Abstract
The phenolic composition and antibacterial and antioxidant activities of the green alga Ulva rigida collected monthly for 12 months were investigated. Significant differences in antibacterial activity were observed during the year with the highest inhibitory effect in samples collected during spring and summer. The highest free radical scavenging activity and phenolic content were detected in U. rigida extracts collected in late winter (February) and early spring (March). The investigation of the biological properties of U. rigida fractions collected in spring (April) revealed strong antimicrobial and antioxidant activities. Ethyl acetate and n-hexane fractions exhibited substantial acetylcholinesterase inhibitory capacity with EC50 of 6.08 and 7.6 μg mL−1, respectively. The total lipid, protein, ash, and individual fatty acid contents of U. rigida were investigated. The four most abundant fatty acids were palmitic, oleic, linolenic, and eicosenoic acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decades, marine algae have received a lot of attention as functional food ingredients because of their richness in protein, vitamins, and minerals. One hundred grams of seaweeds provides more than the daily requirement of vitamins A, B2, B12, and C (Dhargalkar and Pereira 2005). In addition, seaweeds have traditionally been used in several countries in folk medicine for curing infectious diseases, gout, and eczema. Hence, much attention has been paid to the development of innovative projects for the pharmaceutical applications from seaweeds. Marine algal compounds exhibit various biological activities such as anticoagulant (Wijesinghe et al. 2011), antiviral (Artan et al. 2008), antioxidant (Cox et al. 2009), anticancer (Namvar et al. 2012), and antiinflammatory (Kazlowska et al 2010) activities.
Antioxidants are able to protect the human body from several diseases such as cancer, inflammation, diabetes, rheumatoid arthritis, and cardiovascular diseases generated by the reactions of the reactive oxygen species (ROS) with biological molecules. ROS are highly genotoxic/mutagenic and damage cellular macromolecules such as DNA, proteins, and lipids (Mantena et al. 2008). The use of synthetic antioxidants to prevent free radical damage involves toxic side effects (Botterweck et al. 2000). The stability of seaweed to oxidation during storage and the absence of the oxidative damage in the structural components suggest the presence of highly developed protective antioxidant defense systems (Zubia et al. 2007). In addition, the emergence of multiple drug-resistant bacterial infections is an escalating problem worldwide with resistance of pathogenic bacteria to widely used antimicrobial agents (Heuer et al. 2009). Foodborne diseases and food spoilage have been also recognized as two of the most important concerns for the food industry. Therefore, the need to develop additional natural antimicrobial agents is considered a public health priority in both pharmaceutical and food industries. Accordingly, algae extracts and their derived bioactive secondary metabolites like various polyunsaturated and monounsaturated fatty acids, phenolic components, terpenoids, and polysaccharides offer the opportunity in this regard (Suganthy et al. 2009). A strategic extraction of secondary metabolites would increase the potential for new discoveries targeting high value-added products.
Ulva rigida C. Agardh (Ulvaceae) is a substantial part of the total biomass of the benthos along the Tunisian coast. This alga is used in aquaculture since it contains protein, pigments, vitamins, minerals, and unknown growth factors (Nakagawa et al. 1987). For this reason, it is of great interest to investigate the U. rigida secondary metabolites and biological activities in order to promote new industrial applications. However, the production of algae secondary metabolites is often controlled by physical and biological factors such as climate, reproductive state, locality, and seasonality. Sampling throughout the year is essential to characterize biological activity due to seasonal variation. To our knowledge, insufficient attention has been dedicated to evaluating the possible temporal variation in antibacterial and antioxidant activities of U. rigida. The main objectives of the present work are (1) evaluation of the seasonal variation of the antibacterial and antioxidant activities of the hydroalcoholic extracts of U. rigida collected monthly for 12 months from the Tunisian coast; (2) assessment of antioxidant, antimicrobial, and antiacetylcholinesterase activities of the different U. rigida fractions; and (3) investigation of the chemical composition of this alga.
