Abstract
The brown seaweed Sargassum vulgare C. Agardh abounds along the southeast coast of Rio de Janeiro State, Brazil, creating unique habitats, and its polyphenols play an important ecological role as antifouling agents. In order to understand more precisely this defensive strategy of S. vulgare, we collected this macroalga at Ilha de Itacuruçá in May and October 2009. Thalli were separated according to tissue type: pneumatocysts, receptacles, leaflets, and axis. Phenolic extracts from each specific part of the plant were obtained and the associated antifouling activity was tested in order to assess whether a tissue specialisation/antifouling activity pattern exists. Preliminary separation process was carried out on a phenolic extract to separate the polyphenols from the remaining compounds. Such fractionation allowed us to study the involvement of strictly phenolic compounds in antifouling activity. Within-thallus variation in both phenolic content and antifouling activity were highlighted, and seasonal variation in both those characters was also observed. For both experiments, pneumatocyst and leaflet phenolic extracts were the most active against the attachment of Perna perna, but no correlation was observed between phenolic concentration and antifouling activity. On the contrary, the extract containing only phenolic compounds was twice less active than the extract from which 80 % of the polyphenols were removed. These results led us to hypothesise that polar compounds other than polyphenols are involved in the antifouling activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although it has long been postulated that Sargassum tannins could inhibit biofouling (Sieburth and Conover 1965), this assumption has rarely been investigated. Polyphenols are found exclusively in brown macroalgae and they are classified in six different groups in relation to variations in their assemblage from the polymerisation of phloroglucinol (Ragan and Glombitza 1986; Targett and Arnold 2001; Koivikko et al. 2007). Stored in subcellular structures called physodes, they have been found in almost all brown macroalgal orders (Amsler et al. 2008) with concentrations up to 20 % of dry weight in some species (Amsler and Fairhead 2006). The concentrations of polyphenols within brown macroalgae have been found to vary according to season, habitat, and local environmental factors such as salinity, UV irradiation, and light and nutrient availability (Jormalainen and Honkanen 2001; Hemmi and Jormalainen 2004; Fairhead et al. 2005; Connan et al. 2007; Svensson et al. 2007). Variation of the polyphenol concentration also exists within the macroalgae as already reported by Connan et al. (2007).
Concerning the ecological role of polyphenols, these compounds are assumed to be responsible for herbivore deterrence, UV protection, and metal-ion chelation (e.g., antioxidant activity). However, the involvement of these chemicals in the defence against fouling is less documented. Nevertheless, studies have suggested phlorotannins act as antifoulants because they have been found to inhibit settlement of bacteria (Sieburth and Conover 1965; Nagayama et al. 2002), algal spores (Jennings and Steinberg 1997 and references therein; Nagayama et al. 2003), and protists (Langlois 1976). It has been also found that even low polyphenol concentrations can deter settlement of invertebrate larvae (Lau and Qian 1997; Lau and Qian 2000; Wikström and Pavia 2004). Nagayama et al. (2002, 2003) and Jennings and Steinberg (1997) already described algicidal and bactericidal effects of polyphenols from the brown macroalga Ecklonia kurome, and Hellio et al. (2004) suggested a possible correlation between polyphenolic content from Bifurcaria bifurcata and variation in the antifouling activity.
The ability of polyphenols to deter epibionts has been questioned because, as highly polar compounds, they are unlikely to adhere to the surface of the plant (Jennings and Steinberg 1997). However, polyphenols are exuded from the thallus (Koivikko et al. 2005) and these exudates may have deterrent property. In a recent work, Plouguerné et al. (2010a) investigated the antifouling activity of polyphenolic extracts from Sargassum vulgare from Rio de Janeiro State. This study presents a most detailed approach to verify this ecological role, posing the following questions:
-
1.
Does the polyphenols concentration vary within the thallus of S. vulgare, according to tissue specialisation and season?
-
2.
Does the antifouling activity vary within the thallus of S. vulgare, according to tissue specialisation and season?
