Abstract
The aim of this study was to isolate and identify algicidal bacteria against the dinoflagellate Cochlodinium polykrikoides, and to determine the algicidal activity and algicidal range. During the declining period of C. polykrikoides blooms, seven algicidal bacteria were isolated. The algicidal bacteria against C. polykrikoides were enumerated using the most probable number (MPN) method. The number of algicidal bacteria was high (3.7 × 103 mL−1). Algicidal bacteria were identified on the basis of biochemical and chemotaxonomic characteristics, and analysis of 16S rDNA sequences. Seven algicidal bacteria isolated in this study belonged to the genera Bacillus, Dietzia, Janibacter, and Micrococcus. The most algicidal bacterium, designated Micrococcus luteus SY-13, is assumed to produce secondary metabolites. When 5% culture filtrate of this strain was applied to C. polykrikoides cultures, over 90% of C. polykrikoides cells were destroyed within 6 h. M. luteus SY-13 showed significant algicidal activities against C. polykrikoides and a wide algicidal range against various harmful algal bloom (HAB) species. Taken together, our results suggest that M. luteus SY-13 could be a candidate for controlling HABs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Harmful algal blooms (HABs) are natural phenomena that occur across the world. HABs occur frequently and drastically affect the aquaculture industry in coastal waters. Over the past three decades, the occurrence of HABs has increased, likely due to several factors, including environmental pollution and global warming. In the Republic of Korea, extreme blooms of dinoflagellates that cause severe damage to fisheries occur annually. In particular, Cochlodinium polykrikoides blooms have caused heavy damage to fish farming in the Republic of Korea, Japan, and other countries (Onoue and Nozawa 1989; Whyte et al. 2001; Kim et al. 2007). The alleged economic losses caused by C. polykrikoides in the Republic of Korea were estimated at US $95.5 and US $7.2 million in 1995 and 2003, respectively (Kim et al. 1997; NFRDI 2004). Cochlodinium polykrikoides is an unarmored, marine, planktonic dinoflagellate species with a distinctive spiral-shape cingulum and is a photosynthetic alga. Its vegetative cells usually form long cell chains. Since the first outbreak in 1982, HABs have occurred annually in Korean coastal waters (Kim et al. 1999). Cochlodinium polykrikoides generally kills fish and shellfish through massive mucus production and oxygen depletion (Lee 1996; Cho et al. 1999; Kim et al. 2000a).
Many scientists have performed physiological and ecological studies in the hope of reducing damage to fisheries caused by HABs (Yanagi et al. 1995; Uchida et al. 1999; Kim et al. 2000b, 2002). Some chemicals have been used to mitigate HABs, but a search for safer algicidal agents for biological control is needed due to the side effects of chemical agents as environmental pollutants. Recently, numerous investigations on the correlation between the termination of HABs and various bacteria have been conducted (Yoshinaga et al. 1995; Imai et al. 1995, 1998; Fukami et al. 1996; Kim et al. 1998; Park et al. 1998; Doucette et al. 1999). Many algicidal bacteria have been isolated from various sources. These algicidal bacteria include species of various genera, e.g., Alteromonas, Bacillus, Cytophaga, Flavobacterium, Micrococcus, Pseudomonas, Pseudoalteromonas, and Vibrio (Baker and Herson 1978; Imai et al. 1993, 1995; Lovejoy et al. 1998; Park et al. 1998; Yoshinaga et al. 1998; Lee et al. 2000; Jeong et al. 2000a, 2003; Adachi et al. 2002; Zheng et al. 2005). These findings have raised the possibility that HABs can be controlled by algicidal bacteria.
In the course of our screening program of algicidal bacteria, seven marine bacteria against C. polykrikoides have been isolated. Seawater samples were collected during the termination of C. polykrikoides blooms in Kamak Bay, Republic of Korea. The number of algicidal bacteria against C. polykrikoides was high (3.7 × 103 mL−1) in the field. Algicidal bacteria were identified on the basis of biochemical and chemotaxonomic characteristics, and analysis of 16S rDNA sequences. The strongest algicidal bacterium, strain SY-13, is assumed to produce secondary metabolites. In the present study, we describe the taxonomy of algicidal bacteria, and determine the algicidal activity and algicidal range against HAB species.
Materials and methods
Seawater samples from Kamak Bay (southern coast of Korean peninsula) were collected from a depth of 1 m using a Van Dorn sampler. To enumerate and isolate algicidal bacteria against Cochlodinium. polykrikoides, sampling was performed during the termination of C. polykrikoides blooms in mid-September 2002. The numbers of C. polykrikoides cells were counted using an inverted microscope.