Materials and methods
The alga U. rigida was collected monthly for 12 months, between winter (September) 2008 and summer (August) 2009, from Sidi Mansour Sfax (Tunisia, 35°14′58.36″ N, +11°7′17.75″ E). A voucher specimen (LBPes URA01) was deposited in the Laboratory of Biopesticides of the Centre of Biotechnology of Sfax. Extracts of air-dried U. rigida were prepared by two methods. Firstly, 300 g of air-dried U. rigida thallus collected monthly (12 samples) was extracted with 80 % ethanol–water for 48 h at room temperature. The ethanol was evaporated at 40 °C under vacuum with a rotary evaporator and the remaining aqueous phase was lyophilized to produce a hydroalcoholic extract (EtOH–H2O extract) (40 g). These extracts were used to study the seasonal variation of the antibacterial and antioxidant activities. Secondly, 200 g of air-dried thallus of U. rigida collected in spring (April) was extracted (for 24 h) in a Soxhlet apparatus with 1.5 L methanol at 70 °C. The mixture was filtered and then concentrated to produce methanol extract (total extract 26.6 g). Twenty-five grams of the dried methanolic extract was suspended in 100 mL distilled water and was sequentially partitioned with n-hexane (3 × 250 mL), chloroform (3 × 250 mL), ethyl acetate (3 × 250 mL), and n-butanol (3 × 250 mL) to obtain a n-hexane fraction (HexF), chloroform fraction (CHCl3F), ethyl acetate fraction (EtOAcF), butanolic fraction (ButOHF), and water fraction (WF), respectively. Samples were filtered through a filter paper in a Buchner funnel, evaporated at reduced pressure at 40 °C, and then dissolved in the appropriate solvent. The remaining aqueous layer was lyophilized to give a water fraction. The stock solutions were kept at 4 °C in the dark until further analysis.
Antimicrobial, antioxidant, and antiacetylcholinesterase activities
Seasonal variation of the antibacterial activity of the U. rigida hydroalcoholic extracts, prepared monthly for 12 months, was evaluated against two Gram-positive bacteria, Bacillus subtilis ATCC 6633 and Staphylococcus aureus ATCC 25923, and two Gram-negative bacteria, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. The antibacterial activity of spring U. rigida crude extract and fractions was investigated against 11 bacterial strains and six fungal strains obtained from international culture collections (ATCC) and the local culture collection of the Centre of Biotechnology of Sfax, Tunisia. They included seven Gram-positive bacteria, B. subtilis ATCC 6633, Bacillus cereus ATCC 14579, S. aureus ATCC 25923, S. aureus ATCC 6536, S. epidermis ATCC 12228, Enterococcus faecalis ATCC 29212, and Listeria monocytogenes (food isolate 2132), and four Gram-negative bacteria, E. coli ATCC 25922, E. coli ATCC 8739, P. aeruginosa ATCC 27853, and Klebsiella pneumoniae CIP 32147. The following fungal strains were also tested: Aspergillus niger CTM 10099, Fusarium graminearum (ISPAVE 271), Fusarium oxysporum (CTM 10402), Fusarium culmorum (ISPAVE 21 W), Alternaria alternata (CTM 10230), and Candida albicans ATCC 10231. The bacterial strains were cultivated in Muller–Hinton agar (MH) (Oxoid Ltd, UK) at 37 °C except for Bacillus species which were incubated at 30 °C. The fungi were cultured on potato dextrose agar medium and incubated at 28 °C. Working cultures were prepared by inoculating a loopful of each tested bacteria in 3 mL MH broth and were incubated at 37 °C for 12 h. For the test, the turbidity of the overnight broth was adjusted to 0.5 McFarland standards (1–1.5 108 CFU mL−1) by the Densimat spectrophotometer (BioMérieux, Italy) and then diluted in Mueller–Hinton broth to a final inoculum concentration of 107 CFU mL−1. Fungal spore suspensions were collected from the surface of each fungal colony by gently scraping with a loop and suspended in 10 mL potato dextrose broth. Each suspension was mixed vigorously by vortexing for 15–20 min. The spore suspension stock was diluted to obtain a concentration of 106 spores mL−1 (measured by Thoma blade).
Antibacterial and antifungal tests were performed by the agar well diffusion method as described by Andrews (2005) using sterile Mueller–Hinton media (Bio-Rad, France) for bacterial strains and potato dextrose agar (Bio-Rad, France) for antifungal tests. One hundred μL of freshly prepared cell suspension adjusted to 107 CFU mL−1 for bacteria and 106 spores mL−1 for fungi were inoculated onto the surface of agar plates. Thereafter, wells with 6 mm in diameter were punched in the inoculated agar medium with sterile Pasteur pipettes and 50 μL of extracts (50 mg mL−1) was added to each well. Sterile DMSO (20 %) and ethanol (50 %) were negative controls of the solvent used to resuspend the extract also tested in the bioassays. Gentamicin (10 μg/well) was used as positive control for bacteria while amphotericin B (20 μg/well) was used as positive control for fungal strains. The plate was allowed to stand for 2 h at 4 °C to permit the diffusion of the extracts followed by incubation at 37 °C for 24 h for bacterial strains and 72 h for fungi at 28 °C. The antimicrobial activity was evaluated by measuring the zones of inhibition (clear zone around the well) against the tested microorganisms. All tests were repeated three times.
