Abstract
The study compared the species composition of phytoperiphyton (“lab-lab”) present in ponds when gradually filled with water weekly to depths of 5, 10, 15 and 30 cm between the wet and dry seasons, for one month before the stocking of fish was studied. This was done during the dry season (March–April, 2003) and wet season (June–July, 2002). Periphyton was allowed to grow on 24 artificial substrates set at equal distances in a 1000 m2 pond. “Lab-lab” that colonized the artificial substrates and that on the pond surrounding the substrates were scraped off from a measured surface area. Simultaneously, water was collected for the analysis of physical, chemical and biological parameters. Sampling was done bi-weekly coinciding with 2 and 7 days submergence at a desired depth before adjusting the water level. The major algae consisted of the diatoms (Bacilliarophyta), the blue green algae (Cyanobacteria), and the green algae (Chlorophyta). The diatoms were dominant during the dry season while the cyanobacteria dominated during the wet season. Twenty eight genera were observed during the dry season and 25 genera were noted in the wet season. Variation in genera and density that were observed every sampling period, was influenced by environmental conditions and the incoming water. The total algal density ranged from 100.7 × 108 – 855.1 × 108 and to 24.7 × 108 – 83.9 × 108 organisms.m−2 during the dry and wet seasons, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“Lab-lab” is a Filipino term that literally means “bloom”. It is technically known as periphyton which is made up the complex microflora and microfauna attached to the submerged surfaces of ponds (Wetzel 1971). The complex microcosm could be made up of living, senescent or dead autotrophic or heterotrophic microorganisms and fine particulate matter (Neckles et al. 1994). When “lab-lab” is grown in ponds as a food source in the farming of milkfish in brackishwater ponds, such a system is designated as periphyton-based aquaculture. Previous studies on “lab-lab” in brackishwater ponds dealt with identifying the organisms in the algal mat, and the procedures for growing them (Esguerra 1951; Ronquillo and de Jesus 1957; Tang and Chen 1966). Its importance as food of fishes was attested when algal components were found in the stomach of milkfish (Vicencio 1977). In many fishes, food intake per fish per unit time was higher when the natural feed was fed as periphyton than when it was fed as phytoplankton (Dempster et al. 1993). Filter feeding on small planktonic algae is insufficient to provide the bulk needed by most herbivorous fish, like carp and milkfish, for they may require larger food sources such as benthic algae, algal detritus or plant fodder, that can be taken in more efficiently. Fry and fingerlings of milkfish had been grown in periphyton (“lab-lab”) as its natural food in brackishwater ponds (Rabanal 1966). However, studies in brackishwater are lacking on basic studies like the qualitative and quantitative aspect of the algal communities in the “lab-lab”. This is considered important because recent trends on aquaculture incorporates algal communities in farm management to maintain good water quality. Hence, this study aims to determine the effect of season and varying depths of water on algal composition of the “lab-lab”.
Materials and methods
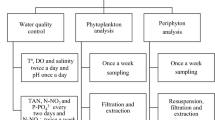
A 1000 m2 earthen pond of the Brackishwater Aquaculture Center, Institute of Aquaculture, University of the Philippines Visayas (UPV) College of Fisheries and Ocean Sciences in Leganes, Iloilo was prepared as follows: 600 kg of chicken manure was broadcast on the pond bottom after it was cracked-dried. Water was allowed to enter the pond up to about 2 cm to initiate growth of “lab-lab”, thereafter, 5 kg of 16-20-0 fertilizer was applied. “Lab-lab” was allowed to grow on artificial substrate installed at the beginning of the study. Periphyton or the algal mat was studied employing biomass accumulation on artificial substrates (Wetzel 1971; Weitzel 1979). Preliminary study was done on sampling techniques to efficiently harvest “lab-lab”. In this study, the artificial substrate (Fig. 1) was made out of a 4-legged plastic stool lined with nylon screen around its sides and an iron ring lined with net that was fitted to its top. This can efficiently collect both the floating and attached “lab-lab” free of mud. The “lab-lab” can be harvested easily by lifting the ring. Twenty four artificial substrates were installed at the bottom of the pond in an upside-down position