Abstract
Intensive fish farming discharges large amount of nutrients, the majority of which are composed of dissolved nitrogen in ammonium form, which promotes eutrophication in coastal waters. Macroalgae have been proven to effectively reduce the nutrients of fish farm effluents and at the same time increase the economic output of the aquaculture system when economically important species are utilized. In this study, the potential of three high value carrageenophytes (Kappaphycus alvarezii, Kappaphycus sp., K. striatum) to extract ammonium in fish farm effluent collected from a milkfish (Chanos chanos) fish cage was investigated. To establish economic viability of the integrated culture system, the effects of elevated total ammonia of fish farm effluent on the growth rate, phycocolloid content, and quality of these seaweeds were determined. Tank cultivation trials showed that the three carrageenophytes substantially reduced the ammonium content of the fish farm effluent (41–66% reduction efficiency) and consequently attained maximum daily growth rates of 4.41%, 2.90%, and 2.75% for K. striatum, Kappaphycus sp., and K. alvarezii, respectively. Their carrageenan content was improved. Carrageenan quality, however, was not significantly enhanced. Elevated ammonium in fish farm effluent did not adversely affect the performance of tank cultivated Kappaphycus; thus, future integration of these seaweeds in fish farms is feasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive fish farming activities have been pointed out as one of the culprits of coastal eutrophication (Naylor et al. 2000). Intensive fish farm effluents which consist of excess feeds and fish excretory products, could cause the formation of harmful algal blooms and anoxia (Wu 1995). Consequences to the environment caused by the shift from traditional to semi-intensive and intensive aquaculture practices are being encountered in the Philippines. In the northern Philippines where semi-intensive and intensive milkfish aquaculture is prevalent, localized fish kills due to overstocking, excessive feeding, and deteriorating water quality in pens and cages have been experienced. These mass mortalities have progressively worsened to such an extent that in January 2002 alone, the town of Bolinao, Pangasinan lost 200 million tonnes of milkfish worth approximately PhP 500 million (US $ 10 million) (Jacinto 2002).
In recent years, the need for environmentally sound aquaculture practices has been emphasized and various methods have been proposed to achieve sustainability (Neori et al. 2004). One viable approach is integrated aquaculture of fish and macroalgae where the ability of macroalgae to take up large amounts of nutrients is being explored. Thus, the waste of one resource user (finfish) becomes a nutrient source for the other (macroalgae). Such a balanced ecosystem approach not only ensures bioremediation but also optimizes efficiency of the system and creates diversification by producing other value-added products (Chopin et al. 2001).
One of the studied macroalgae for bioremediation is the chlorophycean Ulva which has a very efficient biofiltration capability (Jimenez del Rio et al. 1996; Neori et al. 1998, 2000; Schuenhoff et al. 2003). Its drawback, however, lies in the low commercial value of the harvested biomass. Rhodophycean seaweeds, on the other hand, enjoy a considerably high commercial value due to their established markets (phycocolloid and human consumption among others) and have shown potential for bioremediation. The genus Gracilaria has been demonstrated to offer both bioremediation efficiency and commercial value for its agar content (Martinez and Buschmann 1996; Troell et al. 1999).
Most studies on the use of seaweeds as biofilters for marine fish farm effluents are based on ammonium biofiltration (Jimenez del Rio et al. 1996; Cohen and Neori 1991) as ammonium is preferred over nitrate and nitrite by most seaweeds (Harrison and Hurd 2001; Qian et al. 1996; Cohen and Fong 2004). It is usually the major component of fish farm effluents (fish excretion and excess fish feed) (Buschmann et al. 1996; Obliosca et al. 2003; Hall et al. 1992). Lastly, this form of dissolved nitrogen has considerable impact on surrounding waters (i.e., high concentrations of ammonia is toxic to aquatic animals).
