Abstract
The seagrass Posidonia oceanica from the Mediterranean coast and the brown algae Durvillaea antarctica and Lessonia nigrescens from the Chilean coast were analysed for total arsenic and for arsenic species. Arsenic species were extracted with water and measured by high-performance liquid chromatography–inductive coupled plasma mass spectrometry. Arsenite, arsenate, methylarsonate, dimethylarsinate, sulfonate sugar, sulphate sugar and phosphate sugar were analysed with an anion exchange column. Arsenobetaine, arsenocholine and glycerol sugar were separated with a cation exchange column. An extract of Fucus serratus was used to assign the arsenosugar peaks in the chromatograms from the sample extracts. In addition to the four common arsenosugars found in algae, inorganic arsenic was also measured in some samples, with the highest content found in L. nigrescens. The certified reference material (CRM) NIES no. 9 Sargassum fullvellum was analysed for quality assessment of total arsenic, and moreover, arsenosugars were also determined in this CRM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is widely known that the toxic effects of arsenic depend on the chemical form of its compounds occurring in living organisms (Craig et al. 2003). This has led to growing interest in the identification and quantification of arsenic compounds in materials related to human and animal food, among them algae and seagrasses. In these organisms, arsenic can be taken up, bio-accumulated and bio-transformed. Therefore, arsenic concentrations are often much higher (up to four or five orders of magnitude) in such organisms than in their surrounding environment (Edmonds and Francesconi 2003; Robinson et al. 2006). Most of this arsenic occurs as organic forms, which depend on the species of algae, the geographical location and seasonal variations (Lai et al. 1998). The mechanisms that control the transformation of inorganic arsenic into organic forms are not fully understood, although several hypotheses have been proposed (Geiszinger et al. 2001; Dembitsky and Rezanka 2003; Edmonds and Francesconi 2003). Arsenosugars, (dimethylarsinoylribosides or trimethylarsinoylribosides) are reported to be the most abundant compounds in seagrasses and algae (Edmonds and Francesconi 1981). The structures of the four most reported arsenosugars found in algae are presented in Fig. 1, together with their commonly used names. Arsenosugars are considered much less toxic than the inorganic forms of arsenic, although their toxicity has not been well assessed. Several studies based on the presence of arsenic metabolites in humans and in some animals point to indirect toxicity resulting from the ingestion of algae containing arsenosugars (Andrewes et al. 2004; Francesconi et al. 2002; Hansen et al. 2003). In many countries, algae form part of the human diet, either through direct ingestion or as a food supplement, owing to their high contents of iodine, minerals and vitamins. Therefore, we need to quantify the potentially toxic arsenic forms. To do this, analytical speciation data are essential.
The present study reports, for the first time, data on arsenic speciation in three marine organisms: Posidonia oceanica (L.) Delile, a marine flowering plant that is widespread in Mediterranean coastal waters, and two brown algae that are abundant on the Chilean coast, named Lessonia nigrescens Bori and Durvillaea antarctica (Chamisso) Hariot.
To carry out the study, water has been used as extracting reagent, and high-performance liquid chromatography–inductively coupled plasma mass spectrometry (HPLC-ICPMS), using both anionic and cationic chromatographic systems, has been used for quantifying the arsenic species found. An extract from Fucus serratus is used for the identification of arsenosugars (Madsen et al. 2000). The NIES CRM 9 Sargassum fullvellum reference material, certified for total arsenic, is analysed to assess the quality of data, and moreover, the detection and quantification of arsenosugars in this certified reference material (CRM) is carried out for the first time.
The selected organisms to carry out the present study can be considered of great interest as P. oceanica is a well-recognised bioindicator of seawater pollution (Gosselin et al. 2006; Lafabrie et al. 2007). The two algae were selected since L. nigrescens and, especially, D. antarctica are healthy and nutritious edible seaweeds for humans and animals (Ortiz et al. 2006) and are also used as a natural alginate source (Skjermo et al. 2006; Vásquez 2008). It is reported that L. nigrescens has high sorption capacity of arsenic from the surrounding aquatic media, as is shown in samples collected in the Valparaiso Bay, Chile (Hansen et al. 2006). In the present paper, we observed this absorption capacity in samples collected in the same zone, and at the same time, we can compare this capacity with the lower sorption capacity observed in D. antarctica since both species share their habitat in some zones in the Chilean coast and show competitive interactions (Westermeier et al. 1994).
