Abstract
We developed a rapid and effective procedure for scanning electron microscopy of three delicate dinoflagellates, Karlodinium micrum, Akashiwo sanguinea, and Heterocapsa niei. Good results were obtained when the specimens were fixed with a modified Párducz’s fixative (2% osmium tetroxide:saturated mercuric chloride = 5:1 v/v) for 10 min, washed in 0.05 M sodium cacodylate trihydrate buffer for 2 min, dehydrated in tert-butanol for 10 min and dried with hexamethyldisilazane in air for 3 min in a fume hood because reagents are very toxic. This method could be completed in 25 min. Compared with other preparative techniques, the present protocol has significant advantages for SEM observation by limiting distortion of delicate specimens and reducing the preparation time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free-living dinoflagellates are a very old and successful haplontic group of eukaryotic microorganisms adapted to a variety of pelagic and benthic habitats from arctic to tropical seas and estuaries as well as fresh to hypersaline waters (van den Hoek et al. 1995). Dinoflagellates were first described during the late 1800s, based on light microscope observations. However, morphological identification characteristics (i.e., cingular position, sulcal placement, apical groove, and peducle) are difficult to resolve by light microscopy. For accurate identification the use of scanning electron microscopy (SEM) is needed (Steidinger et al. 1996a, b). Moreover, unarmored dinoflagellates lack thick cellulosic plates in the thecal vesicles, and cannot adequately be preserved for SEM studies using a general preparation procedure involving pre- and post-fixation, and critical point drying (CPD; Smith and Finke 1972).
The general preparation procedure for SEM can frequently result in distortion of the cell wall during fixation and drying procedures. To overcome these problems, alternative preparative techniques have been developed such as freezing methods (Huang et al. 1994; Robards and Sleytr 1985; Sargent 1988; Suzuki et al. 1995). A chemical preparation method using new fixing and drying reagents has reduced surface tension during evaporating, and has been effectively used to dry soft materials including delicate microalgae, insect tissues, rat hepatic endothelial cells, and the cilia of rat trachea (e.g., Botes et al. 2002; Braet et al. 1997; Bray et al. 1993; Nation 1983). These methods have the advantage of simplicity, limited equipment requirement and are low cost compared with freezing methods. However, further improvements are needed to enhance the maintenance of original cell morphology and save preparation time. Therefore, the present study aimed to develop a more rapid, simple, and effective technique for preparing samples for SEM.
Materials and methods
Delicate dinoflagellates including the unarmored Karlodinium micrum and Akashiwo sanguinea and the armored Heterocapsa niei were isolated from coastal waters of South Korea using a capillary method. The organisms were incubated in 300-mL flasks containing 100 mL of f/2 medium (Guillard and Ryther 1962) with shaking (30 rpm) at 25°C and 40 μmol photons m−2 s−1 under 12:12 light:dark cycles. Cells were grown in log phase to a density greater than 103 cells mL−1.
The procedure developed is shown in Fig. 1. All reagents were carefully used in a fume hood because the reagents are hazardous. Live cells were fixed with modified Párducz’s fixative (Párducz 1967) at 1:1 v/v for 10 min at room temperature: the fixative comprised 2% solution of osmium tetroxide (75632, Sigma) in filtered seawater and a saturated solution of mercuric chloride (M1136, Sigma) in distilled water mixed in the ratio of 5:1 v/v, respectively. Fixed cells were harvested by gravity filtration) onto a 2.0 μm polycarbonate membrane (TTTP, Millipore). To prevent the formation of NaCl crystals, any seawater remaining associated with the specimen was removed by washing for 2 min at room temperature with drops of distilled water followed by drops of 0.05 M sodium cacodylate trihydrate buffer (pH 8.0). For dehydration, drops of tert-butanol were continuously dripped on the specimen for 10 min at 30°C. Following this procedure, several drops of hexamethyldisilazane (H4875, Sigma) were immediately dispensed onto the membrane to complete the drying process. Finally, the specimens were coated with gold–palladium for 3 min, and examined using a SEM (JSM-5600 LV; Jeol). The entire fixation procedure could be completed in 25 min.
