Abstract
The relationship between median grain size of sediments and the abundance in the wild of green algal mats (Ulva prolifera) on the intertidal flats of Muan, Korea, were investigated. The impact of substratum particle size on the growth and survival of germlings was examined in the laboratory. In the wild, the average annual density of algal mats was 7,950 ind m−2. The algal mats mainly occurred in sands and exhibited patchy distribution. Statistical analysis indicates significant spatial analysis differences and a significant relationship between density and the ratio of sands to silts, suggesting that the distribution and density of this species were related to particle size. In laboratory experiments, the survival rate of U. prolifera germlings was the lowest value (22%) on sediments with a median grain size of 63–125 μm. Laboratory experiments have generally shown a positive relationship between attachment or survival of the alga and substratum particles size. Our laboratory results indicate a clear link between germling settlement/survival and substratum particle size. These results explain the spatial differences in abundance observed in the field in relation to the distribution and ratio of sands to silt on the Muan flats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ulva prolifera O.F. Müller is an edible green alga that grows in almost every bay, intertidal flat, and river mouth in Korea. In 2005, the production of U. prolifera as food was approximately 2,500 tonnes wet weight (Informatization officer 2006). Because of the demand for this seaweed and the need to increase biomass, there have been many efforts to harvest this alga on an industrial scale, but there are few places in Korea where U. prolifera production in the wild is adequate for commercial exploitation, and consequently, the opportunity for increasing production through stock enhancement is being investigated (Yoon et al. 2003).
Tidal flats show a marked longitudinal gradient in environmental factors (temperature, salinity, light, sediment, etc.) and biological factors (herbivory and competition with other intertidal organisms) from the land to the sea (Buschmann et al. 1997). Generally, salinity and wave action decrease, whereas silt, turbidity, light extinction, and nutrient concentrations all increase along this gradient. Consequently, tidal flat macroalgae communities typically exhibit spatial zonation and low species diversity. Attached species such as Ulva spp. often form the dominant algal species in warm intertidal mudflat environments.
Sediment dynamics significantly influence benthic macroalgal distribution patterns on both rocky shores (Seapy and Littler 1982; Kendrick 1991; Renaud et al. 1997; Airoldi and Cinelli 1997; Umar et al. 1998) and soft sediments (Albrecht 1998; Chapman and Chapman 1999) in the coastal environment. On rocky shore habitats, it has been demonstrated for a range of algal species that sediment deposition can interfere with the attachment of microscopic stages of seaweeds (Norton 1978; Littler et al. 1983; Arakawa and Matsuike 1992). In addition to impairing the initial attachment process, burial under sediment may significantly reduce growth and impair the regeneration ability of adults (Lyngby and Mortensen 1996). Scour through sediment movement removes small life stages of macroalgae from the substratum and hence reduces successful recruitment (Devinny and Volse 1978).
Recruitment of seaweeds through small reproductive stages is limited on sediment-inundated rocky shores and largely unsuccessful in soft sediment environments. Burial in sediment has several potentially negative effects for seaweed propagules. Sediment dynamics on intertidal flats may alter ecological assembly composition because species shifts occur in consequence to differential sediment susceptibility and life history responses (Airoldi 1998). In soft sediment-dominated coastal environments (e.g., tidal flats), the effects of dynamic sediments on marine macroalgae are more fundamental than other stable attachment substrate. However, in nature, soft sediment environments with organically enriched muds (e.g., tidal flats and salt marshes) represent habitats that are favorable for colonization through small reproductive stages of seaweeds.
Whereas the effects of sediment depositions on growth and survival of adult seaweeds have been tested experimentally for a number of species (Umar et al. 1998) as well as for species assemblages (Airoldi and Cinelli 1997), direct evidence for the mechanisms by which sediment burial reduces survival and/or growth of macroalgal recruits, especially after settlement, remains scarce.
Investigations of U. prolifera have been mainly carried out on the pattern of distribution on rocky shore habitats, morphology (Pang et al. 2010), physiology (Runcie et al. 2003), and growth (Sfriso and Marcomini 1996). To date, there have been no studies that examine the distribution and population dynamics of U. prolifera on intertidal flats.
In this study, we investigated the relationship between distributions of U. prolifera, a representative species of a dominant intertidal group of seaweeds with a wide distribution in soft sediment environments. The spatial and temporal variation in density and structure of U. prolifera were investigated from Muan intertidal flats and the influence of substrate heterogeneity on its distribution.
