Abstract
The process of metal bioaccumulation in marine food chains is poorly understood because very little data is available on metal concentration at different trophic levels and their temporal or spatial variation. Because of that, we were interested to (1) determine the concentration range of heavy metals in seaweed and seagrasses species in Magdalena Bay; (2) describe the spatial and temporal variation of heavy metal concentrations in the seaweeds and seagrasses. Seasonal collections were done at Estero Banderitas in November 2004, February, and April 2005 wherein we divided the estuary into three major areas (upper, middle, and lower), and within each area, two sites were selected. Our results showed that iron, copper, and magnesium were the most significant metals found in seagrasses, red, and green algae. We found significant more variation in temporal heavy metal concentrations in relation to the maximum abundance in the samples and spatial variation in relation to the studied taxa suggesting that hervibores have a differential intake of the metals. Also, our results suggest that heavy metals might be incorporated regularly in the diet of many herbivorous animals with severe consequences to their health. Management strategies for these species should consider monitoring the levels of metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals occur naturally in the environment (Sparling et al. 2000) as part of the biogeochemical cycles (Sánchez-Rodríguez et al. 2001), and it is often difficult to differentiate between natural and anthropogenic sources (Kieffer 1991; Moreno 2003). In marine systems, natural processes (e.g., upwelling, river runoff) can redistribute and concentrate heavy metals in the environment, occasionally reaching toxic levels (Sparling et al. 2000; Machado et al. 2002). The effects of these processes may vary over seasonal and spatial scales (Sawidis et al. 2001). An understanding of temporal and spatial changes in metal concentrations in the environment can aid in determining the sources as biomonitors (Szefer et al. 1998; Páez-Osuna et al. 2000), and ultimately their effects on wild life (Sparling et al. 2000; Talavera-Saenz et al. 2007). Also, they can be used for biosorption in contaminated waters (Kumar and Kaladharan 2006). Caliceti et al. (2002) found a decrease in Zn and Cd concentrations from the center of a lagoon, close to an industrial district, towards the Venice lagoon (Italy) openings to the sea, suggesting anthropogenic sources, while Villares et al. (2002) found that seasonal and special variation in metals was related to algal growth cycles and river runoff.
The process of metal bioaccumulation in marine food chains is poorly understood because very little data is available on metal concentration at different trophic levels (de la Lanza et al. 1989; Talavera-Saenz et al. 2007) or their temporal (Abdallah et al. 2006; Rodriguez-Castañeda et al. 2006) or spatial variation (Kalesh and Nair 2006) and their effects on the photosynthetic process (Catriona et al. 2002). Previous studies in Magdalena Bay, Mexico (Méndez et al. 2002; Gardner et al. 2006) have found high concentrations of metals in marine vertebrates, despite the lack of obvious anthropogenic sources. For example, Cd, Zn, and Fe concentrations in the herbivorous green turtle, Chelonia mydas, were the highest ever reported in sea turtles globally (Gardner et al. 2006). Rodríguez-Meza et al. (2008) developed an extensive evaluation of the heavy metals in sediments and seaweeds along ten sites in the bay. They suggested that the high levels of some heavy metals are related to terrigenous input from the arroyos and biogenic origin by the upwelling. In order to better understand the sources of heavy metals to marine species, more information is needed on metal concentrations in primary producers that make up the base of the food chain. However, few papers have approached the study of natural levels of heavy metals in seaweed communities and their temporal and spatial variation.
The aims of the present paper are to: (1) determine the concentration range of heavy metals in seaweed and seagrass species in Magdalena Bay; (2) describe the spatial and temporal variation of heavy metal concentrations in the seaweeds and seagrasses.
Methods
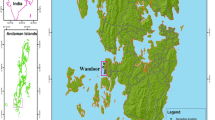
Magdalena Bay is located on the Pacific coast of the Baja California Peninsula between 24° 15′ N and 25° 20′ N and 111° 30′ W and 112° 15′ W. It is a shallow lagoon protected from the Pacific by barrier islands with high productivity resulting from seasonal marine upwelling. Diverse marine habitats within the bay include sandy bottoms and rocky margins, extensive beds of the seagrass, Zostera marina, and a diverse assemblage of macroalgae (Riosmena-Rodríguez unpublished data). A sea turtle refuge area known as Estero Banderitas is a mangrove channel located in the northwest region of the Bay (Fig. 1). Rodríguez-Meza et al. (2008) has found that the presence of heavy metals in the bay is heavily influenced by sediment type, organic material, and carbonates and concluded that there was no evidence of human impacts.