C. polykrikoides, the harmful dinoflagellate used in this study, was isolated from Kamak Bay in mid-September 2002. Axenic clones were obtained by repeated washing with capillary pipettes (Droop 1967) and repeated subcultures using enriched seawater medium containing antibiotic complex (Jeong et al. 2000a). The concentration of antibiotic complex in the medium was: 100 μg mL−1 ampicillin, 10 μg mL−1 streptomycin, 10 μg mL−1 chloramphenicol, 10 μg mL−1 penicillin G, 50 μg mL−1 neomycin, 50 μg mL−1 gentamicin, 10 μg mL−1 kanamycin, and 1.5 μg mL−1 nystatin. All antibiotics and f/2-Si medium (Guillard and Ryther 1962) were purchased from Sigma (St. Louis, MO). Cochlodinium. polykrikoides cultures were routinely maintained in f/2-Si medium made of GF/F-filtered seawater. The composition of f/2-Si medium was: each liter of GF/F-filtered seawater contained 75 mg NaNO3, 5 mg NaH2PO4·H2O, 4.36 mg Na2EDTA, 3.15 mg FeCl3·6H2O, 0.01 mg CuSO4·5H2O, 0.022 mg ZnSO4·7H2O, 0.01 mg CoCl2·6H2O, 0.18 mg MnCl2·4H2O, 0.006 mg Na2MoO4·2H2O, 0.1 mg thiamine·HCl, 0.5 μg biotin, and 0.5 μg vitamin B12. The cultures were grown in disposable sterilized tissue culture flasks (Iwaki, Chiba, Japan) with illumination of 120 μmol photons m−2 s−1 under a 12 h light:12 h dark cycle at 20°C.
Initial screening for algicidal bacteria was performed only on C. polykrikoides cultures. The algicidal bacteria against Cochlodinium. polykrikoides were enumerated using the most probable number (MPN) method (Yoshinaga et al. 1995). Cultures at mid-exponential phase were diluted with f/2-Si medium to ca. 1.0 × 104 cells mL−1, and 0.5 mL portions of C. polykrikoides cultures were pipetted aseptically into 48-well microplates (Costar, Corning, NY). The seawater samples were filtered through a 0.8 or 0.2 μm pore-size membrane filter (Advantec, Tokyo, Japan) and serially diluted with autoclaved seawater. A 0.8 μm filter was used to eliminate particles larger than bacteria (Imai et al. 1993). A portion of each diluted seawater sample (0.5 mL) was inoculated into each well of the 48-well microplates containing C. polykrikoides cultures. The assay cultures in the microplates were incubated under the same conditions described above, and the survival of C. polykrikoides in each well was assessed daily using an inverted microscope. The wells in which more than 99% of C. polykrikoides cells were destroyed were counted as “positive”, and the MPN of the algicidal bacteria against C. polykrikoides was calculated using a microcomputer program (Koch 1994). Autoclaved and filtered (0.1 μm pore-size membrane filter) seawaters were inoculated into assay cultures for negative controls.
The putative algicidal strains against C. polykrikoides were isolated from the positive wells in which the C. polykrikoides cells were completely destroyed. Wells with the highest possible dilution factor were selected to isolate the dominant species among bacteria populations, and subsamples were spread onto Zobell 2216E agar plates. The viable bacterial number was determined by colony-forming unit (CFU) after 1 week of growth at 25°C on Zobell 2216E agar plates. All 53 colonies with different colony color and morphological shape were chosen for isolation. Each strain was cultured and was again inoculated into C. polykrikoides in order to confirm its algicidal activities against C. polykrikoides. As a result, seven algicidal strains were isolated in this study.
To measure the algicidal activities of the seven strains against C. polykrikoides, these strains were cultured in Zobell 2216E medium. Bacterial cultures were grown at 25°C for 3 days at 200 rev min−1 to reach stationary phase. Bacterial cells were then removed by centrifugation (5,000 g for 20 min at 4°C) and filtration (0.2 μm pore-size membrane filter) and the culture filtrates were investigated for algicidal activity. The C. polykrikoides cells were cultured in 300 mL tissue culture flasks containing 100 mL f/2-Si medium. Then, 180 μL C. polykrikoides culture (ca. 1.0 × 104 cells mL−1) at mid-exponential growth phase (12 days after incubation) was inoculated into each well of a 24-well tissue culture plate (NUNCLON, Copenhagen, Denmark). Twenty microliters of each bacterial culture filtrate was added to a 24-well plate containing C. polykrikoides. To exclude the effect of bacterial culture medium, an equal volume of fresh Zobell 2216E medium was added as a control. The bioassay plates were then incubated under the conditions described above. After 6 h, the numbers of surviving cells were counted directly on a Sedgewick-Rafter counting chamber at a magnification of ×200 using the inverted microscope. The algicidal activity was calculated as follows (Byun et al. 2002): Algicidal activity (%) = {1 – (viable individuals of C. polykrikoides after the treatment / initial individuals of C. polykrikoides × 100}. All experiments were repeated in triplicate, and results are given as the mean and standard deviation of raw data.