Macroalgae biomass production, phycocolloid yield, and quality are significantly affected by nutrient-rich waters. For agarophytes, improved gel quality has been noted to accompany an increase in biomass production when cultured in high nutrient waters (Bird et al. 1981; Martinez and Buschmann 1996) whereas phycocolloid yield decreased. However, the increase in biomass production greatly compensates for this decrease in yield (Buschmann et al. 1996; Troell et al. 1997).
The Philippines is the leading exporter of the red alga, Kappaphycus, the raw material for kappa-carrageenan production (FAO 2005). Kappa-carrageenan, the hydrocolloid which makes up the intercellular matrix material of the macroalgae in this genus, enjoys an enormous demand in the world market due to its various applications (Stanley 1990). An attractive alternative to increase production is through integrated aquaculture. Studies have shown that Kappaphycus alvarezii is capable of assimilating nitrogenous waste, ammonium in particular (Qian et al. 1996), and exhibits high growth rate in ammonium-rich waters (Li et al. 1990). This characteristic of Kappaphycus is important in the context of bioremediating intensive aquaculture systems where most of the nitrogen released is in the form of dissolved ammonium (Hall et al. 1992; Buschmann et al. 1996; Troell et al. 1999; Obliosca et al. 2003).
To evaluate the viability of Kappaphycus for future integrated aquaculture in local fish farms, we assessed the bioremediation potential of three Kappaphycus species which constitute the bulk of red algae cultivated commercially for carrageenan production. Kappaphycus alvarezii is among the pioneer species cultivated in the Philippines. Studies on its ecophysiological and phycocolloid characteristics are extensive. Kappaphycus striatum, which has been noted for its resistance to “ice–ice” disease has been the choice of farmers over K. alvarezii in some areas (Villanueva and Montaño 2003). One basic criterion in choosing seaweed species for inclusion in an integrated aquaculture system is their resistance to epiphytes and disease-causing organisms (Neori et al. 2004), other criteria include high growth rate and ease of cultivation. Kappaphycus sp. is among the newly farmed red algae observed to grow well in cultivation. Beyond these basic criteria, the choice of seaweed could be based on the intended application. The optimal system, however, would incorporate both value and bioremediation.
The aim of the present study is to assess the nutrient reduction efficiency, growth rate, and carrageenan yield of Kappaphycus cultivated with fish farm effluent. Further, since the economic value of Kappaphycus is contingent on kappa–carrageenan quality, the effect of fish farm effluent on carrageenan properties is also evaluated.
Materials and methods
Sample specimens of the carrageenophytes Kappaphycus alvarezii (Doty) Doty var. tambalang, Kappaphycus striatum (Schmitz) Doty ‘sacol’ strain, and Kappaphycus sp. (locally known as ‘aring-aring’) (KA, KS and K, respectively) were collected from a seaweed farm in Pasiagan, Bongao, Tawi-Tawi, southern Philippines. The seaweeds were transported in a styrofoam box between layers of paper and towels moistened with seawater to the outdoor hatchery facility of the Marine Science Institute in Bolinao, Pangasinan, northwestern Philippines (16°22.793′ N, 119°54.530′ E). Upon reaching the outdoor hatchery facility, the seaweeds were rinsed with seawater, cleaned of epiphytes, cultured separately under continuous flow of seawater and served as stock culture for the bioremediation experiments. The pre-culture set-up consisted of aerated 57 L aquaria with flow-through seawater pumped directly from the sea in front of the outdoor hatchery facility. During the pre-culture period, epiphytes and plant parts with any signs of depigmentation and necrosis were carefully removed. Healthy specimens were selected from the stock and acclimated for 2 weeks before being subjected to testing. During acclimation, the seaweeds were subjected to the same conditions as with the flow-through set-up, except that the seawater was changed every 24 h.