The antioxidant activity of spring (April) U. rigida extracts and fractions was assayed by radical scavenging activity, β-carotene bleaching test, and DNA nicking assay. Radical scavenging activity was determined by the spectrophotometric method based on the reduction of a methanol DPPH solution (Blois 1958). Briefly, 0.5 mL of the extract, in the concentration range of 0.1–2 mg mL−1, diluted in methanol was added to 1 mL DPPH methanol solution (0.1 mM). The mixture was shaken vigorously and left to stand at room temperature for 30 min in the dark. Then, the absorbance was measured at 517 nm against a blank by a spectrophotometer (Bio-Rad SmartSpec™ plus). DPPH radical scavenging activity (%) was calculated according to the formula: DPPH radical scavenging activity (%) = [(ODblank − ODsample) / ODblank] × 100, where ODblank is the absorbance of the control reaction containing all reagents except the tested compound. ODsample is the absorbance of the test compound. Extract concentration providing 50 % inhibition (EC50) was calculated from the graph plotting inhibition percentage against extract concentration. Ascorbic acid was used as positive control. Tests were carried out in triplicate.
The antioxidant activity of the spring (April) U. rigida extracts was evaluated by the spectrophotometric β-carotene bleaching test (Juntachote and Berghofer 2005). A stock solution of β-carotene–linoleic acid mixture was prepared as follows: 0.5 mg β-carotene was dissolved in 1 mL chloroform (HPLC grade) for which 25 μL linoleic acid and 200 mg Tween 20 were added. Chloroform was completely evaporated using a vacuum evaporator. Then, 100 mL distilled water with oxygen (30 min at a flow rate of 100 mL min−1) was added with vigorous shaking. Aliquots of 4 mL of this mixture were mixed with 200 μL of the extracts in the concentration range of 0.1–4 mg mL−1. The emulsion system was incubated for 3 h at room temperature. The same procedure was repeated with the synthetic antioxidant, butylated hydroxytoluene (BHT), as positive control in the concentration range of 5–150 μg mL−1 and a blank containing 200 μL of ethanol. After this incubation period, absorbance of the mixtures was measured at 470 nm. Antioxidative capacity of the extracts was compared with those of BHT and a blank. Antioxidant activity is expressed as percent inhibition relative to control (0.2 mL ethanol) using the equation:
where OD0 and OD′0 are the absorbance values measured at zero time for the test sample and control, respectively, while ODt and OD′t are the corresponding absorbance values measured after incubation for 3 h.
A DNA nicking assay was performed as described by Lee et al. (2002) with minor changes. DNA damage protective activity of U. rigida fractions was checked on pBluescript II vector. Plasmid DNA was isolated by SpinKlean™ Plasmid MiniPrep Kit (BIOMATIC). The experiments were performed in a volume of 20 μL in a microcentrifuge tube containing 3 μL of intact plasmid DNA (50 ng), 5 μL of each extract (250 μg), and 7 μL 1× TE buffer (10 mM Tris–HCl and 1 mM EDTA pH 7.2). The reaction mixture was incubated firstly for 10 min before adding 5 μL of Fenton’s reagent (30 mM H2O2, 50 μM ascorbic acid, and 80 μL FeCl3), followed by incubation for 45 min at 30 °C. Untreated plasmid DNA, treated plasmid DNA with Fenton’s reagent only, and treated plasmid DNA with Fenton’s reagent and quercitin at 100 mM were used as controls. After incubation, 10 μL of each reaction mixture was mixed with 3 μL of gel loading dye (0.15 % bromophenol blue and 40 % sucrose) and immediately loaded into an agarose gel (1 %) and electrophoresed in a horizontal slab gel apparatus in Tris/acetate/EDTA gel buffer for 1 h (50 V). The gel was photographed on a UV transilluminator 312 nm (Vilber Lourmat, France).