-
3.
Are antifouling chemical defences of S. vulgare correlated with polyphenolic content?
To address these questions, we measured total phenolic contents in S. vulgare collected from Ilha de Itacuruçá in May and October 2009 and its antifouling activity. Furthermore, a preliminary study aiming to separate the polyphenols from the active extract was performed, and the antifouling activity associated with the resulting fractions was also evaluated.
Material and methods
Thalli of S. vulgare were collected in May and October 2009 by hand while free diving in the shallow subtidal zone from Ilha de Itacuruçá, a large near shore island inside Sepetiba Bay (Mangaratiba district, Rio de Janeiro State, Southeast Brazil—22°56′ S, 43°52′ W). These two collection periods represent the beginning of the dry (May) and rainy (October) seasons in the area, with average cumulative rainfall of 85 and 181 mm, respectively (data from the National Meteorological Institute—INMET, Brazil).
Extract preparation
After collection, specimens of S. vulgare were immediately transferred to the laboratory in isothermic boxes filled with local seawater, where they were gently washed in seawater, sorted, and carefully cleaned from associated biota. Thalli were then split in different parts (pneumatocysts, receptacles, leaflets, and axis) and pooled. The different algal tissues were then freeze-dried and ground to a fine powder before performing the polyphenol extractions.
The extraction of polyphenols was by using a mixture of acetone/water (7/3) as solvent combination (Koivikko et al. 2005). All extracts were obtained using 20 mL of solvent per gram of algal tissue (dry weight—DW). Two first extractions of 5 h were followed by one extraction of 2 h. Between each extraction, the mixture was filtered and acetone eliminated under reduced pressure in a rotary evaporator (<40°C), while the remaining water was removed using a freeze-dryer. The remainder was then weighed and stored at −15°C prior to use in bioassays. All procedures were performed in the dark, as polyphenols are sensitive to light (Plouguerné et al. 2006).
Quantitative analysis of polyphenols
Total phenolic contents were determined by colorimetry with an adapted Folin–Ciocalteu assay known to be less affected than other methods by interfering of compounds like aromatic amino acids (Sanoner et al. 1999). Absorbance was read at 700 nm (spectrophotometer SP 2000UV, BEL Photonics, Monza, Italy), and phloroglucinol (1,3,5-trihydroxybenzene, Sigma-Aldrich) was used as a standard.
Extraction and separation of polyphenols from the whole thallus of S. vulgare
In order to fractionate the extract and separate the polyphenols, a protocol adapted from Magalhaes et al. (2010) and Toth and Pavia (2001) was used (Fig. 1). Whole S. vulgare individuals collected in October 2009 were freeze-dried then reduced to powder (30 g) in a mortar and subsequently extracted two times with 500 mL of dichloromethane by ultra-sonication for ca. 30 min and shaken by vortex for 30 min. The extract was filtered in a Whatman no. 1 filter paper on a Büchner funnel, and the remaining residue was kept for further polyphenol extraction. The dichloromethane extract was evaporated at room temperature by rotary evaporation under reduced pressure and reserved for antifouling tests (extract I).
The Sargassum residue was extracted three times with 500 mL of acetone/water (7:3, v/v) by sonication for 15 min and shaking for more 15 min. The extract was then filtered in a Büchner filter (with Whatman no. 1 filter paper). The combined extracts were evaporated at room temperature by rotary evaporation to remove acetone. After organic solvent evaporation, approximately 300 mL of water extract were obtained; 150 mL of initial sample from acetone water (70:30, v/v) were freeze-dried for further analysis and antifouling tests (extract II). The other 150 mL of water extract were kept for further adsorption of phenolic compounds to poly(vinylpolypyrrolidone) (PVPP, Sigma-Aldrich) (Fig. 1).
Water extract (150 mL) (adjusted to pH 4.0) was mixed with 5 g of PVPP (30 mg mL−1) and shaken for 15 min. The extract was filtrated in a Büchner filter (Whatman no. 1 filter paper) and the filtrate was mixed again with a new portion of PVPP (5 g) for 15 min. The operation was repeated one more time. The filtrate was finally lyophilized for further analysis (extract III) (Fig. 1).