Experimental units consisted of four aquarium tanks (60.96 x 29.21 x 41.91 cm) for each species of seaweed and were randomly assigned to treatments (4 aquaria x 3 species = 12 tanks and 2 treatments). The experimental tanks received fish farm effluent water collected from the nearby milkfish culture area (16°23.225′ N, 119°55.544′ E). Water was collected downstream, within 2 m of the outside perimeter of the fish cage, to capture the maximum concentration of nutrients released. In an open system, the macroalgae would be cultured at relatively shallow depth due to light requirement; thus, surface water was collected to simulate this condition. During the period of collection, the fish cage contained approximately 100,000 two-month-old milkfish which were being fed at the rate of 10 sacks of fish feed per day (approximately 50 kg/sack).
The control set-up received the same seawater used during the pre-culture and acclimation period. The water was replaced every 24 h for both the experimental and the control units. Tanks were cleaned every 7 days and epiphytes on the seaweeds were removed.
The seaweeds were kept suspended in the water column with the aid of air diffusers (55 cm PVC pipe, 2.5 cm in diameter, with bored holes 3 cm apart) located at the bottom of the tank. The bubbling rate was regulated to attain good water mixing. Regulation of water motion is essential to ensure uniform light exposure, facilitate dispersion of nutrients, and reduce the undisturbed boundary layer adjacent to the thalli (Trono 1992; Harrison and Hurd 2001). Daylight irradiance of the culture set-up was measured with a LI–193SA spherical quantum sensor attached to a LI-1400 data logger (LI-COR Inc., USA) which was between 80 and 300 μmol photons m−2 s−1 on cloudy days; and 500–900 μmol photons m−2 s−1 on sunny days. Abiotic parameters (pH, temperature and salinity) were monitored three times a day (at 0700, 1300, and 1900 h) for the first 7 days, and then twice a day (early morning and afternoon) for the succeeding days. The range of pH, salinity, and temperature of fish farm effluent set-up during the culture period were 6.6–10.1, 32–35‰, and 23–31°C, respectively. Similar values were also noted for the control set-up.
Monitoring of the growth rate of seaweeds was done during the culture period (28 days) at seven-day intervals. Each experimental unit contained 15 seaweed cuttings, initially weighing approximately 50 g each. Three representative samples were collected at each sampling time, which were pre-selected at random. Measurement of growth was determined using the following equation (Qian et al. 1996):
where Wi is the initial wet weight, Wj is the weight on day j, T is the interval between day i and day j, and R is the daily growth rate of algae. Harvested seaweeds were patted dry with tissue paper to remove excess water after which their fresh weight was measured.
To determine the nutrient reduction efficiency of the macroalgae, water samples were collected at 0 h and 24 h for each unit every day, and were stored in polyethylene bottles. These were later frozen at –4°C until analysis. Total ammonia-N (TAN) was determined following the method of Hansen and Koroleff (1999). The amount of ammonium taken up by the seaweeds was estimated by subtracting the amount of post-algal uptake from that of pre-algal uptake. Total ammonia-N reduction efficiency was computed for each of the seaweeds. The seaweeds harvested after the culture period were cleaned and dried at 60°C in a convective air drying oven for 24 h, then were set aside for carrageenan extraction and analyses. Carrageenans were extracted by boiling the dried seaweed in 5% KOH (100 mL.g−1 dried seaweed) for one hour, then washed and boiled with distilled water for another hour. The mixture was homogenized while hot using an osterizer then pressure filtered with the aid of diatomaceous earth. The extract was then dehydrated with 2-propanol and was oven-dried at 60°C until maintaining constant weight. Polysaccharide yield was determined by dividing the weight of dried carrageenan extract by the weight of dried seaweed.