Results and discussion
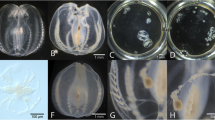
SEM images of the three delicate dinoflagellate species are shown in Fig. 2. Cells of K. micrum were well preserved with very little morphological distortion (Fig. 2a); key identification characteristics of this species were clearly evident, including the apex, apical groove, longitudinal flagellum, transverse flagellum, and sulcus (Fig. 2b). A cell of A. sanguinea had a pentagonal shape with a broadly conical epitheca and a bilobed hypotheca (Fig. 2c), and a transverse flagellum in a transverse cingulum (Fig. 2d). H. niei, a delicate armored dinoflagellate, is shown in ventral and dorsal views in Fig. 2e and f, respectively. The cell had almost equal-sized rounded to conical epitheca and rounded to attenuated hypotheca, and was slightly compressed dorsoventrally; thin thecal plates and triangular-shaped scales were also evident.
SEM photographs of three delicate dinoflagellates fixed using the procedure described in this study. a and b label the taxonomically diagnostic ventral pore on Karlodinium micrum showing the apex (a), apical groove (ag), cingulum (c), longitudinal flagellum (lf), sulcus (s) and transverse flagellum (tf). c, d Akashiwo sanguinea showing the apex, antapex, cingulum, sulcus and transverse flagellum. e and f Label plates on Heterocapsa niei showing the apex, antapex, cingulum, longitudinal flagellum, sulcus, and transverse flagellum. Scale bars are 5 μm
When comparing the efficacy of the fixatives Párducz and OsO4 using the same fixation time (10 min), the Párducz fixative caused little distortion of a cell wall of K. micrum, while the OsO4 caused cell wall damage of the species examined (figure not shown). As a primary fixative, OsO4 causes gross permeabilization of the membrane, with cessation of cytoplasmic movement occurring within seconds to minutes, but this is one of the slowest penetrating fixatives (Dykstra and Reuss 2003). To overcome this problem, Párducz (1967) added HgCl2 to OsO4 as a fixative, to help reduce the fixation time as well as to increase cell hardness. Takahashi et al. (2004), for example, obtained good results after applying this fixative for approximately 1 min (Table 1).
tert-Butanol can effectively remove water in a cell and reduce the dehydration time. Although ethanol continues to have the widest application as a dehydrating agent, its use does not completely remove water from the cell (Luft and Wood 1963). Moreover, cell shrinkage is frequently associated with ethanol use, and the dehydration time may exceed 1 h (Hanstede and Gerrits 1983). The use of tert-butanol has been introduced in freeze drying because of its high freezing point (25.5°C) and vapor pressure (Inoue and Osatake 1987), and it has been recommended as a more effective dehydration agent than ethanol (Handa et al. 1998; Suzuki et al. 1995).
Drying using CPD has been the general approach to biological SEM since its inception and, despite advances in cryopreservation techniques (e.g., Craig and Beaton 1996), continues to dominate the field (Dykstra and Reuss 2003). However, this procedure has the disadvantages of being time-consuming and causing cell shrinkage. HMDS can reduce the collapse and distortion of delicate specimens during drying. The reagent cross-links proteins, reducing the surface tension and giving strength to the sample during air-drying (Nation 1983). In contrast, CPD causes shrinkage due to vigorous solvent exchange with the cells, and temperature and pressure changes (Boyde 1980). The use of HMDS for SEM observations of delicate specimen produced excellent results.
Compared with other preparative techniques (Table 1), the present method has a number of advantages.
-
1.
It is rapid and effective. Our procedure minimized preparation time (approximately 25 min) and cell distortion. To our knowledge, the present study may represent the shortest time consumed for preparing SEM.
-
2.
Air-drying involves HMDS, which reduces the shrinkage of delicate specimens
-
3.
The use of tert-butanol is effective in removing water in a cell as well as decreasing the dehydration time.
-
4.
Párducz’s fixative can enhance rapidly the rigidness of a soft cell wall.