The acetylcholinesterase (AChE) inhibitory activity was determined according to the method described by Mata et al. (2007) using electric eel acetylcholinesterase (EC 3.1.1.7, type VS), acetylthiocholine iodide (AChI), and 5′-dithio-bis-2-nitrobenzoic acid (DTNB). The galanthamine·HBr was used as positive control. The reaction containing 300 μL of Tris–HCl buffer (pH 8), 100 μL of organic fractions of U. rigida collected in spring (April) at different concentrations (1–250 μg mL−1), and 50 μL of the enzyme solution (0.28 UI mL−1) was incubated for 15 min at 37 °C. Subsequently, 75 μL of a solution of AChI (23 μg mL−1) and 475 μL of 3 mM DTNB were added, and the final mixture was incubated for 30 min at 37 °C. Absorbance of the mixture was measured at 405 nm. A control mixture was prepared using 100 μL of a solution, similar to the sample mixture but with the solvent instead of the extract. Percent of inhibition was calculated as: I% = 100 − [A sample / A control] × 100, where A sample is the absorbance of the extracts and essential oil containing reaction and A control is the absorbance of the reaction control. Tests were carried out in triplicate.
Analytical methods
Total phenolic content was determined using the Folin–Ciocalteu method (Waterman and Mole 1994) adapted to a microscale. Phloroglucinol was used as a standard for the calibration curve. The phenolic content was expressed as mg phloroglucinol equivalent/gram of dry extract (mg PHG g−1) using the linear equation based on the calibration curve.
The following three parameters are measured: moisture, protein, and fat content. The moisture was prepared from the dried sample of spring U. rigida (April). The samples were ground into a fine powder with a mechanic grinder (Moulinex, France) and then ashed by heating at 525 °C for 5 h (AOAC 2000). Protein content was calculated from nitrogen content as follows: N (%) × 6.25 using the Kjeldahl method (AOAC 2000). The fat matter was measured from 5 g of spring U. rigida (April) using a Soxhlet extraction apparatus with 150 mL petroleum ether as a solvent at the boiling point for 8 h. Fatty acid composition was determined by the analytical methods described in the European Parliament and of the European Council in EEC regulation 2568/91 (1991). Fatty acids were converted to fatty acid methyl esters (FAMEs) by vigorous shaking of 0.1 g total fat in 3 mL n-hexane with 0.4 mL methanolic potassium hydroxide (2 N). The FAMEs were then analyzed in an Agilent 6890 N Network Gas Chromatograph system (Agilent Technologies, France) equipped with an HP-5MS Phenyl Methyl Siloxane capillary column (30 m × 250 μm × 0.25 μm) and a 5975 B Insert MSD mass selective detector. Helium (constant flow, 1 mL min−1) was used as a carrier gas. The temperatures of the injector and detector were 250 and 240 °C, respectively. The following temperature program was applied: 120 °C for 5 min, increase of 3 °C min−1 to 180 °C, increase of 10 °C min–1 to 220 °C, and 220 °C for 31 min. The identification of compounds was achieved by comparing the retention times with those of authentic compounds and the spectral data obtained from the Wiley and NIST libraries.
Statistical analysis
Experimental results concerning this study were expressed as means ± standard deviation of three parallel measurements. The significance of difference was calculated by Student’s t test, and values p < 0.05 were considered to be significant. Correlation and regression analysis was carried out using Microsoft Excel.
Results and discussion
Seasonal variation of the antibacterial and antioxidant activities
Antibacterial activity against the two tested Gram-positive bacteria was observed throughout the year with differences of the inhibition zones from 1 month to another (Fig. 1a). However, this activity varied monthly against E. coli and P. aeruginosa, with a full attenuation in some periods. The highest inhibitory activity was detected with the material collected during spring and summer, with a decline in activity when the collection was made during autumn and winter (Fig. 1a). Results obtained from the agar well diffusion method indicated that B. subtilis and S. aureus were the most sensitive microorganisms showing the largest inhibition zones in April (spring). Analysis of the variance revealed that both the seasonal and bacterial strain variations exhibited a significant (p < 0.05) effect on the antibacterial activity of U. rigida. Ulva fasciata, collected from the east coast of South Africa, exhibited a strong antibacterial activity in autumn (May), low activity in late winter (July) and spring (September–November), and no activity in summer (Stirk et al. 2007). The discordance in the results could be attributed to the geographic location. There are seasonal and geographical variations in the antimicrobial activity of seaweeds collected from the Mangalore coast of India with the highest activity using algae collected during the post-monsoon season (from October to November) (Vidyavathi and Sridhar 1991).