at approximately equal distances. The pond was filled with water to depths of 5, 10, 15 and 30 cm weekly for a period of one month. Sampling of water and “lab-lab” were done bi-weekly corresponding to 2 and 7 days submergence at the desired depth and before adjusting water level. Three artificial substrates were randomly removed every sampling period. “Lab-lab” which were within the substrate and those around the artificial substrates, were scraped off to get a representative sample. Previous study which compared the community structure of diatoms developed on artificial substrata and natural ones showed no variation (Lane et al. 2003). Hence, sampling was done only on and around the artificial substrates. The samples were placed either in a Petri dish or small basin depending on the quantity of “lab-lab” collected and then mixed thoroughly. Because of the apparent variability of the appearance and structure of the mat, approximately 1 to 2 g were taken from all over the bottom of the container then homogenized to a uniform consistency. From this harvest, a one g sample was diluted with 10 mL of water. A drop of which was examined qualitatively under a compound microscope. Fresh samples were examined on the same day of sampling. However, samples that can not be examined on the same day, were stored in a freezer and analyzed within the week. Some samples were also preserved in 5% formalin solution for diatoms which were later cleaned using concentrated acid oxidation method. The taxa were identified up to the genus level using available taxonomic books in phycology. The small, unicellular algae were enumerated using the haemacytometer following the method of Martinez et al. (1975) while the filamentous algae were counted using a Sedgewick rafter counting chamber (Welch 1949). Then the number of cells per unit area, cells.cm−2 was calculated using the following formula:
where P is the number of cells counted in the haemacytometer or Sedgewick chamber, C is the volume of the concentrate (mL); S is the scraped area in cm−2 and N is number of organisms cm−2. Several counts were made on the sub-sample taken to obtain a reliable count and more accurate estimate of density. Water salinity was determined using an Atago refractometer; dissolved oxygen and temperature with YSI oxygen meter and pH, with a digital pH meter. Soluble reactive phosphorus, ammonia-nitrogen (N) and nitrite-N were determined following procedures described by Strickland and Parsons (1972). This study was undertaken during two seasons: dry (March–April, 2003) and wet (June–July, 2002). The total counts of algal divisions during each sampling period were log transformed and compared using ANOVA, with factors 5, 10, 15, and 30 cm depths (corresponding to time/weeks of colonization) and days of submergence (2 and 7 days) using a 4 × 2 factorial completely randomized design (Gomez and Gomez 1984). The mean values of the water quality parameters between dry and wet seasons were compared using a t-test. SPSS version 10 was used to perform the analyses.
Results
Phytoperiphyton composition of” lab-lab” in wet and dry season
The algal assemblage in “lab-lab” in the brackishwater ponds, consisted of diatoms (Bacillariophyta), blue-green algae (Cyanobacteria) and green algae (Chlorophyta). Their distribution in the dry and wet seasons are shown in Fig. 2. During the dry season, diatoms were observed to be dominant. These were followed by the green algae and cyanobacteria, in decreasing order. During the wet season, the cyanobacteria dominated over the diatoms and the green algae.
A total of 20 genera of diatoms and their densities during the dry and wet seasons are presented in Fig. 3. Sixteen diatoms were found during the dry season: Nitzschia, Cymbella, Navicula, Pleurosigma, Mastogloia, Surirella, Cyclotella, Pinnularia, Amphiprora, Chaetoceros Coscinodiscus, Tabellaria, Diatoma, Amphora, Fragilaria, and Thallasiothrix. During the wet season, 14 diatoms were observed in the following decreasing order of density: Navicula> Nitzschia> Cymbella> Pleurosigma> Amphora> Surirella> Pinnularia> Stauroneis> Coscinodiscus> Cyclotella> Mastogloia> Dactyliosolen> Rhizosolenia> Cocconeis.
A total of seven genera of cyanobacteria, and their densities during dry and wet seasons are shown in Fig. 4. Listed from highest to lowest density during the wet season they were: Oscillatoria> Lyngbya> Anabaena> Plectonema> Nodularia> Agmenellum> Spirulina. Five genera of cyanobacteria, were observed during the dry season Oscillatoria> Lyngbya> Spirulina> Plectonema> Agmenellum.