Seasonal collections were done at Estero Banderitas in November 2004, February, and April 2005. We visited the site on July 2004 but no plant species were present in the visited sites. To collect our samples, we divided the estuary into three major areas (upper, middle, and lower) and within each area, two sites were selected. Seaweed samples were collected along the length of the channel using 16 transects of 30 m length. Every 6 m along the transects, algae was manually collected within a 25-cm2 to 1-m2 area, depending on the density of the algae at that location, for a total of 80 samples per trip. We collected the most common species in the area (Riosmena-Rodríguez, unpublished data). The samples were stored in labeled plastic bags and contents were separated by species using keys provided by Riosmena-Rodríguez (1999). Samples were sun-dried in the field, and in the laboratory, they were pressed to further remove moisture.
Laboratory analyses
Tissue samples (0.5 g) were dried in an oven at 70°C until a dry weight was obtained and then digested in acid-washed Teflon tubes with concentrated nitric acid in a microwave oven. Samples were analyzed by atomic absorption (GBC Scientific equipment, model AVANTA, Australia) using an air–acetylene flame. The certified standard reference material TORT-2 (National Research Council of Canada, Ottawa) was used to verify accuracy, and the analytical values were within the range of certified values. The percentage of recovery was greater than 15% for all metals analyzed. All recoveries of metals analyzed were over 95%. Detection limits were: Zn = 0.0910 μg g−1, Cd = 0.0390 μg g−1, Mn = 0.0680 μg g−1, Cu = 0.043 μg g−1, Ni = 0.0400 μg g−1, Fe = 0.0190 μg g−1, Pb = 0.049 μg g−1.
Quantitative analyses
We analyzed the data based on taxonomic group (red algae, green algae, and seagrass), season, spatial area, and dominant species. Reported statistics are medians (n > 2) and ranges in μg g−1 on a dry weight basis. The Kruskal–Wallis test was used to compare the median metal concentration across all floral species. The null hypothesis was rejected if P ≤ 0.05. Additionally, factorial analysis was used to determine trends in the presence of heavy metals and the relative spatial and/or temporal variation.
Results
Based on our analysis, we found temporal and spatial variations in the concentration in several of heavy metals (Tables 1 and 2). Analyzing all of the species (all sites combined), we found significant seasonal differences in the heavy metal concentrations with the exception of Zn (P = 0.53). Samples collected in April had a higher concentration of Cd (P < 0.001) and Fe (P = 0.002) and a lower concentration of Pb (P < 0.001) and Ni (P = 0.002) than the other months. Mn was highest in November (P = 0.049) and Cu was higher in November compared to February (P = 0.01). In comparisons between the profiles of heavy metals in major plant groups, we found that Ni differed significantly between the major groups (P = 0.01), wherein seagrasses had lower concentrations. In the case of the analysis of green algae using all species combined, we found temporal significant differences of Cd in April (P = 0.01). When comparisons between the five most relevant red algae (Halophila johnstonii, Gracilaria vermiculophylla, G. textorii, Gracilariopsis andersonii, and Laurencia pacifica) are done, significant differences between seasons can be detected (Tables 1 and 2).
However, spatial differences in Pb concentration was significantly different in G. vermiculophylla (P = 0.02) in November but this species also had the highest concentration of Ni (P = 0.03) in relation to the other four species. Also, there were significant differences in the concentrations of Cd (P = 0.001), Fe (P = 0.01), and Ni (P = 0.002), while Pb (P < 0.001) and Cu (P = 0.03) were significantly different than the same metals in November. In the same month, highest Ni concentrations were recorded in Codium amplivesiculatum both from the middle zone. While in April, C. amplivesiculatum, Codium cuneatum, and Caulerpa sertularoides from the middle region had the highest concentrations of Cu (7.3 μg g−1 dw), Ni (11 μg g−1 dw), and Mn (61.4 μg g−1 dw), respectively.