Materials and methods
Standards and reagents
All solutions were prepared with doubly deionised water (18.2 MΩ cm−1 resistivity). Sixty-nine per cent nitric acid (Panreac, Hiperpur), 98% formic acid (Panreac, p.a.), ammonium dihydrogen phosphate (Panreac, p.a.) and 25% aqueous ammonia solution (Panreac, p.a.), pyridine (Scharlau, p.a.), 31% hydrogen peroxide (Merck, Selectipur) were used. Stock standard solutions (1,000 mg L−1) were prepared as follows: arsenite, from As2O3 (NIST Oxidimetric Primary Standard 83d, 99.99%) dissolved in 4 g L−1 NaOH (Merck, Suprapure); arsenate, from Na2HAsO4⋅7H2O (Carlo Erba) dissolved in water; methylarsonate (MA), prepared from (CH3)AsO(ONa)2⋅6H2O (Carlo Erba) dissolved in water; dimethylarsinate (DMA), prepared from (CH3)2AsNaO2⋅3H2O (Fluka) dissolved in water. Arsenocholine (AC) from (CH3)3As+(CH2)CH2OHBr− was supplied by the “Service Central d’Analyse” (CNRS Vernaison, France).
All the stock solutions were kept at 4°C, and further diluted solutions for the analysis were prepared daily. Arsenate, DMA, MA and AC were standardised against As2O3 (NIST Oxidimetric Primary Standard 83d) for our internal quality control.
The following CRM was used for quality control: arsenobetaine (AB), BCR CRM 626, standard solution (1,031 ± 6 mg AB per litre). Arsenic standard solution from High-Purity Standards and with a certified concentration of 1,000 ± 2 mg As per litre was used to determine total arsenic content with ICPMS.
NIES CRM 09 Sargasso (S. fullvellum) seaweed was from the National Institute for Environmental Studies with a certified total arsenic content of 115 ± 9.2 mg As per kilogram.
An aliquot of the extract of F. serratus containing the four most common arsenosugars (phosphate, sulphate, sulfonate and glycerol sugars) was used to assign the arsenosugar peaks in the chromatograms from the sample extracts and from the NIES CRM 9 extracts.
Instrumentation
A microwave digestion system, Milestone Ethos Touch Control, with a microwave power of 1,000 W and temperature control was used for digestion. An Agilent 7500ce ICPMS with a micro-flow nebuliser (Agilent, Germany) was used to measure total arsenic content.
Arsenic species were measured in aqueous sample extracts by HPLC-ICPMS. A Perkin Elmer 250 LC binary pump, equipped with a Rheodyne 7125 injector with a 50-µL loop, was used. The analytical columns Hamilton PRP-X100 (250 × 4.1 mm, 10 μm, Hamilton, USA) and Zorbax-SCX300 (150 × 4.6 mm, 5 μm, Agilent) were protected by guard columns filled with the corresponding stationary phases. The outlet of the HPLC column was connected via PTFE capillary tubing to a T-shape to which 1% nitric acid was added to dilute the mobile phase, and the outlet of the T-shape was connected to the nebuliser (cross-flow type) of the ICPMS system (Agilent 7500ce), which was the arsenic-selective detector. The ion intensity at m/z 75 (75As) was monitored using time-resolved analysis software. Additionally, the ion intensities at m/z 77 (40Ar37Cl and 77Se) were monitored to detect possible argon chloride (40Ar35Cl) interference at m/z 75. The chromatograms were exported, and the peak areas were calculated using homemade software running Matlab. Table 1 summarises the instrumental and experimental analytical conditions used in the study.
Sampling and sample pretreatment
P. oceanica
Approximately 5 kg of the fresh seagrass was collected in May 2006 from the Mediterranean coastline, close to Vandellós (Tarragona, Spain). The seagrass was carefully washed with double deionised water.
D. antarctica from Valparaiso Bay was acquired in a local market, and composite samples were obtained from several commercial units supplied by Dr. H.A. Hansen from the Federico Santamaria University in Valparaiso (Chile).
L. nigrescens
Approximately 10 kg of the fresh alga was collected in the Valparaiso Bay (Acapulco Beach) and washed with deionised water. After drying and homogenising, subsamples were also supplied by Dr. H.A. Hansen.
All subsamples selected for subsequent analysis were dried at 40°C for 24 h and ground to a fine powder in a tungsten carbide disc mill.