The viscosity of a 1.5% (w/w) carrageenan solution was measured at 75°C using a Cole Parmer viscometer (98936 Series, Cole Parmer Instrument Co., USA), a spindle 1 at 60 rpm. A 1.5% (w/w) carrageenan solution with 0.2% KCl was prepared for gel texture, gelling, and melting point determination. The solution was heated until the carrageenan was completely dissolved. Then, 35 mL was transferred to a 50 mL beaker and aged for at least 20 h at room temperature. Samples were covered with parafilm to avoid evaporation loss. Gel texture was determined using the TA-xT2i Texture Analyzer (Stable Microsystems, Surrey, England) equipped with a 1.27 cm diameter plunger which operates at a 2 mm s-1 descent rate. For dynamic gelling temperature determination, a hot carrageenan solution was poured into a test tube fitted with a thermometer and allowed to cool. Glass beads (diameter: 2.85 mm; weight: 30 mg) were introduced at 0.5°C intervals. The temperature at which the beads stayed on the surface was taken as the dynamic gelling temperature. The same gel was allowed to age for at least 20 h at room temperature after which, a lead shot (diameter: 4.30 mm; weight: 430 mg) was placed on the surface, then heated in a water bath. The melting temperature corresponded to the temperature at which the lead shot sank to the bottom of the test tube.
Normal distribution of the data was analyzed using the Shapiro-Wilks test for normality. Homogeneity of variance was analyzed using Levene’s test. Independent sample t-tests (α=0.05) were utilized to determine the effect of fish farm effluent on the different parameters tested, while paired samples t-tests (α =0.05) was used to compare the TAN concentration in seawater on pre and postalgal uptake. One-way ANOVA (α=0.05) was used to determine the response of the different seaweed species as reflected by the aforementioned parameters. For post-hoc comparison of means among species, Tukey’s test was employed. The statistical analyses were carried out using SPSS v13 (SPSS Inc., USA).
Results
Ammonia in culture medium
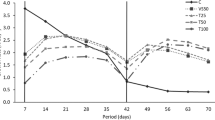
In this study, total ammonia nitrogen (\({\text{NH}}_{{\text{3}}} + {\text{NH}}^{{\text{ + }}}_{{\text{4}}} \)) concentration of fish farm effluent ranged from 3.26 to 47.63 μM, and for control seawater the range was from below detection limit to 15.8 μM during the culture period. Daily monitoring of ammonium concentration in the culture medium showed that all three carrageenophytes significantly reduced the ammonium content of the fish farm effluent (p < 0.05), but there was no significant difference (p > 0.05) in the degree of reduction among the three Kappaphycus species (Fig. 1).
Figure 2 shows the average percentage TAN reduction efficiency of the three seaweeds where K. striatum exhibited the highest percentage reduction efficiency, followed by K. alvarezii, and Kappaphycus sp. (41–66% reduction efficiency).
Growth rate
The highest growth rate was recorded during the first week of culture (Fig. 3). Seaweeds grown in fish farm effluent exhibited slightly higher values (KS: 4.41%, K: 2.90%, KA: 2.75%) than the control (KS: 3.94%, K: 2.68%, KA: 2.63%). Of the three, K. striatum displayed the highest growth rate and was significantly different (p < 0.05). Meanwhile, growth rates of K. alvarezii and Kappaphycus sp. did not differ significantly. Still, the growth rates of the three seaweed species gradually declined in the succeeding weeks.
After four weeks of culture, the weight of K. striatum increased almost four-fold, while K. alvarezii and Kappaphycus sp. exhibited only a three-fold increase in weight. However, there was no significant difference between the growth rate of seaweeds cultured in fish farm effluent and the control (t-test; p>0.05).
Carrageenan quantity and quality
Kappaphycus alvarezii cultured with fish farm effluent had a significantly higher carrageenan content compared to the control (p<0.05). The carrageenan contents of K. striatum and Kappaphycus sp., on the other hand, were not significantly different when grown with fish farm effluent or control seawater. Among the three carrageenophytes grown in fish farm effluent, K. alvarezii produced the highest carrageenan yield (87.47±4.21% dw), followed by K. striatum (72.12±11.35% dw), and then Kappaphycus sp. (69.9±2.57% dw) (Fig. 4 ).