Identification of algicidal bacteria
Seven algicidal strains were grown at 25°C for 3 days on Zobell 2216E agar. Morphological characteristics were observed with a scanning electron microscope (Hitachi S3500N, Japan). Standard physiological and biochemical characteristics were examined according to the methods of MacFaddin (1984). Additional biochemical tests were performed using API kits (API 20E, API 50CHB/E, API 50 CHL, and API Staph; BioMerieux, Marcy L’Etoile, France). Furthermore, cellular fatty acid methyl esters were prepared according to Huys et al. (1997), and were analyzed by the Sherlock Microbial Identification System (MIDI; Hewlett Packard 6890, Los Angeles, CA ).
PCR amplification of the 16S rRNA genes was performed on a Takara PCR Thermocycler. Genomic DNA was prepared using the AccuPrep Genomic DNA Extraction Kit (Bioneer, Daejeon, Republic of Korea), and PCR was conducted using genomic DNA (0.1 μg) as the template and universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′- GATTACCTTGTTACGACTT-3′). These primers were synthesized commercially (Bioneer, Republic of Korea). Genes were amplified by 30 cycles of 94°C for 20 s, 55°C for 1 min, and 72°C for 1 min, followed by extension at 72°C for 10 min. The 1.5 kb PCR product was cloned into the pGEM-T easy vector (Promega, Madison, WI), sequenced using a Termination Sequencing Ready Reaction kit (Perkin Elmer, Foster City, CA), and analyzed using an ABI 377 genetic analyzer (Perkin Elmer).
The near-complete 16S rDNA sequence was aligned using CLUSTAL W software Ver. 1.7 (Thompson et al. 1994). 16S rDNA sequences used for the phylogenetic analysis were derived and compared with those of other bacterial 16S rDNA sequences available in the DDBJ/EMBL/GenBank database. Kimura’s two-parameter model (Kimura 1980) was applied for the calculation of evolutionary distance. A phylogenetic tree was constructed by the neighbor-joining method (Saitou and Nei 1987). Bootstrap analyses of 1,000 replicates were carried out using MEGA version 2.0 (Kumar et al. 2001).
Algicidal effect of culture filtrates of strain SY-13 against C. polykrikoides
The algicidal effect of the culture filtrates of strain SY-13 against C. polykrikoides was investigated at various concentrations of filtrates. The bacterial and C. polykrikoides cultures were prepared as above. The culture filtrates of strain SY-13 were added to C. polykrikoides cultures (ca. 1.0 × 104 cells mL−1) at concentrations of 1.25, 2.5, 5, and 10%, respectively. Zobell 2216E medium was added as the control, and the bioassay plates were incubated as above. After incubation for 3, 6, and 9 h, the viable swimming cells in each well were counted with a Sedgewick-Rafter chamber using an inverted microscope.
Algicidal range of strain SY-13 against other HAB species
The algicidal range of strain SY-13 against other HAB species was investigated using the following algal species: Chattonella sp. and Heterosigma akashiwo were provided by NFRDI (National Fisheries Research & Development Institute, Republic of Korea), and Akashiwo sanguinea, Gymnodinium impudicum, Prorocentrum micans, P. minimum, Scrippsiella trochoidea, and Skeletonema costatum were provided by the South Sea Institute of KORDI (Korea Ocean Research & Development Institute, Republic of Korea). Cultures of five dinoflagellates, two raphidophytes, and one diatom were unialgal, and showed little bacterial contamination.
All algal cultures except S. costatum were cultured in f/2-Si medium under an illumination of 120 μmol photons m−2 s−1 on a 12 h light:12 h dark cycle at 20°C. To investigate the algicidal range of strain SY-13, all of the bacterial and HAB species cultures, except S. costatum, were prepared in the same manner as for the assay with C. polykrikoides. The culture filtrates, at concentrations of 10%, were added to each algal culture at the mid-exponential growth phase. After incubation for 6 h, the viable swimming cells were counted as above.
An agar overlay assay was used to assess the algicidal effect of strain SY-13 on the growth of S. costatum. Sterile 8 mm diameter paper discs (Advantec) that had been soaked in 10% culture filtrates were placed on the center of the S. costatum lawns. The algal lawn plates were incubated in f/2 agar plates for 2 weeks with illumination of 30 μmol photons m−2 s−1 at 20°C. Inhibition of growth was assessed by the size of the clear inhibition zone around the paper discs.
Results
To enumerate and isolate algicidal bacteria, seawater samples were collected during the termination process of C. polykrikoides blooms in Kamak Bay. The number of algicidal bacteria against C. polykrikoides was enumerated by the MPN method. At the field sampling site, the dominant HAB species was C. polykrikoides (1,320 cells mL−1). The number of algicidal bacteria (in the >0.2 μm and <0.8 μm size fractions) against C. polykrikoides was high (3.7 × 103 cells mL−1). At that time, the density of viable bacteria was 4.4 × 104 cells mL−1.