Six genera of green algae and their densities found during the dry and wet seasons are presented in Fig. 5. Arranged from highest to lowest counts during the dry season they were: Chlamydomonas, Chlorella, Ulothrix, Enteromorpha, Chaetomorpha, and Spirogyra. During the wet season Chaetomorpha, Ulothrix, Enteromorpha and Chlamydomonas were found. The dominance of unicellular species during dry season and filamentous types during wet season were noted. The dinoflagellate, Ceratium, was seen only once during the dry season, and its occurrence was negligible compared to the other algae.
Effect of depth of submergence on the algal composition of “lab-lab” after 7 days of submergence
In both dry and wet seasons, there were variations in the generic composition with time, depth and days of submergence (Fig. 6). Statistical analysis using ANOVA showed variation on total counts among major groups of algae in the dry and wet seasons. The variation during the wet season was highly significant (p < 0.01) for the green algae; significant for the cyanobacteria, (p < 0.05) and there was no significant difference (p > 0.05) for the diatoms. During the dry season, the variation was significant (p < 0.05) for the green algae and highly significant (p < 0.01) for both the diatoms and the cyanobacteria. There was interaction between depth and days of colonization during the wet season. It was highly significant (p < 0.01) for the cyanobacteria and significant (p < 0.05) for both the green algae and the diatoms. During the dry season, there was no interaction between depth and days of colonization. The effect of depth on the total counts of algal groups was highly significant (p < 0.01) for diatoms and significant (p < 0.05) for green algae and cyanobacteria. There was highly significant (p < 0.01) interaction between the depth and cell count. The results of ANOVA showed that total density was significantly higher (p < 0.01) during dry season and lower (p < 0.05) during the wet season. Changes per sampling period were highly significant (p < 0.01) during the dry season and significant (p < 0.05) during wet and days of colonization during wet season. However, only depth of the water showed highly significant effect (p < 0.01) on algal count during the dry season.
Water quality parameters
The mean values of the water quality parameters and their level of significance are given in Table 1. The values for salinity, pH, dissolved oxygen, water temperature, soluble reactive phosphorus, and chlorophyll a concentration were higher during the dry season than during the wet season (Table 1). During the wet season nitrite-nitrogen (N), and ammonia-N concentrations were higher than in the dry season.
Amount of rainfall
The amount of rainfall was taken from the PAGASA weather bureau station in Manduriao, Iloilo. The amount of rainfall during the wet season in June–July, 2002 were 192 mm and 598 mm, respectively, while no rainfall was recorded in the dry season, March–April, 2003.
Discussion
The main composition of the “lab-lab” in the brackish water pond included diatoms, cyanobacteria and green algae. Such algal assemblages were also found in a similar brackish water pond (Esguerra 1951), shallow water subtropical systems (Zimba et al. 2003) and in sediment microflora of intertidal mudflats (Hillebrand and Kahlert 2002). The diatom-dominated algal mat during dry season and the cyanobacteria-rich flora during the wet season could be due to the seasonal differences in environmental factors (Table 1). It appears that salinity during the dry season and rainfall largely influenced our results. It has been reported that the distribution and succession in phytoplankton are influenced by seasonal variation in rainfall and its subsequent effect on the spatial distribution of salinity in an estuary (Twomey and John 2001). This also could be true for micro-phytobenthos in a brackish water pond supplied with both freshwater and salt water.