Carrageenan yield (% dry wt) of Kappaphycus alvarezii (KA), Kappaphycus sp (K) and Kappaphycus striatum (KS) after 4 weeks of culture in natural seawater (shaded bars) and fish farm effluent (shaded bars). Same letters above columns indicate no significant difference across the species (ANOVA, p>0.05). (*) denotes significant difference between culture medium (T-test, p<0.05). Data are means +SD (n=3)
Figure 5 summarizes the carrageenan quality of the three species cultured with fish farm effluent and control seawater in terms of viscosity and gel quality indices (gel strength, deformation, cohesiveness, gelling, and melting point). The viscosity of the 1.5% carrageenan extract from the three carrageenophytes were not significantly different (p>0.05). However, KS treated with fish farm effluent displayed a significantly higher viscosity (p<0.05) as opposed to the control. As for the gel quality, kappa-carrageenan from the three carrageenophytes were not significantly different (p>0.05).
Carrageenan quality indices of Kappaphycus alvarezii (KA), Kappaphycus sp (K) and Kappaphycus striatum (KS) grown in natural seawater (unshaded bars) and fish farm effluent (shaded bars). (*) denotes significant difference (t-test, p<0.05) between culture medium; there are no significant differences among species (ANOVA, p>0.05). Data are means +SD (n=3). a Viscosity. b Gel strength. c Gelling temperature. d Melting temperature. e Deformation. f Cohesiveness
Discussion
Red algae are considered efficient in taking up nutrients rapidly due to their mechanisms for storing large reserves of nutrients. This behavior, however, seems species specific as reflected by the wide range of nutrient reduction efficiency of these seaweeds (2–90%) as summarized by Troell et al. (2003). In this study, all three carrageenophytes exhibited significant reduction efficiency. Results also showed that K. striatum was able to strip ammonium better than K. alvarezii, corroborating the fact that nutrient uptake of seaweeds was highly variable within and among species. Kappaphycus alvarezii is suggested to have a low N-requirement (Lluisma 1992), while the nutrient requirement of K. striatum is yet to be investigated.
In agarophytes, several species of Gracilaria were established as efficient biofilters such as G. chilensis (Troell et al. 1997, Buschmann et al. 1996; Martinez and Buschmann 1996), G. gracilis (Hernández et al. 2002), and G. tikvahiae (Kinne et al. 2001). The carrageenophyte, Eucheuma denticulatum, the only carrageenophyte studied for bioremediation potential, was unsuitable as a biofilter since its condition deteriorated when cultivated in fish pond effluent. The unsatisfactory result of E. denticulatum integrated culture was attributed to the high siltation of the effluent whose high nutrient concentration could be toxic to the seaweed (Msuya and Neori 2002). The limited studies exploiting K. alvarezii have shown that this alga could take up nutrients efficiently from seawater (Li et al.1990; Qian et al. 1996). Yet again, the differences in nutrient uptake could be attributed to the N-requirement of the seaweed which could vary from species to species.
In choosing which seaweed species to include in an integrated aquaculture system, the ecophysiological characteristic of the seaweed should match the cultural environment. In the case of K. striatum, its natural habitat is in muddy flats where water clarity can be very low. Thus, it is expected to adapt well in fish farm conditions where turbidity is high due to effluent discharged.
Growth rates of the three Kappaphycus species in tank culture were not significantly different in fish farm effluent and in control seawater; although the N concentration of fish farm effluent was significantly higher compared to natural seawater (Fig. 1). However, an obvious abatement of TAN levels in fish farm effluent was observed in the first week of culture, particularly from days 1 to 4. This could be a reflection of starved algal tissues absorbing N efficiently until they become saturated, thus reducing their uptake. The high growth rate of the tank cultured seaweeds in the first week can be attributed to the efficient absorption and utilization of N.
The gradual decline of growth rate after the first week can also be due to the low N requirement of Kappaphycus as exemplified in K. alvarezii. In another study dealing with the effect of N on a carrageenophyte, the growth rate of tank cultivated Agardhiella subulata (formerly Neoagardhiella baileyi) was not further increased at N concentrations higher than 0.5 μM (DeBoer 1979). Incidentally, the carrageenan yield from KA was highest among the three carrageenophytes (87.5% dw, refined carrageenan), sug-gesting that formation of cell wall structures, of which carrageenans are component, occurred during the decline in growth.