To isolate bacterial strains with algicidal activity, we used the positive wells in which C. polykrikoides cells were completely dead: 53 colonies with different colony color and morphological shape were chosen for isolation. Each strain was cultured and inoculated again into C. polykrikoides cultures to confirm its algicidal activities against C. polykrikoides. As a result, seven algicidal strains were isolated in this study. No algicidal bacterial strains could be isolated from the wells inoculated with the <0.2 μm size fractions of seawater samples.
Algicidal activities of seven algicidal strains against C. polykrikoides
The algicidal activities of seven algicidal strains against C. polykrikoides were investigated. As shown in Fig. 1, all strains significantly inhibited the growth and motility of the dinoflagellate C. polykrikoides. In particular, strain SY-13 showed the strongest algicidal activity, and was selected for further study. After 6 h of treatment, all strains inhibited C. polykrikoides by over 79% at a 10% filtrate concentration. Based on the motility of cells, more than 50% of C. polykrikoides cells lost their motility within 1 h after treatment with the 10% culture filtrates. Interestingly, the algicidal activity of all strains in this study was found in the culture filtrates, not in the cells (data not shown).
The algicidal activities of the 10% culture filtrates of seven algicidal strains against Cochlodinium polykrikoides. All strains were cultured at 25°C for 3 days in Zobell 2216E medium. The 10% culture filtrates of each strain were added to C. polykrikoides cultures (ca. 1.0 × 104 cells mL−1) at the mid-exponential growth phase. The filtrate of Zobell 2216E medium was used as a control. Data are expressed as the mean ± standard deviation from triplicate assays
Identification of algicidal bacteria
The morphological and biochemical characteristics of the seven algicidal bacteria are summarized in Table 1. All strains were Gram-positive and catalase-positive. Scanning electron microscopy revealed that strains SY-11, SY-13, SY-31, and SY-47 were cocci, and strains SY-19, SY-20, and SY-21 were rods (Fig. 2). Strain SY-47 was the smallest, with a cell size of 0.6–0.7 μm. All strains except for strain SY-21 hydrolysed gelatin. Strains SY-19, SY-20, and SY-47 also hydrolyzed starch, casein, and esculin. None of the strains contained arginine dehydrolase or urease. Strains SY-19 and SY-20 assimilated d-glucose, d-maltose, potassium gluconate, N-acetyl-d-glucosamine, malate, and trisodium citrate, and produced acid from d-glucose, d-fructose, glycerol, d-maltose, d-trehalose, and d-sucrose.
The cellular fatty acid profiles of algicidal bacteria are shown in Table 2. Strains SY-11, SY-13, and SY-31 are similar in that they contain major amounts of iso- and anteiso- methyl-branched-chain acids, including iso-C15:0 and anteiso-C15:0. Strains SY-19 and SY-20 contained anteiso-C15:0 and anteiso-C17:0 as the major components of cellular fatty acids. Strain SY-21 contained C17:0 as a major component. The major components of strain SY-47 were iso-C16:0 and anteiso-C17:1ω8c. Based on the fatty acid profiles, strains SY-11, SY-13, and SY-31 were similar to those of the genus Micrococcus. Strains SY-19 and SY-20 exhibited fatty acid profiles close to those of the genus Bacillus. However, strains SY-21 and SY-47 could not be identified to the genus level in the MIDI system.
To identify the seven algicidal bacteria to the species level, 16S rDNA sequencing was performed. The sequences of strains SY-11, SY-13, and SY-31 shared the highest degrees of similarity to sequences of Micrococcus luteus ATCC 381T (98.1%, 98.6%, and 98.7%, respectively). The sequences of strains SY-19 and SY-20 showed over 99% homology with Bacillus atrophaeus ATCC 51189. Strains SY-21 and SY-47 exhibited 16S rDNA sequence similarities of above 98% to Dietzia maris DSM 43102 and Janibacter brevis DSM 13953T, respectively.
Physiological characteristics and phylogenetic analysis of strain SY-13
The strongest algicidal bacterium, strain SY-13, was a Gram-positive, non-motile coccus. Growth of strain SY-13 was observed at 15–45°C and pH 5–10. This strain is tolerant of NaCl concentrations up to 10%, but does not require NaCl for growth. However, this strain showed weak growth at 15°C and 45°C, pH 10 and over at 6% NaCl concentration. The optimal conditions for the growth of strain SY-13 were 25°C, pH 8.0, at 3.0% NaCl. Strains SY-13 and SY-31 showed very similar properties to each other in many aspects. However, strains SY-13 and SY-31 displayed obvious macrostructural and biochemical differences (Table 1). The colony shape of strain SY-13 was convex and smooth, but that of strain SY-31 was umbonate. These strains differed due to the assimilation of N-acetyl-d-glucosamine and phenylacetic acid, and acid production from d-maltose. In addition, strain SY-13 and strain SY-31 exhibited 16S rDNA sequence similarity of 99.3%. Most of all, they revealed significant differences in their algicidal activity (Fig. 1).