The variation in the density of algal groups during the wet and dry seasons could also be attributed to the weather condition. Physico-chemical changes in the environment could have influenced the generic composition. In this study, dissolved oxygen in water was lower during the wet seasons when cyanobacteria were dominant. However, during the dry season diatoms were dominant. During the wet season, the skies were usually overcast but factors other than light intensity could have influenced our results. Salinity was higher during the dry season due to high rates of evaporation. Salinity has been reported to have an indirect effect on algal population by modifying species composition (Kinne 1971). Diatoms were dominant when salinity was high because they were able to withstand high salinity level. Earlier reports noted that the diatoms were the dominant group in the algal mat with higher salinity while the cyanobacteria, and chlorophytes appeared at areas with lower salinity (Herbst and Blinn 1998). Higher temperatures during the dry season were probably caused by a relatively higher amount of heat absorbed by the water during the dry season. Temperature did not vary significantly during the wet season in this study. The effect of temperature is more pronounced in temperate countries than in tropical ones where spring maximum of diatoms prevailed. Temperature was within the limits of optimal growth of algae in this study. Some species are pH sensitive but most species cease to grow when pH range is 9.5–10. The pH levels in this study were below this range. The pH in water governs the solubility of nutrients. Periphyton photosynthesis increases pH by up to 1 unit which can lead to increased precipitation of calcium phosphate (Dodds 2003). Such mechanism could have affected the higher pH and the lower concentration of soluble reactive phosphorus (SRP) during the dry season in this study. Diatoms were favoured by low phosphorus concentration and cyanobacteria tend to dominate at higher SRP. On the other hand, it was found that a taxonomic shift occurred from dominance (by biovolume) of diatoms and cyanobacteria at low total phosphorus (TP) to dominance of chlorophytes at intermediate TP (Liboriussen and Jeppesen 2006). At pH 6.5 to 9, most nutrients are rendered available. Nutrients limit algal growth. Periphyton responded positively to nutrient fertilization in freshwater ponds. (Azim et al. 2001). Phosphorus has been documented as the principal factor affecting periphyton structure and function (McCormick et al. 2001) in Florida wetlands. They found that at elevated phosphorus (6.4–12.8 g.P m−2 y−1) the cyanobacteria and diatoms prevailed and filamentous chlorophytes appeared with lower load (1.6–3.2 g.P m−2 y−1). During the wet season, the rain caused water movement as it fell into the pond, thus disturbing the bottom sediments. Water movement influences species assemblages (Horner et al. 1990; McIntire 1968). Sudden increases in velocity causes the dominance of filamentous cyanobacteria and green algae but the reverse makes diatoms dominant (Horner et al. 1990). This probably explains the observed difference in the dominant algal groups during the dry and wet seasons. Patrick (1948) observed that algae that thrive on swift streams are those that attach firmly to substrate through gelatinous masses or stalks which is a characteristic of cyanobacteria. During the wet season, the cyanobacteria persisted because of their adaptive mechanism. It has been reported that cyanobacteria in tropical wetlands can withstand extreme environmental conditions because of an array of structural and functional adaptations (N-fixing capability; oxygenic vs anoxygenic photosynthesis) (Paerl 1988). Nitrogen-fixation was high in cyanobacterial mats in marshes (Rejmankova and Komarkova 2000). Species composition may vary with the age of the pond. The younger ponds were associated with centric diatoms and green algae. Ponds older than four years however were observed to have a higher density of cyanobacteria (Zimba et al. 2003).
Besides abiotic influences, biotic mechanisms may also be involved in the changes in phytoperiphyton. The development of periphyton community structure was due to a non-interactive mechanism (exchange of organisms between substratum and water column and interactive mechanism (inter-specific competition for surface) and grazing by phytophages (Lukin 2002, 2003). In the Akulovsky water channel, diatoms and filamentous blue green sedimented from plankton at the beginning of succession from April to June. In mid-June, unicellular diatoms and colonial cyanobacteria replaced them due to grazing pressure (Lukin 2002). It has been further mentioned that both mechanisms take part in the development which changes the course of seasonal succession (with phytoplankton sedimentation affecting the early stages of succession when diatoms dominated). During the later stage of succession, when periphyton was represented by colonies of cyanobacteria, inter-specific competition for surface was important. Rotifer populations also have a positive effect on algal biomass in newly constructed ponds. This is possibly through grazing accompanied with rapid recycling of nutrients, resulting in rapid growth of algae (Zimba et al. 2003). In this study, macroinvertebrates in the periphyton samples were observed. Cattaneo and Kalff (1986) found direct effects of grazer manipulation on periphyton communities. It has also been recognized that physiological and morphological characteristics determine competitiveness of algal species in the benthic mat (Asaeda and Son 2001). There are also algae that produce toxins against other species (Paerl 1988). Periphyton mats are not only a source of food but a habitat for organisms. Snails found on the mat tend to disturb it and infaunal bivalves affect benthic algae (Page et al. 1992).