Kappaphycus striatum, on the other hand, behaved differently. Its growth rate, after the first week surge, did not vary much. Further, a slight increase in growth rate during week 4 corresponded to an obvious abatement of TAN levels on culture days, 23–26 days, suggesting that K. striatum has a different N requirement than K. alvarezii. This was further corroborated by its kappa-carrageenan yield (72.1% dw carrageenan) which was lower than K. alvarezii. It is likely that N assimilation in K. striatum after the first week of culture was not only concentrated on synthesis of cell wall structures, but also on the formation of protoplasmic constituents.
Carrageenan yield was found to be inversely related to biomass production for the case of K. striatum, which corroborates the general observation that cultivation in N-rich waters increased growth rate but consequently decreased the phycocolloid content of the algae (DeBoer 1979; Chopin et al. 1995; Troell et al. 1997).
Exposure to nutrient-rich waters induced an increase of approximately 19% in carrageenan content of K. alvarezii, and approximately 7% for both Kappaphycus sp. and K. striatum; thus, when extrapolated in industrial scale, this could translate to a vast escalation in carrageenan production.
Kappa-carrageenan rheological and textural qualities of the three carrageenophytes did not vary significantly although K. striatum grown in fish farm effluent have significantly higher viscosities as compared to the control. The present study noted a mean viscosity of 1,313 cPs for fish farm effluent grown algae and 670 cPs for those grown in natural seawater. Viscosity of carrageenan is dependent on several factors such as temperature, concentration, presence of other solutes, type of carrageenan, and molecular weight (Stanley 1990). All factors are controlled during viscosity measurement, except for the molecular weight of the kappa-carrageenan extract which is not determined. The high viscosity of the kappa-carrageenan from K. striatum grown in fish farm effluent is apparently due to the longer chains of the kappa-carrageenan polymer elaborated by the seaweed.
Slightly higher gel strengths were obtained from Kappaphycus sp. and K. striatum cultured in fish farm effluent. In contrast, K. alvarezii cultured in natural seawater produced carrageenan with higher gel strength. In field conditions, however, K. alvarezii fertilized intermittently with ammonium and, when grown in N-enriched seawater, both exhibited higher gel strengths as opposed to in an unenriched medium (Li et al. 1990).
The observed increase in gel strength corresponding to nitrogen enrichment in K. striatum and Kappaphycus sp., although slight, is consonant with the improvement in phycocolloid quality (e.g., gel strength) in algae which were grown in nitrogen rich waters (DeBoer 1979; Bird et al. 1981; Martinez and Buschmann 1996; Araño et al. 2000).
The gelling and melting temperatures of carrageenan were not significantly affected by fish farm effluent. For agar, the increase in gel strength was accompanied by an increase in gelling and melting properties when fish effluent was used (Martinez and Buschmann 1996). The improvement in gel quality was attributed to less substituted agar present in young tissues of Gracilaria with high growth activity. To duplicate commercial production conditions and to eliminate unwanted effect of sulphation on gel quality, the carrageenan is already alkali modified. Thus, analysis of the actual sulfate and 3,6-anhydrogalactose content of the extracts should be done to verify the implied effect of these constituents on the gel quality of kappa-carrageenan.
Although Kappaphycus are usually cultured in reef flats where low nutrient conditions predominate, tank culture showed that they could survive the high nutrient fish farm effluent conditions. Furthermore, they were able to remove substantial amounts of TAN in the effluent. Tank culture of Kappaphycus in fish farm effluent showed higher carrageenan yield with no apparent adverse effect on the carrageenan quality. Nevertheless, the relationship between carrageenan quality and quantity of nitrogen supplied is still vague and needs to be elucidated to maximize the full potential of the seaweed in bioremediation studies.