Materials and methods
Sampling and measurement
The density of U. prolifera in the wild was tested by collecting samples of U. prolifera from the intertidal flats of Muan, Korea (Fig. 1). Three replicate quadrat samples of 400 cm2 (20 cm in width × 20 cm in length) were taken from five stations spaced at a 50-m interval distance on an orthogonal line to the shore. Sampling was undertaken once a month at low tide between October 2006 and September 2007. In each sample, the total number of individuals of >1-mm thalli length was recorded. This helped remove the bias in density within a given sampling period, caused by recruitment to the sampled population. The maximum thallus length was measured to the nearest 0.1 mm using digital Vernier calipers. Sampled thalli were dried for 24 h at 60°C, and thallus dry weight determined to the nearest 0.1 g using an electronic digital balance.
A crust sample of substratum (500-g wet weight of <5 cm in depth) was taken adjacent to each station for particle size analysis. Fine and coarse grain sizes represented lower and upper limits of size ranges from tidal flats known to support macroalgal populations. Dry weight in each particle grade was measured using Sedigraph 5100 (Micromeretcs, Australia). Classification of particle size and grade were adopted from Friedman and Sanders (1978). Environmental factors, water temperature, salinity, and conductivity were measured by a logging multiparameter probe (YSI-85, USA) at the experimental site. Triplicate samples for the particle size analysis were taken at January 2007 during the peak growing season for the alga.
Zoospore release, settlement, and growth in the laboratory
For the laboratory experiments, U. prolifera thalli were collected at low tide between October and November 2006 from the intertidal flats of Muan, on the Southwest coast of Korea. U. prolifera thalli were placed in filtered seawater, and were allowed to release zoospores at 15°C and 16:8-h L/D cycle (approximately 100 μmol photons m−2 s−1). The solution of suspended zoospores was checked by means of counting the number of zoospore individuals under a microscope, and the concentration of the solution was adjusted to 1,000 individuals mL−1 (Chapman and Fletcher 2002).
The zoospores were inoculated to settle onto sterilized disposable plant culture dishes (SPL310100, 100 × 40 mm), each containing one of the experimental sediment types and Provasoli’s PES culture medium. The sediments were collected from the study area and represented a range of particle size classes (Table 1). For the selection of treatment levels, we used conditions within the range encountered in Muan intertidal flat sediment environments studied previously (size range of grain sizes; Ryu et al. 2000; Kang et al. 2008). A glass slide (used as the control substratum) was placed at the bottom of each culture dish and left for 24 h to allow time for zoospores of U. prolifera to attach to the slide. The number of zoospores attached to each slide was counted under a microscope. The experiment was repeated ten times and the values of zoospores attaching to the glass slides are given as the mean value of the ten replicate trials.
Culture conditions for on-growing the germlings were 15°C at 12:12-h L/D cycle (approximately 50 μmol photons m−2 s−1). The survival rate of germlings was obtained from the following equation: S r = N final/N control × 100 (%), where S r is the survival rate of germlings, N final is the number of germlings after 30 days of cultivation, and N control is the mean number of germlings in the control. After 30 days of cultivation, the number of germlings and young thalli attached to each substrate were counted under a Stereomicroscope and with the naked eye. To avoid systematic error, all experiments were carried out for one set of replicates first then for the second set of replicates.
Statistical analysis
The differences in density of U. prolifera between monthly samples were tested by an analysis of variance (ANOVA). The differences in density of U. prolifera between stations were analyzed by Kruskal–Wallis test, and subsequent a posterior comparisons between all pairwise stations were tested by Tukey’s honestly significant difference test (Sokal and Rohlf 1995) in SigmaStat, version 2.0. The differences in survival rate of zoospores were expressed as a percentage of the control in each of replicate culture dishes. These data were normalized by arcsine transformation before statistical analysis.
Results
Spatial and temporal variation in distribution and biomass
Water temperature varied from 5°C to 25°C during the experimental period. Maximum water temperature was recorded in September 2007 and the minimum in February 2007. Salinity ranged between 29.2 and 32.5 ppt.