The PVPP collected in the Büchner filter (Whatman no. 1 filter paper) was extracted three times with 200 mL of acetone/water (7:3, v/v) by sonication for 15 min and shaking for an additional 15 min. The extract was then filtered in a Büchner filter (Whatman no. 1 filter paper). The combined extracts were evaporated at room temperature by rotary evaporation to remove the acetone. The remaining water extract was lyophilized for further analysis (extract IV) (Fig. 1).
Antifouling assays with the mussel Perna perna
Extracts from S. vulgare were tested in laboratory bioassays against the brown mussel P. perna (Linnaeus 1758). Mussels are relevant fouling organisms in an ecological context, as they are usually found settled on seaweeds (Petersen 1984; Eyster and Pechenik 1988; Davis and Moreno 1995; Lasiak and Barnard 1995; Alfaro et al. 2004). Juvenile of P. perna were collected during low tide from the rocky coastal area of Itaipu (Niterói, Rio de Janeiro, Brazil—22°58′ S, 43°02′ W) and kept in a 400-L recirculating laboratory aquarium equipped with biological filtering, protein skimming, and activated carbon at constant temperature (ca. 20°C), salinity (ca. 35 p.s.u.), and aeration for 12 h. The mussels were then carefully disaggregated by cutting the byssal threads and those exhibiting substratum exploring behaviour (actively exposing their foot and crawling) were selected for experiments. AF activity was measured by the following procedure, described in detail in da Gama et al. (2003). Water-resistant filter paper was cut into 9-cm diameter circles and soaked in solvent (control filter). Another 9-cm diameter set of filter papers (treatment filters) was cut in a chessboard pattern (1-cm squares) and soaked in a natural concentration of extracts (determined as the extract equivalent to the DW of algae = DW of filter paper). All filter paper circles were allowed to air dry. Entire filter circles were then placed in the bottom of sterile polystyrene Petri dishes, over which treated chessboard filters were placed. Dishes were filled with 80 mL of seawater and three mussels (1.5–2.5 cm length) were added. In this way, mussels would have the same area of treated (superior, squared) and control (inferior, entire) filter paper on which to attach. A total of ten replicates of each treatment were always used. Experimental dishes were kept in total darkness, as mussels have been shown to produce more byssal threads when held in the dark (Davis and Moreno 1995). Mussel attachment was recorded 24 h after the beginning of the experiment. Mussels were then placed in plastic boxes tagged according to treatment, and suspended in a marine aquarium for 24 h to check for possible mortality due to exposure to the test substances.
Statistical analysis
To satisfy the criteria of normality and variance homogeneity, data were transformed using the square root of X + 1 prior to ANOVA. The Dunnett one-tailed test was used for post hoc comparisons with controls when the ANOVA indicated significant differences. We adopted the 0.05 significance level (α = 5 %).
Results
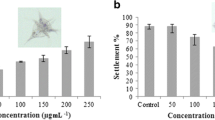
Within-thallus variations of the total phenolic content were observed in S. vulgare. Axis and receptacles presented the highest phenolic content registered for May 2009 (3.61 ± 0.08 and 3.32 ± 0.14 % DW, respectively), while lower phenolic contents were measured in pneumatocysts and leaflets (1.90 ± 0.06 and 2.59 ± 0.12 % DW, respectively) (Fig. 2). The evaluation carried out on specimens collected in October 2009 also highlighted within-thallus variations of the phenolic content in this brown alga (Fig. 3). Higher contents were found in receptacles (3.00 ± 0.08 % DW), followed by axis (1.72 ± 0.07 % DW), leaf tissues (1.24 ± 0.03 % DW), and finally pneumatocysts (0.56 ± 0.02 % DW). The total phenolic contents measured in S. vulgare extracts from May 2009 were higher than those from algae collected in October 2009.