Future integration of these seaweeds with milkfish aquaculture could possibly reduce the nutrient discharge into coastal waters, which could inhibit the potential for outbreaks of devastating and costly hypertrophic events. Integration of these seaweeds could also increase the economic liability of the system by using the discharged nutrients (which represent a loss of money in real terms) in the production of valued kappa-carrageenan. In the broader perspective of coastal management initiatives, removal of nutrient discharge via the production of Kappaphycus would boost the social acceptability of aquaculture since it would not only optimize the efficiency of the system, but also maintain the health of coastal waters.
References
Araño KG, Trono GC Jr, Montaño NE, Hurtado AQ, Villanueva RD (2000) Growth, agar yield and quality of selected agarophyte species from the Philippines. Bot Mar 43:517–524
Bird KT, Hanisak MD, Ryther J (1981) Chemical quality and production of agars extracted from Gracilaria tikvahiae grown in different nitrogen enrichment conditions. Bot Mar 24:441–444
Buschmann AH, Troell M, Kautsky N, Kautsky L (1996) Integrated tank cultivation of salmonids and Gracilaria chilensis (Gracilariales, Rhodophyta). Hydrobiologia 326/327:75–82
Chopin T, Gallant T, Davison I (1995) Phosphorus and nitrogen nutrition in Chondrus crispus (Rhodophyta): effects on total phosphorus and nitrogen content, carrageenan production, and photosynthetic pigments and metabolism. J Phycol 31:283–293
Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Kraemer G, Zertuche-González J, Yarish C, Neefus C (2001) Integrating seaweeds into marine aquaculture systems: a key toward sustainability. J Phycol 37:975–986
Cohen RA, Fong P (2004) Nitrogen uptake and assimilation in Enteromorpha intestinalis (L.) Link (Chlorophyta): using 15N to determine preference during simultaneous pulses of nitrate and ammonium. J Exp Mar Biol Ecol 309:67–77
Cohen RA, Neori A (1991) Ulva lactuca biofilters for marine fishpond effluents: I. Ammonia uptake kinetics and nitrogen content. Bot Mar 34:475–482
DeBoer JA (1979) Effects of nitrogen enrichment on growth rate and phycocolloid content in Gracilaria foliifera and Neoagardhiella baileyi (Florideophyceae). Proceedings from the International Seaweed Symposium, Science Press, Princeton, NJ, vol 9, pp 263–273
FAO (2005) The state of world fisheries and aquaculture 2004. Food and Agricultural Organization, Rome
Hall POJ, Holby O, Kollberg S, Samuelsson M-O (1992) Chemical fluxes and mass balances in a marine fish cage farm. IV. Nitrogen. Mar Ecol Prog Ser 89:81–91
Hansen HP, Koroleff F (1999) Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhart M (eds) Methods of seawater analysis. Wiley-VCH, Weinheim, Germany, pp 188–193
Harrison P, Hurd C (2001) Nutrient physiology of seaweeds: application of concepts to aquaculture. Cah Biol Mar 42:71–82
Hernández I, Martínez-Aragon JF, Tovar A, Pérez-Lloréns JL, Vergara JJ (2002) Biofiltering efficiency in removal of dissolved nutrients by three species of estuarine macroalgae cultivated with sea bass (Dicentrarchus labrax) waste waters 2. Ammonium. J Appl Phycol 14:375–384
Jacinto GS (2002) Bolinao fish kill—looking back. AQUA Beat, 2:2
Jiménez Del Rio M, Ramazanov Z, García-Reina G (1996) Ulva rigida (Ulvales, Chlorophyta) tank culture as biofilters for dissolved inorganic nitrogen from fishpond effluents. Hydrobiologia 326/327:61–66
Kinne PN, Samocha TM, Jones ER, Browdy CL (2001) Characterization of intensive shrimp pond effluent and preliminary studies on biofiltration. North Am J Aquacult 63:25–33