On the basis of morphological, biochemical, and chemotaxonomic analysis, these data support the identification of strain SY-13 as a member of the genus Micrococcus. Furthermore, the near-complete 16S rDNA sequence (1,409 bp) of this strain indicated that it was most closely related to M. luteus ATCC 381T (98.6% homology, M38242) (Fig. 3). Thus, we designated this strain to be M. luteus SY-13, and selected it for further study. The 16S rDNA sequence of strain SY-13 has been deposited in the GenBank database under accession number EU021048.
Algicidal effect of the culture filtrates of M. luteus SY-13 against C. polykrikoides
The algicidal effect of M. luteus SY-13 against C. polykrikoides according to the concentration of bacterial culture filtrate was investigated (Fig. 4). Cochlodinium polykrikoides cells and M. luteus SY-13 were cultured under the conditions described above. The culture filtrates of M. luteus SY-13 were added to C. polykrikoides cultures at mid-exponential phase (ca. 1.0 × 104 cells mL−1) at concentrations of 1.25, 2.5, 5, and 10%, respectively. Slight algicidal activity was observed in the control wells to which 10% filtrate of Zobell 2216E medium was added, but this activity was not significant. At a concentration greater than 1.25%, there were significant differences between the control and culture filtrates after incubation for 3 h. After 6 h, over 90% of C. polykrikoides cells were destroyed at a concentration of 5%. At 10% concentration, over 98% of C. polykrikoides cells were destroyed within 6 h. These results suggest that the culture filtrates of M. luteus SY-13 inhibited the growth of C. polykrikoides in a concentration- and time-dependent manner.
Algicidal activity of the culture filtrate of Micrococcus luteus SY-13 against C. polykrikoides at various concentrations (●, control; ◯, 1.25%; ▼, 2.5%; △, 5%; ■, 10%). M. luteus SY-13 was cultured at 25°C for 3 days in Zobell 2216E medium. Each culture filtrate was added to C. polykrikoides cultures (ca. 1.0 × 104 cells mL−1) at the mid-exponential growth phase. The filtrate of Zobell 2216E medium was used as a control. Data are expressed as the mean ± standard deviation from triplicate assays
In the wells containing the 10% culture filtrates of M. luteus SY-13, C. polykrikoides cells lost their motility; all non-motile C. polykrikoides chains fell apart, followed by rounding up of the resulting single cells, swelling, and finally, lysing of C. polykrikoides within 6 h (Fig. 5). After 6 h, a few C. polykrikoides cells remained in the culture without bursting. However, those remaining cells also rounded up and expanded, and almost all of C. polykrikoides cells were lysed within 9 h after treatment.
Micrographs of the lysing process of C. polykrikoides treated with 10% culture filtrates of M. luteus SY-13. a Living cells of eight chain-forming C. polykrikoides. b Round and expanded cells after 30 min of exposure to 10% culture filtrates. c, d Severely damaged cells after 3 h and 6 h, respectively. Burst and swollen cells were observed (arrow). Note that the nuclei and protoplasms of C. polykrikoides cells are degraded. Once C. polykrikoides cells had burst, they could not be restored to living cells for 2 days. Bar=30 μm
Algicidal range of M. luteus SY-13 against other HAB species
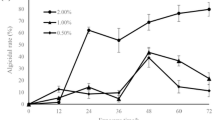
Further algicidal effects of the culture filtrates of M. luteus SY-13 against HAB species were investigated (Fig. 6). We used three armored and three unarmored Dinophyceae, two Raphidophyceae, and one Bacillariophyceae in this study. The 10% culture filtrates of M. luteus SY-13 demonstrated a wide spectrum of algicidal activities against all of the dinoflagellates and raphidophytes tested. The algicidal activities against HAB species were as follows: Chattonella sp. (after 6 h treatment: 90.3%), A. sanguinea (71.8%), G. impudicum (44.3%), H. akashiwo (25.6%), P. micans (47.8%), P. minimum (19.7%), and S. trochoidea (48.2%). However, the culture filtrates of M. luteus SY-13 did not show any obvious inhibitory effects on the growth of the diatom S. costatum. It is desirable that M. luteus SY-13 possesses a wide algicidal range against HAB species, except diatoms.