Intra-thallus phenolic contents in S. vulgare collected at Sepetiba Bay in May 2009 and corresponding antifouling activity. R receptacles, P pneumatocysts, L leaflets, A axis. DW dry weight. The errors bars show the standard deviation. The number of replicates used was n = 3 for phenolic quantification and n = 10 for antifouling assays
Intra-thallus phenolic content and antifouling activity of S. vulgare collected from Sepetiba Bay in October 2009. R receptacles, P pneumatocysts, L leaflets, A axis. The errors bars show the standard deviation. The number of replicates was n = 3 for phenolic quantification and n = 10 for antifouling assays
Variations of the antifouling activity according to tissue specialisation were verified in both seasons of collect (May and October 2009, Figs. 2 and 3). In both moments, the pneumatocysts presented the highest antifouling activity (75 and 99 % inhibition of mussel attachment in May and October, respectively). The second highest antifouling activity was verified for polyphenol content from leaflets of S. vulgare (67 and 80 % inhibition of the byssal adhesion in May and October, respectively). The lower antifouling activities were registered for polyphenols from receptacles (for plants collected in May 2009) and axis (for plants collected in October 2009). Overall, all extracts from specimens of S. vulgare collected in rainy period (October 2009) exhibited a higher antifouling activity than extracts obtained from specimens of this macroalga collected in May 2009 (dry period).
No correlation was found between the total phenolic content and antifouling activity. The most important rise in terms of antifouling activity was observed for receptacles (from 27.64 % in May 2009 to 79.09 % in October 2009), and it was accompanied by a drop in total phenolic content (3.27 to 2.99 % DW). The same trend was also observed for the other thallus parts (pneumatocysts, leaflets, and axis): rise of the antifouling activity and diminution of the total phenolic content.
The highest antifouling activity was observed for the extract III, followed by extract II and extracts IV and I (Fig. 4). Extract II presented the highest concentration of phenolic compounds (1.158 % DW), followed by extract IV (0.919 % DW) and extract III (0.543 % DW).
Discussion
Intra-thallus variations of polyphenol concentrations within macroalgae have already been investigated, although most of these studies focused on temperate Phaeophyceae (Ilvessalo and Tuomi 1989; Tuomi et al. 1989; Keshava Rao and Untawale 1991; Van Alstyne et al. 1999; Connan et al. 2006). Nevertheless, our methodology diverged from previous studies because we separated the thalli according to tissue differentiation (axis, receptacles, pneumatocysts, and leaflets), instead of dividing the thallus in various sections from basal to apical parts. Our aim was to investigate the presence of specific antifouling defences associated with specialised structures. A previous study by Pereira and Yoneshigue-Valentin (1999) also tried to highlight a relationship between tissue specialisation and phenolic content in Sargassum furcatum. They divided the thalli of this brown alga in four parts: main axis, blades, holdfasts, and receptacles. However, they did not find major variation between these parts, registering very low levels of polyphenols (0.2–0.62 % DW). The levels of polyphenols measured in our work were higher in May 2009, ranging from 1.9 to 3.61 %, than in October 2009 (0.56 to 3 % DW). Nevertheless, these values can still be considered low when compared to the higher levels of polyphenols registered for other brown algal species that can reach up to 25 % DW (Targett et al. 1992; Van Alstyne et al. 1999).
Seasonal variations of polyphenolic content have been the topic of various studies regarding temperate brown macroalgae (Connan et al. 2004; Plouguerné et al. 2006; Parys et al. 2009). In tropical countries, such investigations were generally neglected, according to the common, yet disputable concept that the tropical ambient may offer little variation of environmental factors thru the year. Our data highlighted temporal variations of phenolic contents in S. vulgare. Phenolic concentrations measured for October 2009 were lower than the ones quantified for May 2009. This pattern is likely to be linked with both irradiance and nutrient levels. In May, irradiance is higher and precipitation lower than in October. This elevated irradiance may constitute a stressful condition resulting in higher polyphenolic production, as has been observed previously (Cronin and Hay 1996; Pavia et al. 1997; Gomez and Huovinen 2010). On the contrary, higher precipitation in October may result in increased nutrient levels, as Sepetiba Bay has a large drainage basin, leading to lower production of polyphenols (Arnold et al. 1995). Nevertheless, our study is very succinct (only two sampling events) and a more complete study involving a longer monitoring time would be necessary to more precisely evaluate such variations.