Li R, Li J, Wu CY (1990) Effect of ammonium on growth and carrageenan content in Kappaphycus alvarezii (Gigartinales, Rhodophyta). Hydrobiologia 204/205:499–503
Lluisma AO (1992) Effects of nitrogen, phosphorus and photon flux density on growth, photosynthesis and proximate constituents in branch cultures of Kappaphycus alvarezii Doty (Doty). MS Thesis, University of the Philippines Diliman, Quezon City, Philippines, p 98
Martinez LA, Buschmann AH (1996) Agar yield and quality of Gracilaria chilensis (Gigartinales, Rhodophyta) in tank culture using fish effluents. Hydrobiologia 326/327:341–345
Msuya FE, Neori A (2002) Ulva reticulata and Gracilaria crassa: macroalgae that can biofilter effluent from tidal fishponds in Tanzania. Western Indian Ocean J Mar Sci 1:117–126
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Neori A, Shpigel M, Ben-Ezra D (2000) A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 186:279–291
Neori A, Ragg NLC, Shpigel M (1998) The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: II. Performance and nitrogen partitioning within an abalone (Haliotis tuberculata) and macroalgae culture system. Aquacult Eng 17:215–239
Obliosca JM, Jacinto GS, San Diego-McGlone ML (2003) Preliminary results on the development of marine environmental quality criteria for mariculture areas. Philipp Scient 40:73–87
Qian P-Y, Wu CY, Xie YK (1996) Integrated cultivation of the red alga Kappaphycus alvarezii and the pearl oyster Pinctada martensi. Aquaculture 147:21–35
Schuenhoff A, Shpigel M, Lupatsch I, Askenazi A, Msuya FE, Neori A (2003) A semi-recirculating, integrated system for the culture of fish and seaweed. Aquaculture 221:167–181
Stanley NF (1990) Carrageenans. In: Harris P (ed) Food gels. Elsevier Science, New York, pp 79–119
Troell M, Halling C, Neori A, Chopin T, Buschmann AH, Kautsky N, Yarish C (2003) Integrated mariculture: asking the right questions. Aquaculture 226:69–90
Troell M, Halling C, Nilsson, Buschmann AH, Kautsky N, Kautsky L (1997) Integrated marine cultivation of Gracilaria chilensis (Gracilariales, Rhodophyta) and salmon cages for reduced environmental impact and increased economic output. Aquaculture 156:45–61
Troell M, Rönnback P, Halling C, Kautsky N, Buschmann A (1999) Ecological engineering in aquaculture: use of seaweeds for removing nutrients from intensive mariculture. J Appl Phycol 11:89–97
Trono GC Jr (1992) Eucheuma and Kappaphycus: taxonomy and cultivation. Bull Mar Sci Kochi University, Japan, 12:51–65
Villanueva RD, Montaño MNE (2003) Fine chemical structure of carrageenan from the commercially cultivated Kappaphycus striatum (sacol variety) (Solieriaceae, Gigartinales, Rhodophyta). J Phycol 39:513–518
Wu RSS (1995) The environmental impact of marine fish culture: towards a sustainable future. Mar Pollut Bull 31:159–166
Acknowledgements
This study was partially supported by a thesis grant from the University of the Philippines (OVCRD Grant No. 014414 TNSE) to M.R. Rodrigueza. We gratefully acknowledge Dr. R. Villanueva for his valuable comments which significantly improved the manuscript. We also thank M. Ponce, O. Olivar and E. Balbin for their assistance in the field and hatchery work and extraction of carrageenan, respectively. The UP-MSI Bolinao Marine Laboratory is likewise acknowledged for allowing the use of the hatchery facility. This is MSI contribution No. 356.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigueza, M., Montaño, M. Bioremediation potential of three carrageenophytes cultivated in tanks with seawater from fish farms. J Appl Phycol 19, 755–762 (2007). https://doi.org/10.1007/s10811-007-9217-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9217-0