The algicidal range of M. luteus SY-13 against other harmful algal bloom (HAB) species. The 10% culture filtrates of M. luteus SY-13 were added to each algal culture at the mid-exponential growth phase. After incubation for 6 h, the algicidal activity was evaluated. Data are expressed as the mean ± standard deviation from triplicate assays
Discussion
Considerable research has been conducted regarding the control of HABs. However, few advances have been made to control HABs in practice. In the Republic of Korea, yellow clay has been the only means used to control and mitigate HABs. However, this method is limited and is not very effective. In addition, it remains controversial because of the possibility of adverse effects on marine ecosystems. Therefore, alternative countermeasures to control HABs should be considered. Many scientists have recently focused on algicidal bacteria for the control of HABs (Imai et al. 1993, 1995; Yoshinaga et al. 1995, 1997; Kim et al. 1998; Lovejoy et al. 1998; Lee et al. 2000; Jeong et al. 2000b, 2003).
In this study, we investigated the abundance of algicidal microorganisms in the <0.8 μm (including mainly bacteria and viruses) and <0.2 μm (including mainly viruses and virus-like particles) size fractions by the MPN method. In addition, we isolated and identified algicidal bacteria that effectively inhibit the growth of C. polykrikoides. During the termination process of C. polykrikoides blooms, the number of algicidal bacteria against C. polykrikoides was high (3.7 × 103 cells mL−1). On the other hand, the effects of viruses or virus-like particles seemed to be negligible because almost no algicidal activity was detected in the <0.2 μm size fractions. Seven algicidal bacteria isolated in this study belonged to genera Bacillus, Dietzia, Janibacter, and Micrococcus. All known algicidal bacteria fall phylogenetically into either the γ-Proteobacteria or Cytophaga-Flavobacterium-Bacteroides (CFB) groups. γ-Proteobacteria includes Alteromonas, Pseudomonas, Pseudoalteromonas, and Vibrio species. More specifically, the genus Pseudoalteromonas includes many species capable of producing biologically active extracellular products against a wide range of algae (Holmstrom and Kjelleberg 1999). However, our algicidal bacteria, Dietzia, Janibacter, and Micrococcus species, belong to the Actinobacteria, and the Bacillus species belongs to the Firmicutes. The Actinobacteria and the Firmicutes do not commonly exist as algicidal bacteria.
All of our algicidal bacteria showed significant algicidal activity against C. polykrikoides. In addition, algicidal activity against C. polykrikoides was detected in the culture filtrates of all strains, not in the cells. These results indicate that all of the seven algicidal strains release certain algicidal compounds into the culture broth. Thus, all of our algicidal strains were thought to act as indirect attackers. Some bacteria attack algal cells directly. For example, Imai et al. (1993) reported that Cytophaga sp. attack algal cells directly. In general, indirect attacks occur through chemical mediators and are species-specific (Yoshinaga et al. 1995, 1997). The strongest strain, M. luteus SY-13, inhibited the growth of C. polykrikoides indirectly, but this strain did not show species-specificity. M. luteus SY-13 showed particularly strong activity against Chattonella sp. and C. polykrikoides. This strain showed a wide host range against all of the HAB species tested. However, this strain revealed weak activity against relatively smaller algal cells (<20 μm cell size) such as H. akashiwo and P. minimum. In addition, these algal cells recovered within 2 days. Interestingly, M. luteus SY-13 did not show any effect on the growth of the diatom S. costatum. It is desirable that M. luteus SY-13 possesses only HAB species-specific algicidal activity. Therefore, these results indicate that M. luteus SY-13 could play an important role in controlling HABs related to C. polykrikoides.
Algicidal bacteria might not be the best tools for use in the control of large-scale HABs, but could be effective in areas of small contained outbreaks, such as that seen in Maryland in 1997 (Magnien 2001). However, further studies are needed before algicidal bacteria can be practically applied for the regulation of HABs. Screening of algicidal compounds to control HABs has scarcely begun, although knowledge of such compounds is biologically important in marine ecosystems. Before they can be practically applied for bioremediation in natural environments, it is very important to elucidate the properties and functions of the algicidal compounds. Unfortunately, most previous studies concerning the control of HABs through the application of algicidal effects have been confined to freshwater ecosystems, and there have been very few investigations of marine HABs that have few environmental side effects. Recently, characteristics of secondary metabolites purified from algicidal bacteria have been reported (Lee et al. 2000; Jeong et al. 2000b). In addition, there have been reports on algicides that have little effect on marine organisms, such as bacillamide and phlorotannins (Jeong et al. 2003; Nagayama et al. 2003). In conclusion, we have investigated seven algicidal bacteria, including M. luteus SY-13. Our results suggest that algicidal bacteria could be potential candidates for use in the control of HABs. Further studies are needed to identify the structure of the algicidal compound and to elucidate its exact mechanism.