We found variations of the antifouling activity according to tissue specialisation. In both experiments, polyphenols extract from S. vulgare pneumatocysts had the highest antifouling activity. These air vesicles provide buoyancy to macroalgae and allow them to maintain an upright posture of their thallus without the trade-off of investing in expensive structural support (Stewart 2006). Such strategy is particularly important in an environment such as Ilha de Itacuruçá, where the high density of individuals can lead to light limitation (Reed 1990; Holbrook et al. 1991). The buoyancy provided by the pneumatocysts is also essential as algae from Sargassum genus disperse by detached, reproductively mature floating fronds. The phenomena known as algae drift or raft permits the dispersion of the algae and allows the colonisation of new areas. Such rafts of buoyant seaweeds have been reported to float at the surface for up to 6 months and can cover distances up to thousands of kilometres (Helmuth et al. 1994; Stewart 2006). As marine macrofoulers can hinder the role played by pneumatocysts, e.g., increasing weight, damaging the structure (eventually leading to its perforation), we can then suppose that S. vulgare has developed specific defences in order to protect these important structures. Our results seem to confirm such hypothesis, as extracts from pneumatocysts exhibited high antifouling activities against the mussel P. perna compared to other thalli parts.
Moreover, structures that exhibited the second highest antifouling activity were leaflets. These tissues constitute the principal part of the thallus in relation to photosynthesis (they represent the largest surface of the alga). Such observation seems to confirm the involvement of specific defences related to tissue specialisation in S. vulgare in order to ensure the photosynthetic process. According to the optimal defence theory, an organism will allocate resources to defense in order to protect tissues and structures that are more valuable or more vulnerable to attack (Fairhead et al. 2005). It then seems logical to observe highest antifouling activity in valuable structures such as pneumatocysts and leaflets. The consequences of fouling on seaweeds may also include reduced growth and reproduction (Orth and van Montfrans 1984; Brawley 1992; Williams and Seed 1992), increased drag and consequently tissue loss during storms (Dixon et al. 1981; Brawley 1992; Williams and Seed 1992), or increased susceptibility to consumers that are attracted to seaweeds possessing fouling organisms (Bernstein and Jung 1979; Pereira et al. 2003).
Nevertheless, the same defence theory led us to expect high defensive property for the reproductive structures, the receptacles, but it was not the case. One possible hypothesis to explain such outcome is that receptacles are ephemeral tissues and thus may not be submitted to the same fouling pressure, as both pneumatocysts and leaflets would be, as permanent tissues.
As previously underlined, no correlation could be established between total phenolic content and the antifouling activity. It is thus necessary to investigate more precisely the content of the acetone/water extracts. Brown algal polyphenols are polymers of phloroglucinol subunits, and different polymers may have different characteristics (Jormalainen and Honkanen 2008). Therefore, qualitative variation of phenolic compounds may be the key to understand variation of the antifouling activity highlighted in the present study. Another possibility is that compounds other than polyphenols may be present in the acetone/water extracts and be responsible for antifouling activity.