Procedures
Moisture determination
The moisture of all the samples was determined in duplicate by oven drying 0.5 g aliquots at 100 ± 5°C to constant weight. Moisture ranged from 10% to 17%. All the results in the present study refer to dry mass.
Total arsenic analysis
For the digestion, 0.2 g aliquots of the dried samples and the CRM (Sargassum fulvellum) were weighed to 0.1 mg in the digestion vessels, and 8 mL of concentrated nitric acid and 2 mL of hydrogen peroxide were added. Mixtures were digested according to the following programme: 10 min from room temperature to 90°C, maintained for 5 min at 90°C, 10 min from 90°C to 120°C, 10 min from 120°C to 190°C and 10 min maintained at 190°C. After cooling to room temperature, the digested samples were filtered through ash-free filter papers (Whatman 40) and diluted in water up to 20 mL. Total arsenic both in the digested algae and in the aqueous speciation extracts was measured by ICPMS (Agilent 7500ce) with He as the gas in the collision cell. Rh was used as the internal standard. Quantification was carried out using an external calibration curve.
Arsenic species analysis
The dried pulverised samples (0.5 g for Posidonia and 0.1 g for the other samples and the CRM) were weighed to 0.1 mg in 25-mL Teflon tubes. Ten millilitres of water was added to each tube. The tubes were placed in an end-over-end shaker operating at 30 rpm for 16 h at room temperature. The resulting mixtures were centrifuged and the supernatants filtered through PET filters (Chromafil® PET, Macherey-Nagel, pore size 0.22 μm). The extracts were analysed with HPLC-ICPMS for arsenite (As(III)), arsenate (As(V)), dimethylarsinic acid (DMA), methylarsonic acid (MA) and phosphate sugar, sulphate sugar and sulfonate sugar by anion exchange chromatography on the Hamilton PRP-X100 column using an aqueous solution of 20 mM NH4H2PO4 at pH 5.6 as mobile phase under the conditions described in Table 1. AB, AC and glycerol sugar were analysed in the extracts by cation exchange chromatography on the Zorbax 300-SCX using an aqueous solution of 20 mM pyridine at pH 2.6 as the mobile phase under the conditions described in Table 1. External calibration curves were used to quantify MA, DMA, arsenite and arsenate in the anion exchange column with the corresponding standards. AB and AC in the cation exchange column were quantified in the same way. ICPMS performance was optimised with a mobile phase solution containing 10 μg As per litre to give maximum response to the signal (m/z 75). The total As content in the aqueous extracts was also determined by ICPMS. Phosphate sugar, sulphate sugar and sulfonate sugar were quantified with the calibration curve of As(III), and glycerol sugar was quantified with the calibration curve of AC since the elemental response of the ICPMS detection system is independent of the species (Francesconi and Sperling 2005).
Results
The total arsenic contents are reported in Table 2 where it can be observed that the values cover a wide range of concentrations. In the analysis of the CRM (NIES 9), good agreement was obtained between the certified and the measured value, as can be observed in the table.
Table 2 shows the results obtained from the samples studied, which show a wide range of arsenic compounds and concentrations. Extraction efficiencies (calculated as the ratio of total arsenic in the water extract to total arsenic in the algae) ranged from 47% to 99%, with the lowest percentage corresponding to the seagrass P. oceanica. One of the most important parameters in analytical speciation using coupled techniques is the assessment of recovery of the arsenic compounds. Mass balance calculations have to be performed in terms of the so-called column recoveries, which are calculated from the ratios between the sum of the species eluted from the chromatographic column and the total arsenic in the extract. In the present study, the values obtained for column recovery ranged from 84% to 105%. Thus, these high percentages give confidence in the good performance of the chromatographic system applied.
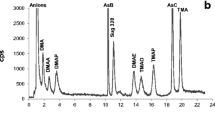
We used F. serratus in which several arsenosugars are well identified to assess accuracy in arsenosugar identification in all the sample extracts by following the described procedure. The results obtained are shown in Table 2 together with those reported by Madsen et al. (2000). As examples, the chromatograms obtained from anion and cation exchange chromatography from F. serratus extract are shown in Fig. 2. It shows the presence of phosphate, sulfonate and sulphate sugar in the anion exchange chromatogram and glycerol sugar in the cation exchange chromatogram.
In the analysis of the CRM NIES 9, S. fullvellum, most of the arsenic was also present as inorganic species (80%). Moreover, the four arsenosugars (sulfonate sugar, sulphate sugar, phosphate sugar and glycerol sugar) were also detected and quantified.