References
Adachi M, Fukami K, Kondo R, Nishijima T (2002) Identification of marine algicidal Flavobacterium sp. 5N-3 using multiple probes and whole-cell hybridization. Fish Sci 68:713–720
Baker KH, Herson DS (1978) Interactions between the diatom Thallasiosira pseudonana and an associated pseudomonad in a mariculture system. Appl Environ Microbiol 35:791–796
Byun HG, Jeong SY, Park YT, Lee WJ, Kim SK (2002) Algicidal activity of substance purified from marine bacteria metabolites against Cochlodinium polykrikoides. J Fish Sci Technol 5:150–155
Cho ES, Kim CS, Lee SG, Chung YK (1999) Binding of alcian blue applied to harmful Microalgae from Korean coastal waters. Bull Natl Fish Res Dev Inst 55:133–138
Doucette GJ, McGovern ER, Babinchak JA (1999) Algicidal bacteria active against Gymnodinium breve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. J Phycol 35:1447–1454
Droop MR (1967) A procedure for routine purification of algal cultures with antibiotics. Brit Phycol Bull 3:295–297
Fukami K, Sakaguchi K, Kanou M, Nishijima T (1996) Effect of bacterial assemblages on the succession of blooming phytoplankton from Skeletonema costatum to Heterosigma akashiwo. In: Yasumoto T, Oshima T, FuKuyo Y (eds) Harmful and toxic algal blooms. Intergovernmental Oceanographic commission of UNESCO, Paris, pp 335–338
Guillard RL, Ryther JH (1962) Studies on marine planktonic diatoms. I. Cyclotella nana (Hustedt) and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Holmstrom C, Kjelleberg S (1999) Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbial Ecol 30:285–293
Huys G, Kämpfer P, Altwegg M, Kersters I, Lamb A, Coopman R, Luthy-Hottenstein J, Vancanneyt M, Janssen P, Kersters K (1997) Aeromonas popoffii sp. nov. a mesophilic bacterium isolated from drinking water production plants and reservoirs. Int J Syst Bacteriol 47:1165–1171
Imai I, Ishida Y, Hata Y (1993) Killing of marine phytoplankton by a gliding bacterium Cytophaga sp., isolated from the coastal sea of Japan. Mar Biol 116:527–532
Imai I, Ishida Y, Sakaguchi K, Hata Y (1995) Algicidal marine bacteria isolated from northern Hiroshima Bay, Japan. Fish Sci 61:628–636
Imai I, Nagagiri S, Nagai K, Nagasaki K, Itakura S, Yamaguchi M (1998) Fluctuations of algicidal microorganisms against the harmful dinoflagellate Heterocapsa circularisquama in Ago Bay, Mie prefecture, Japan. Bull Nansei Natl Fish Res Inst 31:53–61
Jeong SY, Park YT, Lee WJ (2000a) Isolation of marine bacteria killing red tide microalgae. III. Algicidal effects of marine bacterium, Micrococcus sp. LG-5 against the harmful dinoflagellate, Cochlodinium polykrikoides (in Korean). J Korean Fish Soc 33:331–338
Jeong SY, Park YT, Kim MC, Choi SC, Seong HK, Kim JY, Kim TU, Lee WJ (2000b) Isolation of marine bacteria killing red tide microalgae. IV. Characteristics of algicidal substances produced from Micrococcus sp. LG-5 and the effects on marine organisms (in Korean). J Korean Fish Soc 33:339–347
Jeong SY, Ishida K, Ito Y, Okada S, Murakami M (2003) Bacillamide, a novel algicide from the marine bacterium, Bacillus sp. SY-1, against the harmful dinoflagellate, Cochlodinium polykrikoides. Tet Lett 44:8005–8007
Kim HG, Lee SG, An KH, Youn SH, Lee PY, Lee CK, Cho ES, Kim JB, Choi HG, Kim PJ (1997) Recent Red Tides in Korean Coastal Waters (in Korean). Nat Fish Res Dev Agency, Pusan
Kim MC, Yoshinaga I, Imai I, Nagasaki K, Itakura S, Ishida Y (1998) A close relationship between algicidal bacteria and termination of Heterosigma akashiwo (Raphidophyceae) bloom in Hiroshima Bay, Japan. Mar Ecol Prog Ser 170:25–32
Kim HG, Choi WJ, Jung YG, Jung CS, Park JS, An KH, Back CI (1999) Initiation of Cochlodinium polykrikoides blooms and its environmental characteristics around the Narodo Island in the western part of South Sea of Korea (in Korean). Bull Natl Fish Res Dev Inst 57:119–129
Kim CS, Bae HM, Yun SJ, Cho YC, Kim HG (2000a) Ichthyotoxicity of a harmful dinoflagellate Cochlodinium polykrikoides: Aspect of hematological responses of fish exposed to algal blooms. J Fish Sci Technol 3:111–117
Kim CS, Lee SG, Kim HG (2000b) Biochemical responses of fish exposed to a harmful dinoflagellate Cochlodinium polykrikoides. J Exp Mar Biol Ecol 254:131–141
Kim D, Oda T, Muramatsu T, Kim D, Matsuyama Y, Honjo T (2002) Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comp Biochem Physiol C 132:415–423