In order to investigate that last hypothesis, the initial acetone/water extract was fractionated using PVPP. From the four resulting fractions, the highest antifouling activity was related to the extract III. This extract was produced from extract II after removing phenolic compounds by adsorption to PVPP, although this procedure did not remove completely polyphenolics from the extract as a remaining amount was quantified. However, such remainder quantified in extract III cannot be attributed to polyphenols only, as other aromatic compounds may interfere in the Folin–Ciocalteu assay, e.g., ascorbic acid or peptide (Ragan and Glombitza 1986). Moreover, 80 % of the phenolic compounds present in extract II were recovered in extract IV after re-extraction from PVPP. Such result means that extract III must contain less than 20 % of the initial polyphenols concentration. Extract IV, on the contrary, contains only phenolic compounds that were re-extracted from PVPP and exhibited lower antifouling activity than the extract III and the initial acetone/water extract. Such observations allowed us to hypothesise that phenolic compounds are not the key compounds involved in antifouling defence in S. vulgare. Indeed, the aqueous acetone solvent procedure extracts polyphenols but may as well extract other types of compounds, particularly lipids. Previous studies already highlighted the potential ecological role of lipids in brown algae. Deal et al. (2003) demonstrated the involvement of glycolipids as herbivore deterrents in Fucus vesiculosus, while Plouguerné et al. (2010b) characterised galactolipids with anti-microfouling activity from Sargassum muticum. That last result seems particularly promising and encourages us to further investigate the presence of such compounds involved in antifouling defence in S. vulgare.
Another point that our study emphasises is the importance of bioassay-guided investigations. As reported by Deal et al. (2003), many ecologically important compounds may remain undiscovered in polyphenol-containing seaweeds (most brown algae) if these species are studied using chemical investigations of known compounds rather than a bioassay-guided approach to unravel ecologically active metabolites. Focusing on polyphenols only may have led us to mistake the important ecological function played by other compound types. Such result confirms the essential character of bioassay-guided investigations and may lead to reconsider the antifouling role of brown algal polyphenols as described in various studies using extracts instead of isolated compounds.
References
Alfaro AC, Jeffs AG, Creese RG (2004) Bottom-drifting algal/mussel spat associations along a sandy coastal region in northern New Zealand. Aquaculture 241:269–290
Amsler CD, Fairhead VA (2006) Defensive and sensory chemical ecology of brown algae. Adv Bot Res 43:1–91
Amsler CD, McClintock JB, Baker BJ (2008) Macroalgal chemical defenses in polar marine communities. In: Amsler CD (ed) Algal chemical ecology. Springer, Berlin, pp 91–103
Arnold TM, Tanner CE, Hatch WI (1995) Phenotypic variation in polyphenolic content of the tropical brown alga Lobophora variegata as a function of nitrogen availability. Mar Ecol Prog Ser 123:177–183
Bernstein BB, Jung N (1979) Selective pressure and coevolution in a kelp canopy community in Southern California. Ecol Monogr 493:335–355
Brawley SH (1992) Mesoherbivores. In: John DH, Hawkins SJ, Price JH (eds) Plant-animal interactions in the marine benthos. Systematics Association. Clarendon Press, Oxford, pp 235–263
Connan S, Goulard F, Stiger V, Deslandes E, Gall EA (2004) Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot Mar 47:410–416
Connan S, Delisle F, Deslandes E, Gall EA (2006) Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot Mar 49:39–46
Connan S, Deslandes E, Gall EA (2007) Influence of day–night and tidal cycles on phenol content and antioxidant capacity in three temperate intertidal brown seaweeds. J Exp Mar Biol Ecol 349:359–369