In February, like November, we had the highest Fe concentration and several species were responsible for this difference (in H. johnstonii; 567.5 μg g−1 dw) and Zn concentration (in G. textorii; 46.8 μg g−1 dw). However, the lower zone had the highest concentrations of Cd (in G. textorii; 4.4 μg g−1 dw) and Ni (L. pacifica and Chondria nidifica; 13.3 and 13.3 μg g−1 dw). Cu (in L. pacifica; 2.9 μg g−1 dw) and Pb concentrations were highest in G. andersonii from the middle zone (3.8 μg g−1 dw).
Spatial differences in metal concentrations were dependent on the major taxa. In the case of seagrasses, we found a high concentration of Fe (Fig. 2) who was significant different from Mn (in Z. marina; 78.6 μg g−1 dw) concentrations were highest in the upper zone (P = 0.01) because their uneven distribution in the area. Consistent with the above analysis were the multifactorial analysis (Fig. 3) wherein the extreme values are represented by Fe and Mn with no association among seasons or areas.
In the green algae (Figs. 4 and 5), we were able to find many metals in the entire area, but the significant difference was found in Cd in April (P = 0.01), when all species combined, because the low value in relation to other metals are highly concentrated. There is no consistent pattern in relation to the area of the highest concentration of any metal, they tend to present a group lower (Fig. 4) in relation to higher concentration in different areas or times (Fig 5). This is well supported by the multivariate analysis (Fig. 6) wherein most of the observed metals show a combination among them and the areas of sampling.
We found an extremely high variability in the median content in the red algae (Figs. 7 and 8) but there were no significant differences between sites, with the exception of Zn which was significantly higher in the upper zone (P = 0.02). The highest concentration of any metal was Fe in Hypnea johnstonii from the upper zone (1,424.1 μg g−1 dw). The highest concentration of Mn (282.5 μg g−1 dw) and Pb (8.5) μg g−1 dw) were also detected in H. johnstonii from the upper zone. Similarly, Zn (58.8 μg g−1 dw) and Cu (4.8 μg g−1 dw) concentrations were highest in G. textorii in the same zone. The highest Cd concentrations were measured in G. textorii (4.8 μg g−1 dw; Figs. 6 and 7). Multivariate analyses show the same path (Figs. 9 and 10) with the clump of areas within metals and a group of metals with high concentration (Fig. 7) in relation to metals with low concentration (Fig. 8).
Discussion
We found significant spatial and temporal variations in heavy metal concentrations in marine plants as previous spatial studies has shown in the region (Páez-Osuna et al. 2000; Sánchez-Rodríguez et al. 2001; Rodriguez-Castañeda et al. 2006, Rodríguez-Meza et al. 2008). The high concentration of Zn and Fe in the upper region might be related to the isolation of the site (Rodríguez-Meza et al. 2008). Heavy metal concentration was, in some cases, in the levels of toxicity. Temporal variations in metal concentrations, such as high concentrations in Cd and other metals observed in April, may be related to local upwelling events. Surface water Cd concentrations have been strongly correlated with upwelling (Lares et al. 2002) which occurs during spring and early summer off the coast of Magdalena Bay (Zaytsev et al. 2003). These levels of Cd in seaweeds has not been observed in the Gulf of California studied populations but strong species and spatial variations where observed (Páez-Osuna et al. 2000; Sánchez-Rodríguez et al. 2001; Rodriguez-Castañeda et al. 2006).
The differences in heavy metal concentrations that we found in the seaweeds did not generally correspond with patterns of those elements previously observed in the sediment from the same region or seaweed species (Rodríguez-Meza et al. 2008), contrary to the studied sites in the Gulf of California near a mine (Rodriguez-Castañeda et al. 2006) or near industrial ports (Páez-Osuna et al. 2000; Sánchez-Rodríguez et al. 2001; Rodriguez-Castañeda et al. 2006). This finding, together with the observed species differences, suggests that the metabolic condition and life-cycle stage of the individual species might influence metal uptake and accumulation (Lobban and Wynne 1981). Similarly, Riget et al. (1995) found differences between seaweed species Ascophyllum nodosum, Fucus vesiculosus, and Fucus distichus. We found lower levels of Ni and Zn in H. johnstonii than in the environment as reported by Rodríguez-Meza et al. (2008). Based on our data, there are similarities between the composition and concentration of heavy metals between the plant species reviewed and the sediment; except in the case of Cu, Fe, and Mn (Rodríguez-Meza et al. 2008). All those elements are considered critical in the photosynthetic metabolism (Lobban and Wynne 1981). We might assume that those elements are more easily assimilated by the plants because of their use in photosynthesis.