Kim CJ, Kim HG, Kim CH, Oh HM (2007) Life cycle of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides in Korea coastal waters. Harmful Algae 6:104–111
Kimura M (1980) A simple method for estimating evolutionary rates of base substitution through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Koch AL (1994) Growth measurement. In: Methods for general and molecular biotechnology. American Society for Microbiology, Washington, DC, pp 248–277
Kumar S, Tamura K Jakobsen IB, Nei M (2001) MEGA2: Molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Lee JS (1996) Bioactive components from red tide plankton, Cochlodinium polykrikoides. J Korean Fish Soc 29:165–173
Lee SO, Kato J, Takiguchi N, Kuroda A, Ikeda T, Mitsutani A, Ohtake H (2000) Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. strain A28. Appl Environ Microbiol 66:4334–4339
Lovejoy C, Bowman JP, Hallegraeff GM (1998) Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl Environ Microbiol 64:2806–2813
MacFaddin TF (1984) Biochemical tests for identification of medical bacteria. Williams & Wilkins, Baltimore, pp 36–308
Magnien RE (2001) The dynamics of science, perception, and policy during the outbreak of Pfiesteria in the Chesapeake Bay. Bioscience 51:843–852
Nagayama K, Shibata T, Fujimoto K, Honjo T, Nakamira T (2003) Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 218:601–611
NFRDI (2004) Harmful algal blooms in Korean coastal waters from 2002–2003 (in Korean). Nat Fish Res Dev Inst, Korea, Pusan
Onoue Y, Nozawa K (1989) Separation of toxins from harmful red tides occurring along the coast of Kagoshima Prefecture. In: Okaichi T, Anderson DM, Nemoto T (eds) Red tides: biology, environmental science, and toxicology. Elsevier, New York, pp 371–374
Park YT, Park JB, Chung SY, Lim WA, Kim CH, Lee WJ (1998) Isolation of marine bacteria killing red tide microalgae. I. Isolation and algicidal properties of Micrococcus sp. LG-1 possessing killing activity for harmful dinoflagellate, Cochlodinium polykrikoides (in Korean). J Korean Fish Soc 31:767–773
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 24:189–204
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Uchida T, Toda S, Matsuyama Y, Yamaguchi M, Kotani Y, Honjo T (1999) Interactions between the red tide dinoflagellates Heterocapsa circularisquama and Gymnodinium mikimotoi in laboratory culture. J Exp Mar Biol Ecol 241:285–299
Whyte JN, Haigh CN, Ginther NG, Keddy LJ (2001) First record of blooms of Cochlodinium sp. (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 40:298–304
Yanagi T, Yamamoto T, Koizumi Y, Ikeda T, Kamizono M, Tamori H (1995) A numerical simulation of red tide formation. J Mar Syst 6:269–285
Yoshinaga I, Kawai T, Takeuchi T, Ishida Y (1995) Distribution and Fluctuation of bacteria inhibiting the growth of a marine red tide plankton Gymnodinium mikimotoi in Tanabe bay. Fish Sci 61:780–786
Yoshinaga I, Kawai T, Ishida Y (1997) Analysis of algicidal ranges of the bacterial killing the marine dinoflagellate Gymnodinium mikimotoi isolated from Tanabe Bay, Wakayama Pref., Japan. Fish Sci 63:94–98
Yoshinaga I, Kim MC, Katanozaka N, Imai I, Uchida A, Ishida Y (1998) Population structure of algicidal marine bacteria targeting the red tide forming alga Heterosigma akashiwo (Raphidophyceae), determined by restriction fragment length polymorphism analysis of the bacterial 16S ribosomal RNA genes. Mar Ecol Prog Ser 170:33–44
Zheng TL, Su JQ, Maskaoui K, Yu ZM, Hu Z, Xu JS, Hong HS (2005) Microbial modulation in the biomass and toxin production of a red-tide causing alga. Mar Pollut Bull 51:1018–1025
Acknowledgments
We would like to express our appreciation to the Department of Harmful Organisms of NFRDI (National Fisheries Research and Development Institute, Republic of Korea) for providing algal cultures. Thanks are extended to the South Sea Institute of KORDI (Korea Ocean Research & Development Institute, Republic of Korea) for providing additional dinoflagellate cultures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MJ., Jeong, SY. & Lee, SJ. Isolation, identification, and algicidal activity of marine bacteria against Cochlodinium polykrikoides . J Appl Phycol 20, 1069–1078 (2008). https://doi.org/10.1007/s10811-008-9312-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-008-9312-x