The changes in antibacterial activity of the investigated U. rigida hydroalcoholic extracts, with regard to seasonal variations, might be attributed to a possible variation in the quantities and/or qualities of active secondary products. Numerous studies have noticed a wide variation in the production of chemical defense molecules of terrestrial plants associated with physical (temperature and light) and biological factors, like life cycle and herbivore pressure. Season and water pollution influence the sterol composition of U. rigida and Enteromorpha linza (Popov et al. 1985).
Antioxidant activity measured using the DPPH test revealed significant differences (p < 0.05) during the year (Fig. 1). The highest activity was detected in late winter (February) and early spring (March) compared to those of the late summer (August) and the early autumn (September–October). In spring and summer (April–July), there was a decline in the antioxidant activity (Fig. 1b). The scavenging of these ROS is part of U. rigida acclimatization. The antioxidant activity was significantly greater in samples collected during cold months with water temperatures less than 15 °C. In opposition, temperate months, with temperatures greater than 20 °C such as in summer (June–August) and in autumn (September–October), showed a decline in the antioxidant activity. These results indicate that antioxidant activity increases with the decrease of temperature. Acclimatization of algae to low temperatures generally involves several fold increases in the antioxidant enzyme activities (superoxide dismutase, glutathione reductase) and photosynthetic capacity (Dudgeon et al. 1995).
Seasonal variation of the phenolic compounds
The biological activities of U. rigida could be due to the presence of diverse phytochemicals including phenolics, and the Folin–Ciocalteu method was used for the quantification of the total phenolic content. The late winter and early spring extracts (February and March) showed higher phenolic content than those collected in the other months (Table 1). The variation in the activity observed for samples collected monthly for 12 months resembled to the variation observed in total phenolic content. This result might be due to the phenolic compounds that could act as antiradical or antioxidant molecules able to protect cells from biochemical damage caused by ROS. This finding is also in agreement with numerous literature data that reported the effect of phenolic compounds in the protection of the algal thallus against photodestruction by UV radiation (Connan et al. 2006). Analogous up-regulation in antioxidant capacity was observed in algae and many terrestrial plant species (Siatka and Kašparová 2010).
Since the hydroalcoholic extracts of spring U. rigida (April) showed a strong antibacterial activity, we have investigated in more detail the antimicrobial activity of the methanolic extract and derived fractions (HexF, CHCl3F, EtOAcF, ButOHF, WF) against a panel of microorganisms. The antioxidant and antiacetylcholinesterase activities were also investigated.
Total phenolic contents and biological activities of spring U. rigida fractions
The phenolic compounds are commonly found in terrestrial and marine plants and have been reported to have several biological activities including antioxidant and antimicrobial properties. The total phenolic contents of spring U. rigida fractions (April) were determined and are presented in Table 1. The highest content of total phenolic was found in ethyl acetate fraction followed by chloroform fraction, whereas the contents obtained with butanol and water extracts were much smaller. This result showed that the total phenolic content depends on the type and polarity of the extracting solvents.
The in vitro activity of spring U. rigida (April) was qualitatively assessed by the determination of the inhibition zone diameters. Total extract showed an antibacterial activity against 90 % of the tested bacteria and failed to show a prominent antifungal activity (Table 2). The chloroform fraction showed the widest range of antibacterial activity against both Gram-positive and Gram-negative bacteria. Ten out of the 11 tested bacterial strains were inhibited by chloroform fraction followed by the ethyl acetate and n-hexane fractions. Moderate effects were observed using butanol and water fractions against all tested bacteria. Although Perez et al. (1990) found that the extract of Ulva lactuca had no antimicrobial activity, our results showed that the organic extract of U. rigida inhibited most of the tested microorganisms. This discordance could be due to not only to subspecies differences, but also to the production of bioactive compounds related to the seasons and organic solvents used in the extraction. Moreover, results of antibacterial activity indicated that Gram-positive bacteria are more sensitive to the extracts than Gram-negative bacteria. The outer membrane of the Gram-negative bacteria acts as a barrier to many environmental substances including antibiotics (Tuney et al. 2006). Although diffusion agar is a common method of testing the bacterial sensitivity to plant extracts and antibiotics, the use of this method to determine the susceptibility of fastidious or slow-growing organisms present some drawbacks.