The role of seaweeds and seagrasses in coastal lagoons (like Banderitas or any other along the Baja California Peninsula) are relevant because they are feeding grounds for black turtles (C. mydas), loggerhead turtles (Caretta caretta), olive Ridley turtles (Lepidochelys olivacea), and hawksbill turtles (Eretmochelys imbricata) and migratory birds like Brant geese (Branta bernicla; Seminoff 2000; Herzog and Sedinger 2004). All of the species are included in the Mexican endangered species list (NOM ECOL 059) and on the red list in the UICN endangered species (www.uicnredlist.org). They are high productivity areas for fishing all kind of products (CONABIO 2000; Carta Nacional 2005). The fact that we found more significant variation in the spatial than temporal heavy metal concentrations in most of the species show that they might be constantly incorporated in the diet of many herbivorous animals (Gardner et al. 2006) with severe consequences in their health. Management strategies for these species should consider monitoring the levels of metals.
References
Abdallah AMA, Abdallah MA, Beltagy A, Siam E (2006) Contents of heavy metals in marine algae from Egyptian Red Sea coast. Tox Env Chem 88:9–22
Catriona MO, Macinnis-Ng CMO, Peter JR (2002) Towards a more ecologically relevant assessment of the impact of heavy metals en the photosynthesis of the seagrass, Zostera capricorni. Mar Poll Bull 45:100–106
Caliceti M, Argese E, Sfriso A, Pavoni B (2002) Heavy metal contamination in the seaweeds of the Venice Lagoon. Chem 47:443–454
Carta Nacional Pesquera (2005) Carta Nacional Pesquera, SEMARNAT México D.F., p 120
CONABIO (2000) Plan Nacional sobre Biodiversidad. CONABIO México D.F., p 250
Gardner SC, Fitzgerald SL, Acosta Vargas B, Méndez Rodríguez L (2006) Heavy metal accumulation in four species of sea turtles from the Baja California Peninsula, Mexico. Biom 19(1):91–99
Herzog MP, Sedinger JS (2004) Dynamics of foraging behavior associated with variation in habitat and forage availability in captive black Brant (Branta bernicla nigricans) Goslings in Alaska. Auk 121:210–23
Kalesh NS, Nair SM (2006) Spatial and temporal variability of copper, zinc, and cobalt in marine macroalgae from the southwest coast of India. Bull Environ Contam Toxicol 76:293–300
Kieffer F (1991) Seaweeds. In: Merian E. (ed.). Metals and their compounds in the environment. VCH, Weinheim, p 481
Kumar VV, Kaladharan P (2006) Biosorption of metals from contaminated water using seaweed. Curr Sci 90:1263–1267
de la Lanza G, Ortega MM, Laparra JL, Carrillo RM, Godinez JL (1989) Chemical analysis of heavy metals (Hg, Pb, Cd, As, Cr and Sr) in marine algae of Baja California. An Inst Biol Univ Nac Auton Mex (Bot) 59(1):89–102
Lares ML, Flores-Munoz G, Lara-Lara R (2002) Temporal variability of bioavailable Cd, Hg, Zn, Mn and Al in an upwelling regime. Environ Pollut 120:595–608
Lobban CS, Wynne MJ (1981) The biology of seaweeds. Botanical monographs, vol. 17. Blackwell, Oxford, p 786
Machado W, Silva-Filho EV, Oliveira RR, Lacerda LD (2002) Trace retention in mangrove ecosystems in Guanabara Bay, SE Brazil. Mar Poll Bull 44:1277–1280
Méndez L, Álvarez-Castañeda ST, Acosta B, Sierra-Beltrán AP (2002) Trace metals in tissues of Gray whale (Eschrichtius robustus) carcasses from the Northern Pacific Mexican Coast. Mar Poll Bull 44:217–221
Moreno M (2003) Toxicología ambiental, evaluación de riesgo para la salud humana. Mc Graw-Hill, Spain, p 370