The average annual density of the whole U. prolifera population was 7,950 ind m−2. Density ranged from 1,950 ind m−2 at station V (lowest mean density) to 15,475 ind m−2 at station II (highest mean density; Figs. 2 and 3). There was a significant difference in mean density between stations (p < 0.001), with the greatest density being >87% of that at the station with the lowest mean density. The density distribution of the algal population relative to the profile of the Muan intertidal flats is presented as a kite diagram in Fig. 2.
The mean thallus length (TL) and dry weight per square meter (DW) of U. prolifera ranged from 25 to 141 mm and 5.0 to 1,307.5 g m−2, respectively. It spatially ranged from 25 mm in station IV (lowest mean TL) to 141 mm in station II (highest mean TL) and 5.0 g m−2 in station V (lowest mean DW) to 1,307.5 g m−2 in station II (highest mean DW; Table 2). The period of minimal growth of U. prolifera occurred during the summer and autumn, and the period of maximal growth occurred during winter. The biomass of U. prolifera varied between sites, with the deepest (seaward) site recording the least (5.0 g m−2) biomass and site II recording the highest biomass (1,307.5 g m−2) values. The mean length of U. prolifera was not significantly different between sites, but the mean density of U. prolifera varied significantly between sites both spatially (p < 0.001) and temporally (p < 0.001).
Substratum heterogeneity
An analysis of particle size class from substratum samples shows a varied composition and a variable ratio of sand to silt, dominated by fine sands in the study area at the Muan intertidal flats (Table 1). In all the stations, very fine sands (3–4 Ø) outweighed coarse sands (0–1 Ø). From station I through station V, the percentage of sand decreased, with fine sand (2–3 Ø) outweighing coarse sands (0–1 Ø), while that of silt (>4 Ø) increased. Consequently, this led to the increased ratio of silt to sand. The ratio appeared to correspond closely with the population density of U. prolifera. Thus, when the relationship between U. prolifera density and ratio of sand to silt was investigated, by plotting logarithm of density against ratio, a significant relationship was obtained (p < 0.001, Fig. 4). The regression equation is: loge density = 0.7869x + 7.2247 loge ratio (r 2 = 0.6597).
Mean in spatial and temporal density (ind m−2) of U. prolifera during the study period between October 2006 and September 2007 on the intertidal flats of Muan, Korea. Legend from I to V represents stations in Fig. 2
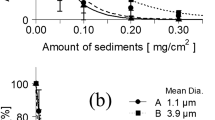
Growth and survival of U. prolifera in the laboratory
Ulva prolifera zoospores were allowed to germinate during exposure to the experimental conditions. Survival of U. prolifera zoospores was calculated from the number of germlings present in each culture dish after 30 days (Fig. 5). Survival rates ranged from 0% in the <63 μm Ø (silt) to a maximum of 88% in the 500–1,000 μm Ø (coarse sand) substrates (Table 3). Three of the four sediment types (very fine sand, fine sand, coarse sand) generally supported high survival rates of zoospores. The density of U. prolifera on the experimental culture dish ranged from 0 to 15 ± 6 ind cm−2. One-way ANOVA showed significant differences in density between the four sediment treatment groups (F3,12 = 35.429, p < 0.001), with the coarse sediment exhibiting the highest density.
Discussion
The density of U. prolifera plants on the intertidal mudflat shows a significant spatial and temporal variation, with peak density in station II. In the study area, U. prolifera inhabits a wider range of substrata containing from 44% to 93% sand (>2.0 mm) to 7–56% silt (<0.063 mm), suggesting that the Ulva occurred in sediment dominated by coarse particles. It has been observed for Ulva at Kochi, Japan (Ohno and Miyanoue 1980).
Studies on sediment dynamics in seaweed beds have focused mainly on depositional processes (Madsen et al. 2001). It has been shown that sediments may reduce survival and growth of seaweed propagules after settlement through the processes of scour and burial (D’Antonio 1986). Few studies describe the influence of substrate particle size on seaweeds without the smothering effects of sediment deposition. On the Muan flats, the population density of U. prolifera shows significant spatial variation, with the highest density occurring at station II, which also had the coarsest substrate (Fig. 2). However, the algal population inhabits a wide range of substrata containing from 54% to 93% sand (>0.063 mm) to 7% to 56% silt (<0.063 mm). The interaction between sediment dynamics, hydrodynamics, and macrophytes is complex. By a multitude of interactions, the sedimentary environment and hydraulic environment affect macrophytes, which in turn have direct effects on the physical environment (Best et al. 2001; Van Duin et al. 2001). Despite this complexity, most of the basic interactions have at least been conceptualized at both the individual plant and stand levels. However, there are only limited studies that provide numerical data to quantify the conceptual relationships. In several instances, results from field or laboratory are not only disparate in numerical value, but also often contradictory in result.
