to identify where the specific significant differences occurred (p < 0.05). Data are reported as mean ± standard deviation.
Lipid extraction, lipid classes, fatty acid, and sterol
Total lipids were extracted from algae crushed (1 kg) with a mixture of dichloromethane/methanol (1:1, v/v). The lipid content was determined by the gravimetric method and as percentage of the algae dry weight (dw). One part of lipids (1 g) was fractionated into neutral lipids (dichloromethane), glycolipids (acetone), and phospholipids (PL) (methanol) on a silica gel column (30 cm × 2.5 cm, 40–63 μm). Fractions were evaporated to dryness, and the percentage was determined as percentage of 1 g of lipids. Another part of lipids (50 mg) was saponified with 2 M ethanolic potassium hydroxide. A part of the unsaponifiable matter was acetylated using acetic anhydride and pyridine, giving a mixture containing sterol acetates. The aqueous phase containing potassium salts of FA was acidified by 2 M HCl (pH = 4–5), and FA were obtained using dichloromethane. Fatty acid methyl esters (FAME) were prepared by methylation (1 h at 80 °C with 6 % methanolic hydrogen chloride). A part of these FAME was heated at 85 °C in a mixture of pyrrolidine and acetic acid for 1 h in order to obtain the N-acyl pyrrolidides (NAP). Total FA derivatives (FAME and NAP), sterols (as free forms and acetates), and neutral lipids were analyzed by gas chromatography coupled with mass spectrometry.
The samples were analyzed using a Hewlett Packard 6890 series GC system coupled with an MS HP 6890 series, equipped with silica capillary column SLBTM-5 ms (60 m × 0.25 mm × 0.25 μm); the carrier gas was helium (1 mL min−1). The analyses were carried out in electron impact (70 eV). The detector was set at 280 °C, and the injector at 250 °C. The samples were injected in splitless mode. Three different temperature gradients were used as followed for FAME analysis: temperature was held at 170 °C for 4 min and programmed to 300 °C at 3 °C min−1; for NAP analysis, 200 °C for 4 min then 3 °C min−1 up to 310 °C and held for 20 min; and for sterol analysis, 200 °C then 3 °C min−1 to 310 °C and held for 25 min. The solvent delay was 7 min for FAME and NAP analyses and 8 min for sterols.
Thin layer chromatography (TLC) was carried out for the whole of sterols and glycolipids in order to visualize the families of products. Thin layer chromatography was carried out on a 20 × 20 cm plate, consisting of an analytical polyester support and of silica gel (60 F254, 60 Å, 15 μm) of 0.25 mm thickness. For the sterols, visualization was by pulverization of sulphuric vanillin, followed by heating in an oven. Standards were cholesterol and cholesterol acetate. The mobile phase was hexane, diethyl ether, and acetic acid (85:15:0.1, v/v/v) in double elution. Glycolipids were visualized using orcinol. In this case, standards used were monogalactosyl diacylglycerols (from spinach leaves and identified by nuclear magnetic resonance), digalactosyl diacylglycerols, sulfoquinovosyl monoacylglycerols, and sulfoquinovosyl diacylglycerols purchased from Sigma-Aldrich (France). The mobile phase was dichloromethane/methanol (85:15, v/v) in double elution.
Phospholipid class composition
Standards of PL, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylglycerol (PG), phosphatidylinositol (PI), lysophosphatidylcholine (LPC) were purchased from Sigma-Aldrich Co. PL separations were performed on a modular UltiMate® 3000 RS HPLC System (Dionex, France) coupled to an evaporative light scattering detector Sedex 85 (Sedere S.A., France). Chromeleon® Chromatography Management Software (Dionex) was used for system control and data processing.
Chromatographic analysis of PL was carried out according to the method of Stolyhwo et al. (1987). The nebulizer gas pressure (dried and filtered air) was maintained at 3.5 bars, and the drift tube temperature was set at 50 °C. The analytical column (150 mm × 4.6 mm, I.D. 3 μm) was packed with a silica normal-phase Previal (Alltech Associates Inc., Belgium). A precolumn with the same packing and internal diameter was used.