The scavenging activity of the spring U. rigida extracts was dose dependent. Ascorbic acid, used as positive control, exhibited 100 % inhibition of the DPPH radical at 0.01 mg mL−1. The antioxidant efficiency can be also evaluated by the determination of the EC50 value corresponding to the amount of the extract or fraction required to scavenge 50 % of DPPH radicals present in the reaction mixture. High EC50 values indicated low antioxidant activity. The most potent radical scavenger fraction was the n-hexane fraction followed by those of chloroform and ethyl acetate fractions (Table 1). Butanol and water fractions showed higher EC50 values (> 2 mg mL−1) and consequently a lower antioxidant capacity. Therefore, compounds with the strongest radical scavenging activity in U. rigida are of low to medium polarity. Furthermore, the highest content of total phenolic was found in ethyl acetate and chloroform fractions. These results were in favor of the implication of phenolic compounds in the antioxidant activity of U. rigida extracts by hydrogen transferring reaction.
In order to provide additional data on the antioxidant potential of spring U. rigida fractions, β-carotene bleaching assay was carried out. This test simulates the polyunsaturated fatty acid oxidation of the membrane lipid components. The linoleic acid free radical attacks the highly unsaturated β-carotene models. The ability of spring U. rigida extracts and the positive control, BHT, to prevent lipid peroxidation is presented in Table 1. The order of the antioxidant activity comparing the amount of extract required to the inhibition of 50 % linoleic acid peroxidation present in the mixture (EC50) was found to be n-hexane > ethyl acetate > chloroform > water (Table 1). Butanol fraction showed a weak antioxidant activity compared to the BHT used as positive control. There was a significant positive association (p < 0.05) between the DPPH radical scavenging ability and β-carotene bleaching capacity. Furthermore, a positive correlation (R 2 = 0.98) between the antioxidant activity and the phenolic contents was also observed. From these results, it is clear that U. rigida exhibited a capacity to reduce lipid oxidation and may have a therapeutic effect. Algae components present benefits in controlling inhibition of lipid oxidation in the rat and liver and inhibition of apoptosis and other protective mechanisms in cancer patients (Shon et al. 2003).
In order to study the protective effects of spring U. rigida fractions on hydroxyl radical mediated DNA strand breaks, a free pBluescript II vector DNA breaks system in vitro was used. The supercoiled plasmid DNA was broken by hydroxyl radical generated from the Fenton reaction into three forms (supercoiled, open circular, and linear form). The effects of U. rigida ethyl acetate and chloroform fractions on pBluescript II DNA cleavage were demonstrated by the gel electrophoretogram (Fig. 2). The plasmid DNA was mainly of the supercoiled form in the absence of Fenton’s reagent and converted into the relaxed circular and linear form after Fenton’s reaction. The effects of U. rigida fractions and quercitin, known as free radical scavenger metal chelators, on the free radical-induced DNA damage were investigated in lanes 3, 4, 5, and 6 (Fig. 2). Quercitin and U. rigida fractions have a protective effect on the cleavage of DNA at least by 50 % except for butanol fraction which was inactive at the tested concentration. Thus, U. rigida-derived fractions are able to protect DNA from damage caused by the hydroxyl radical. This effect might be due to their chelating activity on iron or hydroxyl radical scavenging activity or both.
Effect of U. rigida extracts on Fe2+/H2O2-mediated DNA strand breaks: lane 1, control of pBluescript II DNA; lane 2, DNA damage control (in the presence of Fenton reagents); lane 3, DNA in the presence of the quercitin used as positive control (100 μM); lanes 4–7, DNA in the presence of n-hexane, chloroform, ethyl acetate, and n-butanol fractions, respectively
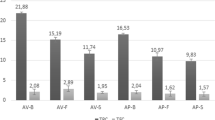
Cholinesterase inhibitors represent the most promising therapeutic agents for Alzheimer’s disease. Inhibition of AChE is considered as a promising approach for the treatment of Alzheimer’s disease and for possible therapeutic applications in the treatment of Parkinson’s disease, aging, and myasthenia gravis. Cholinesterase plays a key role in the hydrolytic degradation of acetylcholine, which is the primary neurotransmitter in the sensory and neuromuscular system regulation of cholinergic nervous transmission (Howes and Houghton 2003). Therefore, spring U. rigida total extract and derived fractions were evaluated for their ability to inhibit acetylcholinesterase. The n-hexane and ethyl acetate fractions showed a strong AChE enzyme inhibition (Fig. 3). At this concentration, total extract and butanol and water fractions exhibited low activity. Due to the high activity of the ethyl acetate and n-hexane fractions in the AChE assay, the inhibition effect of these two fractions at different concentrations (1–100 μg mL−1) was evaluated, and the EC50 values (the inhibitory dose that reduced 50 % of AChE activity) determined. The AChE inhibition showed an increase in inhibition with increasing concentrations of the U. rigida fractions. The best inhibitory activity was exhibited by the EtOAc fraction with an EC50 of 6.08 μg mL−1 followed by the n-hexane fraction (EC50 of 7.6 μg mL−1). The activity of both EtOAc and n-hexane fractions indicated that the inhibitory principles have low to moderate polarity. A number of studies have recently shown AChE inhibitory activity of several marine algae species. Among the most potent algae, the red Ecklonia stolonifera showed an inhibition of 45.97 % toward AChE at 100 μg mL−1 (Yoon et al. 2008). Hypnea valentiae and Ulva reticulata, two species from Tamil Nadu, India, were able to inhibit AChE activity with EC50 values of 2.6 and 10 mg mL−1, respectively (Suganthy et al. 2009). Thus, we noted the strong antiacetylcholinesterase of the Tunisian U. rigida compared to the U. reticulata collected from India. Algae can be used to enhance cognitive function and to alleviate other symptoms associated nowadays with Alzheimer’s disease.