With respect to the arsenic compounds in P. oceanica, although the total arsenic content was very low, the major species found were arsenite and arsenate. It must be stressed that AB was also detected in this sample, accounting for 6% of the extracted arsenic.
The main species found in D. antarctica were the sulfonate sugar and glycerol sugar. These accounted for more than 90% of the arsenic extracted, and the percentage of inorganic arsenic was low. As some examples, chromatograms of P. oceanica and D. antarctica extracts with both anion and cation exchange chromatography are shown in Fig. 3. They reveal the presence of AB in the cation exchange chromatogram of P. oceanica extract and the major contribution of sulfonate sugar in the anion exchange chromatogram of D. antarctica.
In L. nigrescens, only the inorganic species arsenite and arsenate were found, with a higher proportion of the former (85%).
Discussion
Lessonia nigrescens showed the highest total arsenic content, exceeding the few values reported in the literature for this species, such as the range 38.5–53.51 mg kg−1, according to local and temporal variability (Vásquez and Guerra 1996) or 80 ± 14 mg kg−1 (Hansen et al. 2006). D. antarctica showed much lower levels of arsenic, and this is in agreement with other reported values found in the literature for the same species: Almela et al. (2006) reported 15.2 mg kg−1 and Runcie and Riddle (2004) 32 ± 8 mg kg−1 .The lowest level of arsenic in the present study was detected in the seagrass P. oceanica, which is also consistent with the values reported in the literature, such as 5 mg kg−1 (Grauby et al. 1991) or the range 14–21 mg kg−1 reported by Gosselin et al. (2006) for this seagrass collected in the west Mediterranean zone.
With respect to the experimental conditions for analytical speciation, we choose water as extractant since it is recommended for the more polar or ionic arsenic species in algae. With respect to the wide range of extraction efficiency of the arsenic species found, the values reported in the literature also cover wide ranges of percentages since this parameter, even using the same extractant, is highly dependent on the algae species (Shibata et al. 1990; Van Hulle et al. 2002); for example, 13.3–64.1% in algae from several geographical zones (Nischwitz and Pergantis 2005), 9.3–91.2% in algae from Hiroshima Bay (Hirata and Toshimitsu 2007) or 20.7–97% in algae from the Adriatic Sea (Slejkovec et al. 2006) have been reported.
Regarding quality control in the analyses, we would emphasise the following: with the aim of checking the proper quantification of the arsenosugars in all the extracts throughout the study, we also quantified these compounds in the extract of F. serratus, currently the only material available for quality control purposes, and the results in Table 2 show good agreement with those reported by Madsen et al. (2000). As mentioned before, the NIES CRM 9 S. fullvellum reference material, certified for total arsenic, is also analysed in terms of arsenic speciation, and the detection and quantification of arsenosugars in this CRM is investigated for the first time. Thus, the information obtained in the present study contributes to enlarge the knowledge on the arsenic compounds occurring in this material.
With respect to the presence of arsenic compounds found in the samples analysed, the following aspects can be highlighted. From the studies reported in the literature, no arsenic speciation studies have been carried out on P. oceanica, D. antarctica and L. nigrescens. It is interesting, however, to compare the results with some others reported in the literature on arsenic speciation in some seagrasses or algae of different species but belonging to the same genus. Thus, if we compare the results obtained for P. oceanica with those reported for Posidonia australis (Thomson et al. 2007), low levels of total arsenic are found in both species, and the presence of inorganic arsenic and some arsenosugars are in common. Also, the AB is detected in both, and we agree with the authors in the hypothesis that this compound could be attributed to the epiphytes, which are difficult to remove from the sample with the usual washing procedures (Slejkovec et al. 2006). This hypothesis would agree with others (Grotti et al. 2008; Nischwitz and Pergantis 2005). It is reported that in some periods of the year, the leaves of P. oceanica reach the maximum size and certain numbers are still complete and fully covered by epiphytes (Dauby and Poulicek 1995). In D. antarctica, the low value of inorganic arsenic is consistent with a study in which only total and inorganic arsenic was determined (Almela et al. 2006). The predominance of sulfonate sugar would be in agreement with reported data in which this sugar is predominant in brown algae in general (Francesconi et al. 1998). Regarding this alga, it has to be pointed out that the information in the literature on another species of the same genus, Durvillaea potatorum, a Tasmanian Kelp, in which inorganic arsenic, methylated species and the common four arsenosugars are also found (Kirby et al. 2004), although in this species, the total arsenic content is one order of magnitude higher than the value obtained in the present study for D. antarctica.