Chromatographic separation was carried out using a binary gradient according to the following scheme: t0 min, 0 % B; t3 min, 20 % B; t12 min, 100 % B; and finally isocratic conditions (100 % B) for 3 min. Eluent A consisted of chloroform, and eluent B, of methanol/28 % ammonia in water/chloroform (92:7:1, v/v/v). The mobile phase was brought back to the initial conditions, and the column was allowed to equilibrate until the next injection. The total chromatographic run time was 20 min per sample. The flow rate was maintained at 1.5 mL min−1. Samples were dissolved in chloroform (2 mg min−1), and the injection volume was 10 μL per sample. The samples and the column were respectively thermostatized at 10 and 25 °C.
PL identification was carried out by comparison with the retention time of pure standards. In order to obtain a quantitative evaluation of the PL, calibration curves were determined from the area values obtained by injecting 10 μL of chloroform serial-diluted solutions of PE, PC, LPC, and PG (1–10 μg), and PI and PS (0.5–5 μg). Calibration curves were calculated by applying the equations of the power model to the area and concentration values. The sum of the PL concentrations was regarded as total PL concentration. PL species were expressed as percentage of the total PL.
Results and discussion
Lipid contents and lipid class distribution
The total lipid content of G. turuturu was 3.6 % of the dw (3.3–4.1 % dw) (Table 1) which is in agreement with literature concerning seaweeds, 1–6 % dw (Fleurence et al. 1994) and low compared to vegetables such as soy or sunflower.
Denis et al. (2010) found a lipid content of 2.6 % dw, this level is lower than that reported for our study; and Hotimchenko (2002) found 0.7 % gross mass for algae collected in the Sea of Japan. Moreover, 3.6 % is higher than that reported for tropical species of Grateloupia: G. indica 1.47 % dw and G. wattii 1.43 % dw (Kumari et al. 2010), and it is also higher than in other red seaweeds: Porphyra umbilicalis (3.4 % dw), Chondrus crispus (0.6 % dw), Palmaria palmata (1.6 % dw), and Gracilaria verrucosa (1.3 and 1.5 % dw) (Fleurence et al. 1994). Furthermore, it is also higher than in the brown alga Eisenia arborea (0.60 % dw), which is the second most abundant brown alga along the western coast of the Baja California Peninsula of Mexico and is a good candidate to be tested as supplement food for animals, including humans (Hernández-Carmona et al. 2009).
The lipid contents vary according to taxonomic entity, season, location, and growth conditions in both macroalgae and microalgae (Khotimchenko 2005). No significant variation of the total lipid content of G. turuturu was observed for the four seasons, contrary to some algae where the lipid level increases in winter and decreases in summer (Rodríguez-Montesinos and Hernández-Carmona 1991).
The major lipid class was glycolipids (45 %), and no significant variation during the four seasons was observed (Table 1). The glycolipid classes identified by TLC were monogalactosyldiacylglycerols, digalactosyldiacylglycerols, sulfoquinovosyl monoacylglycerols, and sulfoquinovosyl diacylglycerols.
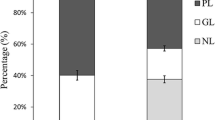
Neutral lipids and PL represented 33 and 22 % (of total lipids), respectively. There are fluctuations between neutral lipids (storage lipids) and PL (structural lipids). The highest neutral lipids content was observed in winter (42 % of total lipids) and the lowest in summer (20 %). In contrast, the highest PL content was recorded in summer (33 % of total lipids) and the lowest in winter (11 %). This could be due to the use of lipid reserves for the growth of the thalli during the summer.
Lipid composition of G. turuturu
Neutral lipid and sterol composition of G. turuturu
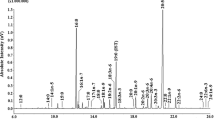
Hydrocarbon composition and compounds of interest found in unsaponifiable matter during the four seasons are given in Table 2. There are variations during the year. Hydrocarbons represented 6.8 % of unsaponifiable matter, among which squalene was the most abundant (3.1 %). It is known in the literature that dietary squalene is able to increase the efficiency of the immune system, enhance the antitumor action of chemotherapeutic agents, and lower blood cholesterol. Moreover, there is some evidence that squalene reduces colon cancer and skin cancer. This activity is likely related to its antioxidant effect (Das 2005). Thus, squalene may be very beneficial, and it has a future in preventive therapy and integrative medicine.