The total densities of algae in lab-lab obtained in this study were 100.7 x108 to 855.1 × 108 org.m−2 during the dry season and 24.8 × 108 to 294.6 × 108 org.m−2 during the rainy season. The densities of sediment algae were 5.85 × 108 to 5.19 × 1010 org.m−2 (Shimmel and Darley 1985). The higher density obtained during the dry season could be due to higher solar radiation. The lower standing crop during the rainy season could be attributed to the rain agitating the water and sediments causing turbidity. The biomass of periphyton decreases nutrient-rich water in lakes (Havens et al. 2001). Horner et al. (1990) found that instantaneous loss rates occur when there were sudden increases in velocity causing temporarily reduced biomass, however re-colonization and growth after biomass reduction were apparently rapid. This possibly explains the results of the irregular decrease and increase in the density of various algal groups with depth observed during the wet season in this study. Variation in algal counts could be affected by depth changes. The pond may have been filled with water carrying new organisms that could either proliferate or die. As depth increased the volume and the water column also increased, which affects the penetration of light to the bottom. Consequential dilution and changes in pond conditions during flooding probably caused changes in the composition and density of the algae. The brackish water pond at Leganes, Iloilo used in the experiment had both saltwater and freshwater sources. When water entered the pond, it caused a current that disturbed the sediments. Similar studies have shown that algal biomass is affected by sand movements (Delgado et al. 1991). At the same time, the water entering probably carried new organisms that may compete, displace and dominate over those those that had colonized earlier.
The effect of depth on densities of algal groups was demonstrated by the the ANOVA results, with depth affecting algal densities during the dry season. This can be attributed to the relatively more stable depth during the dry season. However, during the rainy season when depth was affected by intermittent rains, the time of colonization affected the algal density and not depth. During the dry season there was an apparent increase in the algal density with depth and this could be due to increased habitat volume. In littoral plant communities, larger habitat area resulting from increased water levels provides a larger range of environmental gradients thus promoting species diversity. This can lead to the coexistence of more species with different environmental tolerances and optimum (Riis and Hawes 2002). The results shown in Fig. 6 also demonstrate the growth pattern of the algal groups in the “lab-lab” mat with time. Growth of diatoms and green algae initially was low and peaked exponentially on the 4th week at a depth of 30 cm. In a study done in a reservoir in Brazil for 4 months it was observed that higher biomass was obtained during the 4th week of colonization, it reached maximum values on the 60th day after which it declined (Moschini et al. 2000). A similar trend was observed during the 4th week at 30 cm depth for diatoms and green algae during the dry season. This study was conducted for one month only and growth was not observed over a prolonged period. In an experimental mesocosm, where the effects of deposit feeders had been reduced, benthic algal biomass followed a logistic type curve and converged towards a maximum value called biotic capacity of the environment (Blanchard et al. 2001).
As far as the technology is concerned the study suggests that 4 weeks was sufficient to obtain the amount of food required for the fish to be stocked. During the wet season however, algal growth was characterized by an irregular rise and fall which could be due to uncontrolled depth caused by the rainfall, water turbulence and increased sediment suspension in the water. The experiments of Horner et al. (1990) demonstrated that an elevation in velocity above that to which periphyton are accustomed led to increase loss rates and temporarily reduced biomass after which recolonization and rapid growth can occur again. This may explain our results. Also when the depth exceeded our desired depth treatments after the first week, the pond was drained. This could have caused the decline in algal density during the second week. The differences in the total count of species observed every sampling period could also be attributed to changes in physico-chemical conditions. It has been reported that there were weak inverse relationships between the densities of periphyton and the trophic indicators such as total P and total N and algal chlorophyll, but a positive relationship with Secchi depth (Bachmann et al. 2002). Azim et al. (2002, 2003) reported that a periphyton-based freshwater pond system was dominated by changes caused by periphyton biomass. The observation that algal counts increased towards the end of the study can be attributed to the algal uptake of nutrients which resulted in the decreased levels of nitrogen and phosphorus in water. This result indicates the potential of periphyton to remove nutrients can be explored to maintain good water quality in ponds and to treat the wastewater that comes out from the fishponds. The study also shows that discharges from periphyton-based aquaculture ponds are environment-friendly.