In L. nigrescens, the finding that this alga contains high levels of inorganic arsenic, which includes the most toxic arsenic species, makes it necessary to take special care when it is introduced into the diet.
Although the major arsenic compounds found in most of the algae studied in recent years are arsenosugars, high levels of inorganic arsenic species have also been reported in some algae species (Almela et al. 2006). We think that the results obtained in the present study contribute to increase the existing data on the presence of arsenic compounds in seagrasses and marine algae, especially those used for human consumption. This is especially important since nowadays, although it is known that high amounts of algae are consumed worldwide, a lack of legislation exists, as for example in the European Union, on the limits of arsenic and inorganic arsenic in edible seaweeds, as is claimed in the most recent literature (Besada et al. 2009)
References
Almela C, Jesus Clemente M, Velez D, Montoro R (2006) Total arsenic, inorganic arsenic, lead and cadmium contents in edible seaweed sold in Spain. Food Chem Toxicol 44:1901–1908
Andrewes P, DeMarini DM, Funasaka K, Wallace K, Lai VWM, Sun H, Cullen WR, Kitchin KT (2004) Do arsenosugars pose a risk to human health? The comparative toxicities of a trivalent and pentavalent arsenosugar. Environ Sci Technol 38:4140–4148
Besada V, Andrade JM, Schultze F, González JJ (2009) Heavy metals in edible seaweeds commercialised for human consumption. J Mar Syst 75:305–313
Craig PJ, Eng G, Jenkins RO (2003) Organometallic compounds in the environment. Wiley, Chichester
Dauby P, Poulicek M (1995) Methods for removing epiphytes from seagrasses: SEM observations on treated leaves. Aquat Bot 52:217–228
Dembitsky VM, Rezanka T (2003) Natural occurrence of arseno compounds in plants, lichens, fungi, algal species, and microorganisms. Plant Sci 165:1177–1192
Edmonds JS, Francesconi KA (1981) Arseno-sugars from brown kelp (Ecklonia radiata) as intermediates in cycling of arsenic in a marine ecosystem. Nature 289:602–604
Edmonds JS, Francesconi KA (2003) Organoarsenic compounds in the marine environment. In: Craig PJ (ed) Organometallic compounds in the environment, 2nd edn. Wiley, New York, pp 195–222
Francesconi KA, Sperling M (2005) Speciation analysis with HPLC-mass spectrometry: time to take stock. Analyst 130:998–1001
Francesconi KA, Goessler W, Panutrakul S, Irgolic KJ (1998) A novel arsenic containing riboside (arsenosugar) in three species of gastropod. Sci Total Environ 221:139–148
Francesconi KA, Tanggaard R, McKenzie CJ, Goessler W (2002) Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem 48:92–101
Geiszinger A, Goessler W, Pedersen SN, Francesconi KA (2001) Arsenic biotransformation by the brown macroalga Fucus serratus. Environ Toxicol Chem 20:2255–2262
Gosselin M, Bouquegneau J, Lefebvre F, Lepoint G, Pergent G, Pergent-Martini C, Gobert S (2006) Trace metal concentrations in Posidonia oceanica of North Corsica (northwestern Mediterranean Sea): use as a biological monitor? BMC Ecology 6:12. doi:10.1186/1472-6785-6-12
Grauby A, Augier H, Lion R, Charlent O (1991) Neutron activation analysis of elemental composition in a marine phanerogam, Posidonia oceanica (L.) Delile: a biological indicator of pollution. Environ Exp Bot 31:255–265
Grotti M, Soggia F, Lagomarsino C, Goessler W, Francesconi KA (2008) Arsenobetaine is a significant arsenical constituent of the red antarctic alga Phyllophora antarctica. Environ Chem 5:171–175
Hansen HR, Raab A, Feldmann J (2003) New arsenosugar metabolite determined in urine by parallel use of HPLC-ICP-MS and HPLC-ESI-MS. J Anal At Spectrom 18:474–479
Hansen HK, Ribeiro A, Mateus E (2006) Biosorption of arsenic(V) with Lessonia nigrescens. Miner Eng 19:486–490
Hirata S, Toshimitsu H (2007) Determination of arsenic species and arsenosugars in marine samples by HPLC-ICP-MS. Appl Organomet Chem 21:447–454