Moreover, α-tocopherol, which represents 1.8 % of unsaponifiable matter (Table 2), is an important natural antioxidant (Christie 2012). The phytonadione (vitamin K1) (0.5 %) is an essential component of the human diet. In animal tissues, the only known function of vitamin K is to act as a cofactor specific to the vitamin K-dependent enzyme γ-glutamyl carboxylase in the formation of γ-carboxyglutamic acid, which is involved in the activation of prothrombin and other proteins involved in blood clotting (Christie 2012). Furthermore, phytol, which represents 43.7 % of the unsaponifiable matter (Table 2), is usually used as a precursor for the industrial synthesis of vitamins E and K (Daines et al. 2003; Netscher 2007).
Total sterol composition (Table 2) varies during the year. Cholesterol (38 %) was the major sterol in all seasons with a lower content in spring and summer. Matsuhiro and Alejandro (1984) showed that Grateloupia doryophora had cholesterol as unique sterol, and Plouguerné et al. (2006) had also found cholesterol in G. turuturu. Cholesterol is one of the sterols present in the seaweeds, and its content in green and brown algae is 2–76 % of total sterol mixture and is lower than in red algae where it is the major sterol (Gancheva et al. 2003).
The cholest-4-en-3-one (1.2 %) (Table 2), found from G. turuturu for the first time, is known as a cholesterol derivative occurring in both plant and animal tissues. It may result from biosynthesis or be due to autoxidation of cholesterol. It is also a key intermediate in steroid chemistry. This compound was isolated and characterized as the first intermediate in a study on mycobacterial degradation of cholesterol (Naghibi et al. 2002). Cholest-4-en-3-one, known as an intestinal catabolite of cholesterol, has an anti-obesity effect on animals (Suzuki et al. 1998). The cholesta-4,6-dien-3-one (0.8 %) found from G. turuturu for the first time (Table 2) is a biologically important intermediate in the biosynthetic pathway of cholestanol in cerebrotendinous xanthomatosis, a rare genetic metabolic disorder of cholesterol and bile acid metabolism. Moreover, it has been used as an intermediate in the synthesis of various steroidal derivatives. A recent study described an interesting synthesis of this compound (Hosokawa et al. 2010). The best period to harvest this species for industrial upgrading seems to be winter and autumn.
Interestingly, cholest-5-en-3-ol formate (0.02 % of total neutral lipids) was identified in neutral lipids by TLC examination and revealed the presence of spots having retention factor value as 0.55, which was closed to those of cholesteryl acetate (0.61), as well as the mass spectrum which exhibited characteristic peaks at m/z 414 (cholesteryl formate molecular ion) and m/z 368 ([M-HCOOH]+). Cholest-5-en-3-ol formate found in neutral lipids (0.02 %) seems to be a chemotaxonomic marker of G. turuturu (Plouguerné et al. 2006; Yang et al. 2010).
Fatty acid composition of total lipids
The FA composition of total lipids given in Table 3 shows variations over the four seasons. The FA mixture was constituted by about 60.4 % saturated FA, 12.5 % monounsaturated FA, and 25 % of PUFA. A significant difference of saturated FA content was observed during winter and summer (p < 0.05). The highest content was in winter (68.1 % of total FA) and the lowest in summer (55.1 %). In contrast, a significant difference of content of unsaturated FA was observed during winter and summer (p < 0.05). The highest content was in summer (31.1 % of total FA) and the lowest in winter (20.4 % of total FA). The level of saturated FA is higher than that described for G. turuturu (33 % of the FA fraction in July–August) by Hotimchenko (2002) and is lower than that described for G. turuturu (67 % of the FA fraction) by Denis et al. (2010).