The laboratory experiments demonstrated that the granular composition of sediments affected the survival of U. prolifera germlings: Germlings did not survive in the fine silt treatment, which may be because the fine sediments used in this experiment had particle sizes on the same scale or smaller than the germlings and were so densely packed that few interstices remained in which the germlings could establish. In contrast, grains of coarse sand sediments (500–1,000 μm) were significantly larger than germlings at the beginning of the experiment and, even when compacted, which led to a structure rich in interstitial spaces. Sediments in the Petri dishes were not subjected to resuspension, and therefore, survival rates were not affected by burial or scouring effects that plants may be subject to in the wild. The fact that macroalgal assemblages can spatially dominate sediment-influenced rocky shore habitats despite such negative effects has been attributed to specific morphological and life cycle features, to life history strategies, and to indirect advantages in the presence of herbivores (Chapman and Fletcher 2002).
Regression analysis for U. prolifera survival indicated a significant inverse relationship between loge ratio of sand to silt and loge density in the field (Fig. 4). This confirmed the preference of sandy substrata with a ratio of sands to silt in favor of sand. Other in situ studies of U. prolifera have also shown that community biomass increases with increasing substratum particle size (Ohno and Miyanoue 1980). Undaria pinnatifida and Ecklonia cava exhibited a 50% reduction in settlement efficiency when a very thin (0.008 mm) layer of fine sediment was present (Arakawa 2005; Arakawa and Matsuike 1992). Devinny and Volse (1978) reported that Macrocystis spores, which were released into a Petri dish together with fine sand filtered through a 74-μm mesh strainer, hardly attached when sand was deposited at 10 mg cm−2. Our results show that benthic macroalgae distribution patterns in soft bottom coastal environments influenced at least one of two categories. First, sediment deposition on settlement surfaces may prevent the attachment of propagules. Second, sediments may reduce survivorship and growth of seaweed propagules after settlement through the processes of scour and burial (D’Antonio 1986).
Marked differences on survival rate of the algal zoospores were apparent among the experimental sediment types having different particle sizes (Table 3). The survival rate of the zoospores decreased with decreasing particles size, falling to 67% when the particle size was 125–250 μm and 22% at 63–125 μm. In silt treatments with a mean particle size <63 μm, no growth was observed after30 days of culture.
The ability of seaweed spores to attach to a substrate has been shown to vary depending on the particle size on the substrate. The study has demonstrated that attachment and survival of zoospores of U. prolifera is dependent on particle size. By analyzing the quality of sediment in the intertidal flats, it should therefore be possible to estimate the potential for successful stock enhancement of this species within a given estuary.
References
Airoldi L (1998) Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 79:2759–2770
Airoldi L, Cinelli F (1997) Effects of sedimentation on subtidal macroalgal assemblages: an experimental study from a Mediterranean rocky shore. J Exp Mar Biol Ecol 215:269–288
Albrecht AS (1998) Soft bottom versus hard rock: community ecology of macroalgae on intertidal mussel beds in the Wadden Sea. J Exp Mar Biol Ecol 229:85–109
Arakawa H (2005) Lethal effects caused by suspended particles and sediment load on zoospores and gametophytes of the brown alga Eisenia bicyclis. Fish Sci 71:133–140
Arakawa H, Matsuike K (1992) Influence on insertion of zoospores, germination, survival, and maturation of gametophytes of brown algae exerted by sediments. Nippon Suis Gakk 58:619–625
Best EPH, Buzzelli CP, Bartell SM, Wetzel RL, Boyd WA, Doyle RD, Campbell KR (2001) Modeling submersed macrophyte growth in relation to underwater light climate: modeling approaches and application potential. Hydrobiologia 444:43–70