References
Asaeda T, Son D (2001) A model of the development of periphyton community: resource and flow dynamics. Ecol Model 137:61–75
Azim ME, Wahab MA, van Dam AA, Beveridge MCM, Milstein A, Verdegam MCJ (2001) Optimization of fertilization rate for periphyton production on artificial substrates and the implications for periphyton based aquaculture. Aquacult. Res. 32:749–760
Azim ME, Wahab MA, van Dam AA, van Rooij JM, Beveridge MCM, Verdegam MCJ (2002) The effects of artificial substrate on freshwater pond productivity and water quality and the implications for periphyton-based aquaculture. Aquat. Living Res. 15:231–241
Azim ME, Milstein A, Wahab MA, Verdegam MCG (2003) Periphyton-water quality relationships in fertilized fishpond with artificial substrates. Aquacult 228:169–187
Blanchard GF, Guarini JM, Orvain F, Sauriau PG (2001) Dynamic behaviour of benthic microalgal biomass in inter-tidal mudflats. J Exp Mar Biol Ecol 264:85–100
Bachmann, RW, Horsburgh CA, Hoyer MV, Mataraza LK, Canfield DE Jr (2002) Relations between trophies state indicators and plant biomass in Florida lakes. Hydrobiol 470:219–234
Cattaneo A, Kalff J (1986) The effect of grazer size manipulation on periphtyon communities. Oecologia 69:612–617
Delgado M, de Jonge VN, Peletier H (1991) Effect of sand movement on growth of benthic diatoms. J Exp Mar Biol Ecol 145:221–231
Dempster PW, Beveridge MCM, Baird DJ (1993) Herbivory in tilapia Orechromis niloticus (L): a comparison of feeding rates on periphyton and phytoplankton. J Fish Biol 47:7–17
Dodds WK (2003) The role of periphyton phosphorus retention shallow freshwater aquatic systems. J Phycol 39:840–849
Esguerra RS (1951) Enumeration of algae in the Philippine bangus fishponds and in the digestive tract of the fish with notes on conditions of favorable growth. Phil J Fish 1:171–192
Gomez KA, Gomez AA (1984) Statistical Procedures for Agricultural Research, 680p. Wiley, New York
Havens KE, Hauxwell J, Tyler AC, Thomas S, Mc Glathery KJ, Cebrian J, Valiela I, Steinman AD, Hwang SJ (2001) Complex interaction between autotrophs in shallow marine and freshwater ecosystems: implications for community responses to nutrient stress. Environ Pollut 113:95–107
Herbst DV, Blinn DW (1998) Experimental mesocosm studies of salinity effects on the benthic algal community of a saline lake. J Phycol 34:772–778
Hillebrand H, Kahlert M (2002) Effect of grazing and water column nutrient supply on biomass and nutrient content of sediment microalgae. Aquat Bot 72:143–159
Horner RR, Welch EB, Seeley MR (1990) Responses of periphyton to changes in current velocity, suspended sediment and phosphorus concentration. Fresh Biol 24:215–232
Kinne O (1971) Salinity. In: Kinne O (ed) Marine Ecology. Academic Press, Cambridge, U.K. 1(2):821–995
Lane CM, Taffs K, Corfield JL (2003) A comparison of diatom community structure on natural and artificial substrata. Hydrobiologia 493:65–79
Liboriussen L, Jeppesen E (2006) Structure, biomass, production and depth distribution of periphyton artificial substratum in shallow lakes with contrasting nutrient concentrations. Freshw Biol 51:95–109
Lukin VB (2002) Changes in phytoperiphyton community during seasonal succession: influence of plankton sedimentation and grazing by phytophages (Chironomid larvae). Zh Obshch Biol 63:418–425
Lukin VB (2003) Mechanisms responsible for the development of periphyton community structure during seasonal succession: the role of interspecies competition and plankton sedimentation. Zh Obshch Biol 64:263–272
Martinez MR, Chakroff CL, Pantastico JF (1975) Direct phytoplankton counting techniques using the haemacytometer. Phil Agri 55:43–50
McCormick PV, O’Dell MB, Shuford III RBE, Backus JG, Kennedy WC (2001) Periphyton responses to experimental phosphorus enrichment in a subtropical wetland. Aquat Bot 71:119–139