The most abundant FA was palmitic acid; nearly 45 % of the total FA (Table 3). This result is different from those reported for the same species (Hotimchenko 2002; Denis et al. 2010), and it is also higher than that reported for edible red seaweeds such as P. umbilicalis, P. palmata, G. verrucosa, and C. crispus (Fleurence et al. 1994). G. turuturu also contains PUFAs such as C18:3 ω3 (linolenic acid), C18:2 ω6 (linoleic acid), C20:4 ω6 (arachidonic acid), and C20:5 ω3 (eicosapentaenoic acid) (EPA), which have already been reported in other seaweeds (Dawczynski et al. 2007). Eicosapentaenoic acid is the main PUFA accounting for 13.5 % of the total FA. It is higher than in commercial fish oils and could be valuable in the fields of health and nutrition. This content is lower than that previously reported for G. turuturu, which was 22.6 % (during July and August) (Hotimchenko 2002) and for Porphyra (nearly 50 % of the total FA) (Noda 1993). Its content is similar to those reported for edible red seaweeds such as C. crispus or G. verrucosa (Fleurence et al. 1994). So, the percentages of FA of G. turuturu in our study showed a difference with those of Hotimchenko (2002) and Denis et al. (2010). Furthermore, EPA in G. turuturu was higher compared to that in the brown algae E. arborea (4.9 mg 100 g−1 dw) (Hernández-Carmona et al. 2009). An undetermined C16:3 acid was also found in our samples, and the 3-hydroxyheptadecanoic acid was identified in G. turuturu for the second time (Denis et al. 2010) up to 3.6 %. Such hydroxylated short-chain acids are well known as typical bacterial FA. Variations in the FA composition can be attributed to biogeographical and environmental conditions, and genetic status (Nelson et al. 2002). Al-Hasan et al. (1991) reported variations in macroalgae FA concentrations, but not in the composition pattern, when the temperature varied. An increased desaturation of FA also was detected in various species of brown macroalgae during the winter season (Pohl and Zurheide 1979). According to Hotimchenko (2002), the environmental conditions of the algae, differing by illumination, influences their lipid contents and ratios of FA.
Interestingly, marine algae are rich in PUFA of the ω3 and ω6 series, which are considered essential FA since they are not biosynthesized by mammals and must be taken via food chains. Seaweeds are not used as a conventional energy source because of the low level of lipids. However, seaweeds contain significantly higher levels of PUFA than land vegetables. The World Health Organization currently recommends that the ω6/ω3 ratio should not exceed 10 in the diet (Matanjun et al. 2009). So G. turuturu may be used for the reduction of ω6/ω3 ratio, as the ω3 PUFA (14.4 %) are higher than the ω6 PUFA (10.3 %), and the ω6/ω3 ratio was established at 0.7.
Phospholipid class composition of G. turuturu
The PL class composition, given in Table 4, shows variations over the four seasons. The major PLs were PC (32 %), PS (30 %), and PG (12 %). A high level of PC and a low level of PE are typical of red algae (Khotimchenko et al. 1990). It can be observed that PG, PE, and PI levels are roughly halved between winter and summer, contrary to PC whose level is doubled at the same seasons (% PC summer/% PC winter = 2.18 ± 0.01) (Table 4). During the year, PS content remained unchanged, whereas LPC content changed. Khotimchenko et al. (1990) demonstrated that the most characteristic features of the Rhodophyta are dominance of PC among PLs. The PL classes PG, PI, PC, and PE were found in Porphyra imbilicalis, while Polysiphonia harveyi contained PG, PI, and PE. The polar lipid composition of two red algae, C. crispus and Polysiphonia lanosa, showed that PC was the main PL in both species, and PG was also found in considerable amounts. PE was a minor component. This article did not mention PI. PC, PG, and PE have a role in photosynthetic machinery and biosynthetic pathways (Sanina et al. 2004), and PC is known to play an important and general role in cell signaling (Cyberlipid Center 2012).
This is the first time that PS was identified in G. turuturu and in any Rhodophyta. Phosphatidylserine, an important PL in the plasma membrane and the endoplasmic reticulum, is known to be able to counteract the pathological dysfunctions associated with age-related disorders in humans. Occurring mainly in the brain, PS is the precursor of PC, which is the primary source of choline for acetylcholine synthesis. Recently, it was shown that krill PS improves learning and memory in aged rats (Lee et al. 2010). In addition, PS is an essential cofactor that binds to and activates protein kinase C, a key enzyme in signal transduction, and it is involved in many other biological processes, including blood coagulation and apoptosis (Christie 2012).