Páez-Osuna F, Ochoa-Izaguirre MJ, Bojórquez-Leyva H, Michel-Reynoso IL (2000) Macroalgae as biomonitors of heavy metal availability in coastal lagoons from the subtropical Pacific of Mexico. Bull Env Cont Tox 64:846–851
Riget F, Johansen P, Asmund G (1995) Natural seasonal variation of cadmium, copper, lead and zinc in brown seaweed (Fucus vesiculosus). Mar Poll Bull 30:409–414
Riosmena-Rodríguez R (1999) Vegetación subacuatica. In: Gaytán, J.,Informe Final de Actividades del Proyecto Bahía del Rincón . UABCS-S&R, p 350
Rodriguez-Castañeda AP, Sánchez-Rodriguez I, Shumilin EN, Sapozhnikov D (2006) Element concentrations in some species of seaweeds from La Paz Bay and La Paz Lagoon, south-western Baja California, México. J Appl Phycol 18:399–408
Rodríguez-Meza G D Choumiline E, Méndez-Rodríguez L, Acosta-Vargas B, Sapozhnikov D (2008) Composición química de los sedimentos y del Complejo Lagunar Bahía Magdalena. Almejas. In: J. Gómez Gutiérrez, R. Palomares García R. Funes Rodríguez (eds) Bahía Magdalena Estudios Ecológicos” Chapter 7IPN-CICIMAR, p 250
Sánchez-Rodríguez I, Huerta- Díaz MA, Choumiline E, Holguín-Quiñones O, Zertuche-Gonzáles JA (2001) Elemental concentration in different species of seaweeds from Loreto Bay, Baja California Sur, Mexico: implications for the geochemical control of metals in algal tissue. Environ Poll 114:145–160
Sawidis T, Brown MT, Zachariadis G, Sratis I (2001) Trace metal concentrations in marine macroalgae from different biotopes in the Aegean Sea. Env Inter 27:43–47
Seminoff J A (2000) Biology of the East Pacific green turtle, Chelonia mydas agassizii, at a warm temperate feeding area in the Gulf of California, Mexico. Ph.D. Diss., University of Arizona, Tucson
Sparling D, Bishop C, Linder G (2000) Ecotoxicology of amphibians and reptiles. Society of Environmental Toxicology and Chemistry, Pensacola, FL, p 145
Szefer P, Geldon J, Anis-Ahmed A, Paéz-Osuna F, Ruiz-Fernandez AC, Guerrero-Galvan SR (1998) Distribution and association of trace metals in soft tissue and byssus of Mytela strigata and other benthal organisms from Mazatlan Harbour, Mangrove Lagoon of the northwest coast of Mexico. Envir Internat 24:359–374
Talavera-Saenz AL, Gardner SC, Riosmena-Rodríguez R, Acosta-Vargas B (2007) Metal profiles used as environmental markers of Green Turtle (Chelonia mydas) foraging resources. Sci Tot Env 373:94–102
Villares R, Puente X, Carballeira A (2002) Seasonal variation and background levels of heavy metals in two green seaweeds. Envir Poll 119:79–90
Zaytsev O, Cervantes-Duarte R, Montante O, Gallegos-Garcia A (2003) Coastal upwelling activity on the Pacific shelf of the Baja California Peninsula. J Ocean 59:489–502
Acknowledgments
Funding for this project was provided by a grant to SC Gardner from the Consejo Nacional de Ciencia y Tecnología (Conacyt, SEP-2004-CO1-45749) and the Centro de Investigaciones Biológicas del Noroeste, S.C. (CIBNOR). The authors express their appreciation to Dr. Wallace J. Nichols, Griselda Peña (CIBNOR), Rodrigo Rangel, and the Grupo Tortuguero for their assistance in this project. This research was conducted in accordance with Mexican laws and regulations, under permits provided by the Secretaria de Medio Ambiente y Recursos Naturales (SGPA/DGVS/002-2895). We deeply thank the comments from two anonymous reviewers to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riosmena-Rodríguez, R., Talavera-Sáenz, A., Acosta-Vargas, B. et al. Heavy metals dynamics in seaweeds and seagrasses in Bahía Magdalena, B.C.S., México. J Appl Phycol 22, 283–291 (2010). https://doi.org/10.1007/s10811-009-9457-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9457-2