Buschmann AH, Briganti F, Retamales CA (1997) Intertidal cultivation of Gracilaria chilensis (Rhodophyta) in southern Chile: long term invertebrate abundance patterns. Aquaculture 156:269–278
Chapman AS, Chapman ARO (1999) Effects of cordgrass on saltmarsh fucoids: reduced desiccation and light availability, but no changes in biomass. J Exp Mar Biol Ecol 238:69–91
Chapman AS, Fletcher RL (2002) Differential effects of sediments on survival and growth of Fucus serratus embryos (Fucales, Phaeophyceae). J Phycol 38:894–903
D’Antonio CM (1986) Role of sand in the domination of hard substrata by the intertidal alga Rhodomela larix. Mar Ecol Prog Ser 27:263–275
Devinny JS, Volse LA (1978) Effects of sediments on the development of Macrocystis pyrifera gametophytes. Mar Biol 48:343–348
Friedman GM, Sanders JE (1978) Principles of sedimentology. Wiley, New York
Informatization Officer (2006) Statistical yearbook of maritime affairs and fisheries. Ministry of Maritime Affairs and Fisheries, Seoul
Kang TH, Yoo SH, Lee SW, Choi OI, Lee CB (2008) A study on the habitat use of waterbirds and grading assessment of the tidal flat at Muan Bay in Jeollanamdo, Korea. Kor J Env Ecol 22:521–529
Kendrick GA (1991) Recruitment of coralline crusts and filamentous turf algae in the Galapagos archipelago: effect of simulated scour, erosion and accretion. J Exp Mar Biol Ecol 147:47–63
Littler MM, Martz DR, Littler DS (1983) Effects of recurrent and deposition on rocky intertidal organisms: importance of substrate heterogeneity in a fluctuation environment. Mar Ecol Prog Ser 11:129–139
Lyngby JE, Mortensen SM (1996) Effects of dredging activities on growth of Laminaria saccharina. Mar Ecol PSZNI 17:345–354
Madsen JD, Chambers PA, James WF, Koch EW, Westlake E (2001) The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444:71–84
Norton TA (1978) The factors influencing the distribution of Saccorhiza polyschides in the region of Lough Ine. J Mar Biol Assoc UK 58:527–536
Ohno M, Miyanoue K (1980) The ecology of the food alga Enteromorpha prolifera. Rep Usa Mar Biol Inst 2:11–17
Pang SJ, Lin F, Shan TS, Xu N, Zhang ZH, Gao SQ, Chopin T, Sun S (2010) Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar Env Res 69:207–215
Renaud PE, Riggs SR, WGJr A, Schmid K, Syster DA (1997) Biological–geological interactions: storm effects on macroalgal communities mediated by sediment characteristics and distribution. Continental Shelf Res 17:37–56
Ryu SK, Kim JY, You HS (2000) Seasonal variation and transport pattern of suspended matters in semiclosed Muan Bay, southwestern coast of Korea. J Kor Ear Sci Soc 21:128–136
Runcie JW, Ritchie RJ, Larkum AWD (2003) Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat Bot 76:155–174
Seapy RR, Littler MM (1982) Population and species diversity fluctuations in a rocky intertidal community relative to severe aerial exposure and sediment burial. Mar Biol 71:87–96
Sfriso A, Marcomini A (1996) Decline of Ulva growth in the lagoon of Venice. Biores Technol 58:299–307
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practices of statistics in biological research, 3rd edn. W.H. Freeman, San Francisco
Umar MJ, McCook LJ, Price IR (1998) Effects of sediment deposition on the seaweed Sargassum on a fringing coral reef. Coral Reefs 17:169–177
Van Duin EHS, Blom G, Los FJ, Maffione R, Zimmerman R, Cerco CF, Dortch M, Best EPH (2001) Modeling underwater light climate in relation to sedimentation, resuspension, water quality and autotrophic growth. Hydrobiologia 444:25–42
Yoon JT, Cho YC, Gong YG (2003) A study on the cultivation of Enteromorpha prolifera (Müller) J. Agardh, Chlorophyta in Korea. J Aquault 16:44–50
Acknowledgments
We are grateful to Professor J.H. Chang (Mokpo National University, Korea) for analyzing particle size and Dr. Philip Heath (NIWA, New Zealand) for reviewing the English. This work was supported by NFRDI (RP-2010-AQ-014) and a grant from Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093828).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, C.S., Hwang, E.K. An investigation of the relationship between sediment particles size and the development of green algal mats (Ulva prolifera) on the intertidal flats of Muan, Korea. J Appl Phycol 23, 515–522 (2011). https://doi.org/10.1007/s10811-010-9620-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9620-9