McIntire CD (1968) Structural characteristics of benthic algal communities in laboratory streams. Ecology 49:520–537
Moschini CV, Henry R, Pompeo LM (2000) Seasonal variation of biomass and productivity of the periphytic community of artificial substrata in the Jurumirim reservoir [2pt] (SaoPaulo, Brazil). Hydrobiologia 434:35–40
Neckles HA, Koepler ET, Haas LW, Wetzel R, Orth RJ (1994) Dynamics of epiphytic photoautotrophs in Zostera marina (eel grass) microcosms: response to nutrient enrichment and grazing. Estuaries 17:567–605
Paerl HW (1988) Nuisance phytoplankton blooms in coastal estuarine and inland waters. Limnol Oceanogr 33:823–847
Page HM, Dugan JE, Hubbard DM (1992) Comparative effects of in faunal bivalves on an epibenthic community. J Exp Biol Ecol 157:247–262
Patrick R (1948) Factors affecting the distribution of diatoms. Bot Rev 14:473–524
Rabanal HR (1966) The culture of lab-lab, the natural food of milkfish or bangus (Chanos chanos Forskal) fry and fingerlings under cultivation. Phil Fishing J 22–26
Rejmankova E, Komarkova J (2000) A function of cyanobacterial mats in phosphorus limited wetlands. Hydrobiologia 431:135–153
Riis T, Hawes I (2002) Relationships between water level fluctuations and vegetation diversity in shallow water of New Zealand lakes. Aquat Bot 74:133–148
Ronquillo IA, de Jesus C (1957) Notes on the growing of lab-lab in nursery ponds. Phil J Fish 5:99–102
Shimmel SM, Darley WM (1985) Productivity and density of soil microalgae in an agricultural system. Ecology 66:1439–1447
Strickland JD, Parsons TR (1972) A Practical Handbook of Seawater Analysis, 310p, 2nd edn. Fisheries Research Board of Canada, Ottawa, Canada
Tang YA, Chen SH (1966) A survey of algal pasture soils of milkfish ponds in Taiwan. In: Pillay TVR (ed) Proc. FAO Symposium on Warm-water Fish Culture. FAO Fish. Rep. 44:41–45
Twomey L, John J (2001) Effects of rainfall and salt-wedge movement on phytoplankton succession in the Swan-Canning Estuary, Western Australia. Hydrobiol Proc 15:2655–2669
Vicencio ZT (1977) Studies on the food habits of Milkfish in Chanos chanos (Forskal). Fish Res J Philipp 2:1–18
Welch P (1949) Limnological Methods, 380p. Mc Graw Hill, New York
Weitzel RL (1979) Periphyton measurements and applications. In: Weitzel RL (ed.) Methods and Measurements of Periphyton Communities, pp.3–33
Wetzel RG (1971) Periphyton: Methods of measuring production rates. In: Vollenweider RA (ed) A Manual on Methods of Measuring Primary Production in Aquatic Environments. Blackwell Scientific Publications, Oxford, pp.41–133
Zimba P, Mischke CC, Brashear S (2003) Pond age-water column trophic relationships in channel catfish Ictalurus punctatus production ponds. Aquaculture 219:291–301
Acknowledgments
The authors would like to thank the College of Fisheries and Ocean Sciences, University of the Philippines Visayas for financial support, the Brackishwater Aquaculture Center, Institute of Aquaculture, University of the Philippines Visayas for the pond used, Dr. Romeo D. Fortes for his help in the manuscript preparation, Dr. Roman Sanares for statistical advice and Mr. Pedro Fernandez for the weather data, Ms. Mercy Fabrigas for computer works, and support staff and laborers for their help in purchasing, sampling pond, and for all the work they did to complete this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortes, N., Pinosa, L. Composition of phytobenthos in “lab-lab”, a periphyton-based extensive aquaculture technology for milkfish in brackishwater ponds during dry and wet seasons. J Appl Phycol 19, 657–665 (2007). https://doi.org/10.1007/s10811-007-9225-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9225-0