Chemical composition of U. rigida
Moisture content, protein, and lipid of the spring U. rigida were investigated. The fatty acid composition of U. rigida showed nine fatty acids, and five were unsaturated (Table 3). The most abundant fatty acids were palmitic acid, oleic acid, and linolenic acid. Among the detected fatty acids, unsaturated acids constitute 40.18 % of the total composition. These values are close to those obtained with U. lactuca and Durvillaea antarctica (Ortiz et al. 2006). These fatty acids make U. rigida specifically prone to oxidation and may have beneficial effects in health and in the control of chronic diseases. The chemical composition and properties of Ulva species had not been well investigated compared to the red and brown algae. The main constituent of Ulvaceae species is the typical polysaccharide, known as ulvan. Moreover, a survey of the chemical composition showed a wide range of compounds, particularly fatty acids, terpenes, sterols, and polyphenolic compounds. Seven labdane diterpenoids with potential antibacterial activity were isolated from U. fasciata and an antiinflammatory agent identified as 3-O-β-glucopyranosylstigmasta-5,25-diene from U. lactuca (Chakraborty et al. 2010). Polyunsaturated fatty acids isolated from U. fasciata and U. pertusa showed a strong algicidal activity on phytoplankton (Alamsjah et al. 2008).
Conclusion
In conclusion, significant variation in antimicrobial and antioxidant activities was demonstrated by the hydroalcoholic extract of the green alga U. rigida collected monthly for 12 months. The highest antimicrobial activity was detected with the material collected during spring and summer, with a decline in activity in autumn and winter. A strong antioxidant activity was detected in late winter (February) and early spring (March). The variation in the activity corresponds to the variation observed in total phenolic content. These differences can be attributed to the seasonal changes in abiotic factors such as climate, salinity, temperature, pollution, and epiphytic organisms and also to different stages of plant metabolism. Methanol extract and derived fractions of spring U. rigida were tested for antifungal, antibacterial, and antioxidant activities and AChE inhibitory capacity. U. rigida showed a strong antimicrobial activity against a panel of microorganisms and potent antioxidant and anti-AChE activities. Further investigations to the isolation of specific bioactive compounds through bioassay-guided fractionation and their characterization as well as studies evaluating their safety are needed. U. rigida is an ideal candidate for cost-effective, readily exploitable natural phytochemical compounds and new therapeutic drugs. This seaweed is also rich in proteins and has low total lipid content and polyunsaturated fatty acids. These properties make the green alga U. rigida a healthy food for human and animal nutrition.