References
Bergholtz T, Daugbjerg N, Moestrup Ø, Fernándex-Tejedor M (2005) On the identity of Karlodinium veneficum and description of Karlodinium armiger sp. nov. (Dinophyceae), based on light and electron microscopy, unclear-encoded LSU rDNA, and pigment composition. J Phycol 42:170–193
Botes L, Price B, Waldron M, Pitcher GC (2002) A simple and rapid scanning electron microscope preparative technique for delicate “Gymnodinioid” dinoflagellates. Microsc Res Tech 59:128–130
Boyde A (1980) Electron Microscopy. Vol. II. In: Brederoo P, de Priester W (eds) Review of basic preparation of animal tissues for surface-scanning electron microscopy. Electron Microscopy Foundation, Leiden, pp 768–777
Braet F, De Zanger R, Wisse E (1997) Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Microsc 186:84–87
Bray DR, Bagu J, Koegler P (1993) Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens. Microsc Res Tech 26:489–495
Craig S, Beaton CD (1996) A simple cryo-SEM method for delicate plant tissues. J Microsc 182:102–105
de Salas MF, Laza-martinez A, Hallegraeff GM (2008) Novel unarmored dinoflagellates from the toxigenic family Kareniaceae (gymnodiniales): five new species of Karlodinium and one new Takayama from the Australian sector of the southern ocean. J Phycol 44:241–257
Dykstra MJ, Reuss LE (2003) Biological electron microscopy: Theory techniques and troubleshootingd, 2nd edn. Klumer, New York
Eisenback JD (1986) A comparison of techniques useful for preparing nematodes for scanning electron microscopy. J Nematol 18:479–487
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Handa A, Takahashi K, Kuroda N, Froning GW (1998) Heat-induced egg white gels as affected by pH. J Food Sci 63:403–407
Hanstede JG, Gerrits PO (1983) The effects of embedding in water-soluble plastics on the final dimensions of liver sections. J Microsc 131:79–86
Huang CX, Canny MJ, Oates K, McCully M (1994) Planing frozen hydrated plant specimens for SEM observation and EDX microanalysis. Microsc Res Tech 28:67–74
Inoue T, Osatake H (1987) An epoch-making specimen-drying method superceding a critical point drying one. Arch Histol Cytol 51:53–59
Iwataki M, Botes L, Sawaguchi T, Sekiguchi K, Fukuyo Y (2003) Cellular and body scale structure of Heterocapsa ovata sp. nov. and Heterocapsa orientalis sp. nov. (Peridiniales, Dinophyceae). Phycologia 42:629–637
Kageyama A, Poonwan N, Yazawa K, Mikami Y, Nishimura K (2004) Nocardia asiatica sp. nov. isolated from patients with nocardiosis in Japan and clinical specimens from Thailand. Int J Syst Evol Microbiol 54:125–130
Luft JH, Wood RL (1963) The extraction of tissue protein during and after fixation with osmium tetroxide in various buffer systems. J Biophys Biochem Cytol 19:46a
Nation JL (1983) A new method using hexamethyldisilazane for preparation of soft insect tissues for SEM. Stain Technol 58:347–351
Párducz B (1967) Ciliary movement and coordination in ciliates. Int Rev Cytol 21:91–128
Robards AW, Sleytr UB (1985) Practical methods in electron microscopy. In: Glauert AM (ed) Low temperature methods in biological electron microscopy. Elsevier, Amsterdam, pp 5–146
Sargent JA (1988) Low temperature scanning electron microscopy: advantages and applications. Scanning Microsc 2:835–849
Smith ME, Finke EH (1972) Critical point drying of soft biological material for the scanning electron microscope. Invest Ophthalmol Vis Sci 11:127–132
Steidinger KA, Burkholder JAM, Glasgow HB, Hobbs CW, Garrett JK, Truby EW, Noga EJ, Smith SA (1996a) Pfiesteria piscicida gen. et sp. nov. (Pfiesteriaceae fam. nov.) a new toxic dinoflagellate with a complex life cycle and behavior. J Phycol 32:157–164
Steidinger KA, Landsberg JH, Truby EW, Blakesley BA (1996b) The use of SEM for identification of small gymnodinioid dinoflagellates. Nova Hedwigia 112:415–422
Sutton NA, Hughes N, Handley PS (1994) A comparison of conventional SEM techniques, low temperature SEM and the electroscan wet scanning electron microscope to study the structure of a biofilm of Streptococcus crista CR3. J Appl Microbiol 76:448–454
Suzuki T, Shibata M, Tanaka K, Tsuchida K, Toda T (1995) A new drying method: low vacuum SEM freeze drying and its application to plankton observation. Bull Plankton Soc Japan 42:53–62
Takahashi T, Miyoshi N, Suzuki T, Sunahara T (2004) Growth and encystment of the ciliate Tetrahymena sp. Found in a dead mosquito’s larva. JEOL news 39:16–19
van den Hoek C, Mann DG, Jahns HM (1995) Algae: an introduction to phycology. Cambridge University Press, Cambridge
Acknowledgment
We thank Park, Sung Hwan in Sangmyung University and Cho, Soo-Yeon in Hanyang University for technical assistance. This work was supported by “The survey of Indigenous Biology of Korea” from National Institute of Biological Resources (NIBR) of South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, S.W., Joo, H.M., Park, J.S. et al. Development of a rapid and effective method for preparing delicate dinoflagellates for scanning electron microscopy. J Appl Phycol 22, 313–317 (2010). https://doi.org/10.1007/s10811-009-9461-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9461-6