Cronin G, Hay ME (1996) Effect of light and nutrient availability on the growth, secondary chemistry, and resistance to herbivory of two brown seaweeds. Oikos 77:93–106
Da Gama BAP, Pereira RC, Soares AR, Teixeira VL, Yoneshigue-Valentin Y (2003) Is the mussel test a good indicator of antifouling activity? A comparison between laboratory and field assays. Biofouling 19:161–169
Davis AR, Moreno CA (1995) Selection of substrata by juvenile Choromytilus chorus (Mytilidae): are chemical cues important? J Exp Mar Biol Ecol 191:167–180
Deal MS, Hay ME, Wilson D, Fenical W (2003) Galactolipids rather than phlorotannins as herbivore deterrents in the brown seaweed Fucus vesiculosus. Oecologia 136:107–114
Dixon J, Schroeter SC, Kastendiek J (1981) Effects of the encrusting bryozoan, Membranipora membranacea, on the loss of blades and fronds by the giant kelp, Macrocystis pyrifera (Laminariales). J Phycol 17:341–345
Eyster LS, Pechenik JA (1988) Attachment of Mytilus edulis L. larvae on algal and byssal filaments is enhanced by water agitation. J Exp Mar Biol Ecol 114:99–110
Fairhead VA, Amsler CD, McClintock JB, Baker BJ (2005) Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol 28:680–686
Gomez I, Huovinen P (2010) Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the Kelp Lessonia nigrescens. Photochem Photobiol 86:1056–1063
Hellio C, Marechal JP, Veron B, Bremer G, Clare AS, Le Gal Y (2004) Seasonal variation of antifouling activities of marine algae from the Brittany Coast (France). Mar Biotechnol 6:67–82
Helmuth B, Veit RR, Holberton R (1994) Long-distance dispersal of a subantarctic brooding bivalve (Gaimardia trapesina) by kelp-rafting. Mar Biol 120:421–426
Hemmi A, Jormalainen V (2004) Geographic covariation of chemical quality of the host alga Fucus vesiculosus with fitness of the herbivorous isopod Idotea baltica. Mar Biol 145:759–768
Holbrook SJ, Denny MW, Koehl MAR (1991) Intertidal trees-consequences of aggregation on the mechanical and photosynthetic properties of seapalms Postelsia palmaeformis Ruprecht. J Exp Mar Biol Ecol 146:39–67
Ilvessalo H, Tuomi J (1989) Nutrient availability and accumulation of phenolic compounds in the brown alga Fucus vesiculosus. Mar Biol 101:115–119
Jennings JG, Steinberg PD (1997) Phlorotannins versus other factors affecting epiphyte abundance on the kelp Ecklonia radiata. Oecologia 109:461–473
Jormalainen V, Honkanen T (2001) Multiple cues for phenotypic plasticity in phlorotannin production of the bladder wrack Fucus vesiculosus. Phycologia 40:59–60
Jormalainen V, Honkanen T (2008) Macroalgal chemical defenses and their roles in structuring temperate marine communities. In: Amsler CD (ed) Algal chemical ecology. Springer, Berlin, pp 57–89
Keshava Rao C, Untawale AG (1991) Polyphenol contents of Indian seaweeds. Mahasagar 24:99–102
Koivikko R, Loponen J, Honkanen T, Jormalainen V (2005) Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J Chem Ecol 31:195–212
Koivikko R, Loponen J, Pihlaja K, Jormalainen V (2007) High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem Anal 18:326–332
Langlois G (1976) Effects of algal exudates on substratum selection by the motile marine telotrochs Vorticella marina. J Protozool 22:115–123
Lasiak TA, Barnard TCE (1995) Recruitment of the brown mussel Perna perna onto natural substrata: a refutation of the primary/secondary settlement hypothesis. Mar Ecol Prog Ser 120:147–153
Lau SCK, Qian PY (1997) Phlorotannins and related compounds as larval settlement inhibitors of the tube-building polychaete Hydroides elegans. Mar Ecol Prog Ser 159:219–227
Lau SCK, Qian PY (2000) Inhibitory effect of phenolic compounds and marine bacteria on larval settlement of the barnacle Balanus amphitrite amphitrite Darwin. Biofouling 16:47–58
Magalhaes PJ, Vieira JS, Goncalves LM, Pacheco JG, Guido LF, Barros AA (2010) Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: characterization by high-performance liquid chromatography-diode array detection-electrospray tandem mass spectrometry. J Chromatogr A 1217:3258–3268
Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T (2002) Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother 50:889–893
Nagayama K, Shibata T, Fujimoto K, Honjo T, Nakamura T (2003) Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 218:601–611
Orth RJ, van Montfrans J (1984) Epiphyte–seagrass relationships with an emphasis on the role of micrograzing: a review. Aquat Bot 18:43–69
Parys S, Kehraus S, Pete R, Kupper FC, Glombitza KW, Konig GM (2009) Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur J Phycol 44:331–338
Pavia H, Cervin G, Lindgren A, Aberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Pereira RC, Yoneshigue-Valentin Y (1999) The role of polyphenols from the tropical brown alga Sargassum furcatum on the feeding by amphipod herbivores. Bot Mar 42:441–448
Pereira RC, Da Gama BAP, Teixeira VL, Yoneshigue-Valentin Y (2003) Ecological roles of natural products of the Brazilian red seaweed Laurencia obtusa. Braz J Biol 63:665–672
Petersen JH (1984) Larval settlement behavior in competing species Mytilus californianus Conrad and Mytilus edulis L. J Exp Mar Biol Ecol 82:147–159
Plouguerné E, Le Lann K, Connan S, Jechoux G, Deslandes E, Stiger-Pouvreau V (2006) Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat Bot 85:337–344
Plouguerné E, Hellio C, Cesconetto C, Thabard M, Mason K, Veron B, Pereira RC, da Gama BAP (2010a) Antifouling activity as a function of population variation in Sargassum vulgare from the littoral of Rio de Janeiro (Brazil). J Appl Phycol 22:717–724
Plouguerné E, Ioannou E, Georgantea P, Vagias C, Roussis V, Hellio C, Kraffe E, Stiger-Pouvreau V (2010b) Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. Mar Biotechnol 12:52–61
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. Prog Phycol Res 4:129–241
Reed DC (1990) An experimental evaluation of density dependence in a subtidal algal population. Ecology 71:2286–2296
Sanoner P, Guyot S, Marnet N, Molle D, Drilleau JF (1999) Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J Agric Food Chem 47:4847–4853
Sieburth JM, Conover JT (1965) Sargassum tannin, an antibiotic which retards fouling. Nature 208:52–53
Stewart HL (2006) Morphological variation and phenotypic plasticity of buoyancy in the macroalga Turbinaria ornata across a barrier reef. Mar Biol 149:721–730
Svensson CJ, Pavia H, Toth GB (2007) Do plant density, nutrient availability, and herbivore grazing interact to affect phlorotannin plasticity in the brown seaweed Ascophyllum nodosum. Mar Biol 151:2177–2181
Targett NM, Arnold TM (2001) Effects of secondary metabolites on digestion in marine herbivores. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. CRC, Boca Raton, pp 391–411
Targett NM, Coen LD, Boettcher AA, Tanner CE (1992) Biogeographic comparisons of marine algal polyphenolics: evidence against a latitudinal trend. Oecologia 89:464–470
Toth GB, Pavia H (2001) Removal of dissolved brown algal phlorotannins using insoluble polyvinylpolypyrrolidone (PVPP). J Chem Ecol 27:1899–1910
Tuomi J, Ilvessalo H, Niemelä P, Sirén S, Jormalainen V (1989) Within-plant variation in phenolic content and toughness of the brown alga Fucus vesiculosus L. Bot Mar 32:505–509
Van Alstyne KL, McCarthy JJ III, Hustead CL, Kearns LJ (1999) Phlorotannin allocation among tissues of northeastern Pacific kelps and rockweeds. J Phycol 35:482–492
Wikström SA, Pavia H (2004) Chemical settlement inhibition versus post-settlement mortality as an explanation for differential fouling of two congeneric seaweeds. Oecologia 138:223–230
Williams GA, Seed R (1992) Interactions between macrofaunal epiphytes and their host algae. In: John DH, Hawkins SJ, Price JH (eds) Plant–animal interactions in the marine benthos. Systematics Association. Clarendon Press, Oxford, pp 189–211
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plouguerné, E., Cesconetto, C., Cruz, C.P. et al. Within-thallus variation in polyphenolic content and antifouling activity in Sargassum vulgare . J Appl Phycol 24, 1629–1635 (2012). https://doi.org/10.1007/s10811-012-9826-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9826-0