LPC, found in small amounts in this study, has pro-inflammatory properties in vitro and is known to be a pathological component of oxidized lipoproteins in plasma and atherosclerotic lesions. Recently, it has been found to have some functions in cell signaling, and specific receptors (coupled to G proteins) have been identified. It also activates the mitogen-activated protein kinase in certain cell types (Christie 2012).
In conclusion, the lipid content of the red macroalgae G. turuturu (French Atlantic coast) was relatively low, like in all red seaweeds used in human nutrition, and no significant seasonal variations in lipid and glycolipid contents were observed. G. turuturu is characterized by the presence of PUFAs, including EPA at high levels, similar to other edible red seaweeds such as C. crispus or G. verrucosa, but higher than in commercial fish oils. Rare FAs were identified as minor components such as 3-hydroxyheptadecanoic (a typical bacterial FA). This alga contained interesting minor compounds such as α-tocopherol (vitamin E), phytonadione (vitamin K1), and squalene. Phytosterols were identified, namely brassicasterol, chondrillasterol, and fucosterol. The sterol composition showed also the presence of cholest-4-en-3-one and the rare cholesta-4,6-dien-3-one, which are biologically important. Of great interest is the first report of PS in G. turuturu at high levels in all seasons. Moreover, G. turuturu is characterized by its richness in glycolipids. It would be of interest to isolate and identify the most important ones in terms of biological activities. Work is in progress in the laboratory to determine FA composition in PL classes and to isolate the main glycolipids.
References
Al-Hasan RH, Hantash FM, Radwan SS (1991) Enriching marine macroalgae with eicosatetranoic (arachidonic) and eicosapentaenoic acids by chilling. Appl Microbiol Biotechnol 35:530–535
Bocanegra A, Bastida S, Benedí J, Ródenas S, Sánchez-Muniz FJ (2009) Characteristics and nutritional and cardiovascular-health properties of seaweeds. J Med Food 12:236–258
Christie WW (2012) The lipid library, http://lipidlibrary.aocs.org/. Accessed 20 February 2012
Cyberlipid Center, http://www.cyberlipid.org/. Accessed 20 February 2012
Daines AM, Payne RJ, Humphries ME, Abell AD (2003) The synthesis of naturally occurring vitamin K and vitamin K analogues. Curr Org Chem 7:1–15
Das B (2005) The science behind squalene-the human antioxidant, 2nd edn. Toronto Medical Publishing, Canada
Dawczynski C, Schubert R, Jahreis G (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103:891–899
Denis C, Morançais M, Li M, Deniaud E, Gaudin P, Wielgosz-Collin G, Barnathan G, Jaouen P, Fleurence J (2010) Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem 119:913–917
Fleurence J, Gutbier G, Mabeau S, Leray C (1994) Fatty acids from 11 marine macroalgae of the French Brittany coast. J Appl Phycol 6:527–532
Gancheva KZ, Dimitrova-Konaklieva SD, Ljubomirov KS, Simeonof PS (2003) A comparative study on the sterol composition of some brown algae from the Black Sea. J Serb Chem Soc 68:269–275
Gavio B, Fredericq S (2002) Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. Eur J Phycol 37:349–360
Hernández-Carmona G, Carrillo-Domínguez S, Arvizu-Higuera DL, Rodríguez-Montesinos YE, Murillo-Álvarez JI, Muñoz-Ochoa M, Castillo-Domínguez RM (2009) Monthly variation in the chemical composition of Eisenia arborea J.E. Areschoug. J Appl Phycol 21:607–616
Hosokawa YY, Hakamata H, Murakami T, Kusu F (2010) Electrosynthesis of cholesta-4,6-dien-3-one from cholesterol on a laboratory synthetic scale. Tetrahedron Lett 51:129–132
Hotimchenko SV (2002) Fatty acid composition of algae from habitats with varying amounts of illumination. Russ J Mar Biol 28:218–220