Kirby J, Maher W, Ellwood M, Krikowa F (2004) Arsenic species determination in biological tissues by HPLC-ICP-MS and HPLC-HG-ICP-MS. Aust J Chem 57:957–966
Lafabrie C, Pergent G, Kantin R, Pergent-Martini C, Gonzalez J (2007) Trace metals assessment in water, sediment, mussel and seagrass species—validation of the use of Posidonia oceanica as a metal biomonitor. Chemosphere 68:2033–2039
Lai VW, Cullen WR, Harrington CF, Reimer KJ (1998) Seasonal changes in arsenic speciation in Fucus species. Appl Organomet Chem 12:243–251
Madsen AD, Goessler W, Pedersen SN, Francesconi KA (2000) Characterization of an algal extract by HPLC-ICP-MS and LC-electrospray MS for use in arsenosugar speciation studies. J Anal At Spectrom 15:657–662
Nischwitz V, Pergantis SA (2005) First report on the detection and quantification of arsenobetaine in extracts of marine algae using HPLC-ES-MS/MS. Analyst 130:1348–1350
Ortiz J, Romero N, Robert P, Araya J, Lopez-Hernandez J, Bozzo C, Navarrete E, Osorio A, Rios A (2006) Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem 99:98–104
Robinson B, Marchetti M, Moni C, Schroeter L, van den Dijssel C, Milne G, Bolan N, Mahimairaja S (2006) Arsenic accumulation by aquatic and terrestrial plants. In: Naidu R et al (eds) Managing arsenic in the environment: from soil to human health. CSIRO, Adelaide, pp 235–252
Runcie JW, Riddle MJ (2004) Metal concentrations in macroalgae from east Antarctica. Mar Pollut Bull 49:1114–1119
Shibata Y, Jin K, Morita M (1990) Arsenic compounds in the edible red alga, Porphyra tenera, and in nori and yakinori, food items produced from red algae. Appl Organomet Chem 4:255–260
Skjermo J, Storseth TR, Hansen K, Handa A, Oie G (2006) Evaluation of b-(1->3, 1->6)-glucans and high-M alginate used as immunostimulatory dietary supplement during first feeding and weaning of Atlantic cod (Gadus morhua L.). Aquaculture 261:1088–1101
Slejkovec Z, Kapolna E, Ipolyi I, van Elteren JT (2006) Arsenosugars and other arsenic compounds in littoral zone algae from the Adriatic Sea. Chemosphere 63:1098–1105
Thomson D, Maher W, Foster S (2007) Arsenic and selected elements in inter-tidal and estuarine marine algae, south-east coast, NSW, Australia. Appl Organometal Chem 21:396–411
Van Hulle M, Zhang C, Zhang X, Cornelis R (2002) Arsenic speciation in Chinese seaweeds using HPLC-ICP-MS and HPLC-ES-MS. Analyst 127:634–640
Vásquez JA (2008) Production, use and fate of Chilean brown seaweeds: resources for a sustainable fishery. J Appl Phycol. doi:10.1007/s10811-007-9308-y
Vásquez JA, Guerra N (1996) The use of seaweeds as bioindicators of natural and anthropogenic contaminants in northern Chile. Hydrobiologia 326(327):327–333
Westermeier R, Müller DG, Gómez I, Rivera P, Wenzel H (1994) Population biology of Durvillaea antarctica and Lessonia nigrescens (Phaeophyta) on the rocky shores of southern Chile. Mar Ecol Prog Ser 110:187–194
Acknowledgements
The authors thank the DGICYT (project no. CTQ2007-62261/BQU) for financial support. We also thank Dr Toni Padró from the Serveis Scientifico Tècnics de la Universitat de Barcelona for his valuable support with ICPMS measurements. The authors also thank Dr. Kevin A. Francesconi for the kind donation of the F. serratus extract. The authors thank Dr. H. Hansen for the kind donation of the algae samples from the Chilean coast. The authors are very grateful to Dr. M. Barbero from the Vegetal Biology Department of the Universitat de Barcelona for her invaluable help on botanical knowledge. Dr. M.J. Ruiz-Chancho is grateful to the Universitat de Barcelona for the support given through a pre-doctoral grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz Chancho, M.J., López Sánchez, J.F. & Rubio, R. Occurrence of arsenic species in the seagrass Posidonia oceanica and in the marine algae Lessonia nigrescens and Durvillaea antarctica . J Appl Phycol 22, 465–472 (2010). https://doi.org/10.1007/s10811-009-9480-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9480-3