References
Alamsjah MA, Hirao S, Ishibashi F, Oda T, Fujita Y (2008) Algicidal activity of polyunsaturated fatty acids derived from Ulva fasciata and Ulva pertusa (Ulvaceae, Chlorophyta) on phytoplankton. J Appl Phycol 20:713–720
Andrews JM (2005) BSAC standard disc susceptibility testing method (version 4). J Antimicrob Chemother 56:60–76
AOAC (2000) Official methods of analysis of AOAC International, 17th edn. Association of Official Analytical Chemists, Gaithersburg
Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, Kim SK (2008) Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem 16:7921–7926
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Botterweck AAM, Verhagen H, Goldbohm RA, Kleinjans J, Van den Brandt PA (2000) Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands cohort study. Food Chem Toxicol 38:599–605
Chakraborty K, Lipton AP, Paul Raj R, Vijayan KK (2010) Antibacterial labdane diterpenoids of Ulva fasciata Delile from southwestern coast of the Indian Peninsula. Food Chem 119:1399–1408
Connan S, Delisle F, Deslandes E, Ar Gall E (2006) Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot Mar 49:39–46
Cox S, Abu-Ghannam N, Gupta S (2009) An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int Food Res J 17:205–220
Dhargalkar VK, Pereira N (2005) Seaweed: promising plant of the millennium. Sci Cult 71:60–66
Dudgeon SR, Kübler JE, Vadas RL, Davison IR (1995) Physiological responses to environmental variation in intertidal red algae: does thallus morphology matter? Mar Ecol Prog Ser 117:193–206
Heuer OE, Kruse H, Grave K, Collignon P, Karunasagar I, Angulo FJ (2009) Human health consequences of use of antimicrobial agents in aquaculture. Clin Infect Dis 49:1248–1253
Howes MJR, Houghton PJ (2003) Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 75:513–527
Juntachote T, Berghofer E (2005) Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem 92:193–202
Kazlowska K, Hsu T, Hou CC, Yang WC, Tsai GJ (2010) Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J Ethnopharmacol 128:123–130
Lee J, Kim H, Kim J, Jang Y (2002) Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. Saboten. J Agric Food Chem 50:6490–6496
Mantena RKR, Wijburg OLC, Vindurampulle C, Bennett-Wood VR, Walduck A, Drummond GR, Davies JK, Robins-Browne RM, Strugnell RA (2008) Reactive oxygen species are the major antibacterials against Salmonella typhimurium purine auxotrophs in the phagosome of RAW 264.7 cells. Cell Microbiol 10:1058–1073
Mata AT, Proença C, Ferreira AR, Serralheiro MLM, Nogueira JMF, Araujo MEM (2007) Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem 103:778–786
Nakagawa H, Kasahara S, Sugiyama T (1987) Effect of Ulva meal supplementation on lipid metabolism of black sea bream, Acanthopagrus schlegeli (Bleeker). Aquaculture 62:109–121
Namvar F, Mohamed S, Fard SG, Behravan J, Mustapha NM, Alitheen NBM, Othman F (2012) Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem 130:376–382
Ortiz J, Romeroa N, Roberta P, Arayab J, Lopez-Hernándezc J, Bozzoa C, Navarretea E, Osorioa A, Riosa A (2006) Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem 99:98–104
Perez RM, Avila JG, Perez G (1990) Antimicrobial activity of some American algae. J Ethnopharmacol 29:111–118
Popov SS, Marekov NL, Konaklieva MI, Panayotova MI, Dimitrova-Konaklieva S (1985) Sterols from some black sea Ulvaceae. Phytochemistry 24:1987–1990
Shon MY, Kim TH, Sung NJ (2003) Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem 82:593–597
Siatka T, Kašparová M (2010) Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules 15:9450–9461
Stirk WA, Reinecke DL, Staden J (2007) Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J Appl Phycol 19:271–276
Suganthy N, Karutha Pandian S, Pandima Devi K (2009) Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci Lett 468:216–219
Tuney I, Cadirci BH, Unal D, Sukatar A (2006) Antimicrobial activities of the extracts of marine algae from the coast of Urla (Azmir, Turkey). Turk J Biol 30:1–5
Vidyavathi N, Sridhar KR (1991) Seasonal and Geographical variation in the antimicrobial activity of seaweeds from the Mangalore coast of India. Bot Mar 34:279–284
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. In: Lawton JH, Likens GE (eds) Methods in ecology. Blackwell, Oxford, pp 36–43
Wijesinghe WAJP, Athukorala Y, Jeon YJ (2011) Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr Polym 86:917–921
Yoon N, Chung H, Kim H, Choi J (2008) Acetyl and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish Science 74:200–207
Zubia M, Robledo DY, Freile-Pelegrin Y (2007) Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J Appl Phycol 19:449–458
Acknowledgments
This work was supported by grants from the Tunisian “Ministry of Higher Education, Scientific Research and Technology”. We wish to thank Pr. Adnane Hammami Head of the Research Laboratory “Micro-organismes et Pathologie Humaine”, EPS Habib Bourguiba, Sfax, Tunisia for providing some of the pathogenic bacterial strains and technical assistance. We thank Mrs Lobna Jlail, technical assistant, for excellent technical GC–MS support in these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trigui, M., Gasmi, L., Zouari, I. et al. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and assessment of antiacetylcholinesterase potential. J Appl Phycol 25, 319–328 (2013). https://doi.org/10.1007/s10811-012-9866-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9866-5