Hudson JB, Kim JH, Lee MK, DeWreede RE, Hong YK (1999) Antiviral compounds in extracts of Korean seaweeds: Evidence for multiple activities. J Appl Phycol 10:427–434
Khotimchenko SV, Klochkova NG, Vaskovsky VE (1990) Polar lipids of marine macrophytic algae as chemotaxonomic markers. Biochem Syst Ecol 18:93–101
Khotimchenko SV (2005) Lipids from the marine alga Gracilaria verrucosa. Chem Nat Compd 41:285–288
Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B (2010) Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem 120:749–757
Lee B, Sur BJ, Han JJ, Shim I, Her S, Lee HJ, Hahm DH (2010) Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog Neuropsychopharmacol Biol Psychiatry 34:1085–1093
Matanjun P, Mohamed S, Mustapha NM, Muhammad K (2009) Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 21:75–80
Matsuhiro B, Alejandro U (1984) Sterols of some Chilean algae. Biochem Syst Ecol 2:145–147
Naghibi F, Tabatabai Yazdi M, Sahebgharani M, Noori Daloii MR (2002) Microbial transformation of cholesterol by Mycobacterium smegatis. J Sci 13:103–106
Nelson MM, Phleger CF, Nichols PD (2002) Seasonal lipid composition in macroalgae of the northeastern Pacific Ocean. Bot Mar 45:58–65
Netscher T (2007) Synthesis of Vitamin E. Vitam Horm 76:155–202
Noda H (1993) Health benefits and nutritional properties of nori. J Appl Phycol 5:255–258
Pang SJ, Xiao T, Shan TF, Wang ZF, Gao SQ (2006) Evidences of the intertidal red alga Grateloupia turuturu in turning Vibrio parahaemolyticus into non-culturable state in the presence of light. Aquaculture 260:369–374
Plouguerné E, Kikuchi H, Oshima Y, Deslandes E, Stiger-Pouvreau V (2006) Isolation of cholest-5-en-3-ol formate from the red alga Grateloupia turuturu Yamada and its chemotaxonomic significance. Biochem Syst Ecol 34:714–717
Plouguerné E, Hellio C, Deslandes E, Veron E, Stiger-Pouvreau V (2008) Anti-microfouling activities in extracts of two invasive algae: Grateloupia turuturu and Sargassum muticum. Bot Mar 51:202–208
Pohl P, Zurheide F (1979) Fatty acids and lipids of marine algae and the control of their biosynthesis by environmental factors. In: Hoppe HA, Levring T, Tanaka Y (eds) Marine algae in pharmaceutical science. Walter de Gruyter, Berlin, pp 473–523
Rodríguez-Montesinos YE, Hernández-Carmona G (1991) Seasonal and geographic variations of Macrocystis pyrifera chemical composition at the western coast of Baja California. Cienc Mar 17:91–107
Sanina NM, Goncharova SN, Kostetsky EY (2004) Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry 65:721–730
Stolyhwo A, Martin M, Guiochon G (1987) Analysis of lipid classes by HPLC with the evaporative light scattering detector. J Liq Chromatogr 10:1237–1253
Suzuki K, Shimizu T, Nakata T (1998) The cholesterol metabolite cholest-4-en-3-one and its 3-oxo derivatives suppress body weight gain, body fat accumulation and serum lipid concentration in mice. Bioorg Med Chem Lett 18:2133–2138
Yang JL, Liu LL, Wang BG, Shi YP (2010) Secondary metabolites from Grateloupia turuturu and their chemotaxonomic significance. Biochem Syst Ecol 38:850–852
Acknowledgments
The authors thank Dr Claire Denis, Mr Pierre Gaudin, and Mrs Vony Rabesaotra from University of Nantes, Laboratory Mer-Molécules-Santé (MMS), EA 2160, for their technical assistance, in collecting alga, measurement, and in data analysis. This work is part of Melha Kendel’s Ph.D., supported by a grant from the Conseil Général de la Loire Atlantique, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kendel, M., Couzinet-Mossion, A., Viau, M. et al. Seasonal composition of lipids, fatty acids, and sterols in the edible red alga Grateloupia turuturu . J Appl Phycol 25, 425–432 (2013). https://doi.org/10.1007/s10811-012-9876-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9876-3