Abstract
Juveniles of the European sea bass Dicentrarchus labrax were exposed to both cell-free medium and whole cell cultures of the dinoflagellate Prorocentrum lima strain PL2V. Fish were also fed a commercial fish diet in tanks containing live P. lima, and Artemia that had ingested the alga. Fish exposed to the cell-free medium and to whole cell cultures were stressed and behaved abnormally when compared to the behaviour of control fish, fish in normal seawater. Stress-related behaviours included hyperactivities (jumps, fast let-right turns, surface swims, etc), poor feeding reflexes and abstinence from feeding. Fish that directly ingested the alga or that ingested Artemia containing the alga died. Histological studies revealed that gills and liver of treated fish were impacted, as opposed to the normal conditions of same tissues in control fish. The diseased organs could have been responsible for the abnormal behaviours and death of treated fish. The aquaculture and ecological implications of the results are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgal species are especially attractive in aquaculture operations for rearing fish juveniles and shellfish larvae. A mixture of algal species (Phycopure) is employed to raise shrimps (Heerbrand and Lin 2006), while a good number of other microalgal species that include Isochrysis galbana and Pavlova lutheri are acceptable starter food for winged pearl oyster Pteria sterna (Martínez-Fernández et al. 2004). Similarly, juveniles of the tilapia Oreochromis niloticus raised on Spirulina platensis demonstrated enhanced growth (Lu et al. 2004). In natural waters fish also readily graze on microalgal species. For example, gizzard shad Dorsoma cepadianum suppresses Ceratium spp populations by feeding massively on them (Drenner et al. 1984), while Tilapia galilea suppresses populations of Peridinium spp by also extensively feeding on them (Drenner et al. 1987). Not all algae are beneficial to fisheries or aquaculture. Some are harmful. Prince et al. (2006) observed that microalgae exhibit a diversity of morphologies, nutritional values, and potential chemical defences (the harmful species) that could affect the feeding and fitness of predators.
Blooms of harmful microalgal species constitute a serious threat to fisheries and aquaculture in many ways, which include phycotoxin-induced fish diseases and mortalities. Cell concentration and cell toxicity play important roles in determining the level of impacts associated with phycotoxins (Runge et al. 1992; Kim et al. 2000a,b). When phycotoxins appear, fish behaviour is first impacted, leading to a cascade of events that result in fish kills. Fish may stop feeding or show signs of suffocation. Fish behaviour, then, is used as an indicator that give fish farmers early warnings on changes in environmental or fish health conditions (Linden and Al Houari 1993; Juell 1995; Ajuzie 1998).
Phycotoxins kill fish by altering tissue structure of organs and, by so doing impose some physiological disturbances on the fish. Gill, liver and intestinal tissues are the most attacked (Jones et al. 1982; Takayama and Adachi 1984; Phillips et al. 1985; Okaichi 1989; Toyoshima et al. 1985; Arzul et al. 1998; Lush et al. 1998; Ajuzie and Houvenaghel 2003). Herbivorous, zooplanktivorous and detritivorous fish can directly or indirectly ingest phycotoxin-producing algae and/or phycotoxins in the aquatic food webs (e.g. White 1981a,b; Kelly et al. 1992).
In the wild or in fish farm units several microalgal species have been identified as fish killers. These include phytoplanktonic Prorocentrum species like Prorocentrum balticum, P. concavum and P. dentatum, P. micans, P. minimum, P. sigmoides and P. triestinum (Rabbani et al. 1990; Ho and Hodgkiss 1993; Steidinger 1993; Grzebyk et al. 1997; Noga 1998; Rangel 2002; Lu and Hodgkiss 2004; Kudela et al. 2005). However, the benthic and epiphytic P. lima has not been associated with wild or cultured fish kills (Ajuzie 2002). The animal impact is unknown (see http://waves.marine.usf.edu/redtide_menu/dinos.html). P. lima is a producer of diarrheic shellfish poisoning (DSP) toxins, which include okadaic acid (OA) and dinophysistoxins (DTXs) (Lee et al. 1989; Bravo et al. 2001). These toxins readily accumulate in shellfish and provoke DSP in humans eating such tainted seafood. P. lima has been shown to be toxic to P. micans (Ajuzie and Houvenaghel 2001) and to the brine shrimp Artemia (Ajuzie 2007). OA also accumulates in fish tissues after appearing in the food chain (Edebo et al. 1992). Therefore, a hypothesis that P. lima can negatively impact the behaviour of juvenile fish and cause their death was investigated using juveniles of the European sea bass Dicentrarchus labrax. The hypothesis that fish behaviour alters because toxic algae impact on body organs was investigated histologically. Lastly, the hypothesis that feeding trapped or farmed fish during harmful algal bloom (HAB) events would facilitate their death was also investigated in P. lima’s cell-free medium and in whole cell cultures.
Materials and methods
One hundred day old juveniles of the European sea bass (Dicentrarchus labrax) (hereafter referred-to as fish) were acquired from a commercial fish hatchery in France. They were transported to Université Libre de Bruxelles, Belgium in an oxygenated enclosure with water salinity at 12‰. During transportation and acclimatization in the laboratory, no fish died. The fish were acclimatized at 18 ± 2°C room temperature and in natural seawater diluted with dechlorinated tap water to 24‰. They were fed the same commercial fish diet that was employed at the hatchery in France, 24 hours after arriving the laboratory, by which time they had recovered from the handling stress.
Prorocentrum lima and seawater
The PL2V strain of Prorocentrum lima used for this study was obtained from Instituto Español de Oceanografia, Vigo, Spain. Various workers that include Pillet et al. (1995), Barbier et al. (1999) and Bravo et al. (2001) have determined the toxin profile of this strain and reported that it consists mainly of okadaic acid (OA) and dinophysistoxin-1 (DTX-1). They, however, reported the quantity of OA to be higher than that of DTX-1. Rausch de Traubenberg and Morlaix (1995), in addition, reported that for the PL2V strain 19 to 29% of its toxin is present in the cell-free medium. The acquired inoculum was cultured in bacteria-free K-medium enriched seawater (Keller et al. 1987), at 24 ± 1°C and at 60.19 μmol photons s−1 m−2 at a distance of ca. 10 cm from the light source, on a 12:12 h light and dark cycle. Seawater was collected from the English Channel, filtered under low vacuum on Whatman GF/C filters, and autoclaved for one hour before use.
Experiments with P. lima cell-free medium and live cells in culture
Prorocentrum lima cell-free medium was obtained through the gravity filtration method on cultures with about 9 × 103 cells mL−1. This was achieved by gently filtering the cultures on Whatman GF/C filters to avoid rupturing of the cells (Lush and Hallegraeff 1996; Tang and Dam 2001; Ajuzie 2007). Two litres of the obtained cell-free medium was poured separately into two 4 L glass tanks, after which 10 fish were introduced into each of the tanks. In another set of experiments, 2 L of P. lima culture (cell concentration: ca. 9 × 103 cells mL−1) were introduced into two 4 L glass tanks. Ten fish were introduced into each of the tanks after stirring the medium. The medium was stirred once a day throughout the duration of the investigation. In both sets of experiments, the two study tanks for each set were separated by a piece of plywood, thus preventing fish from one tank from seeing fish in the other tank. The observer who recorded the behaviours of the treated fish could see the two tanks at the same time. To avoid disturbing the fish and any possible interference on the fish behaviour, fish used in these experiments were not fed during the study period, which lasted one week. Air pumps supplied air to all tanks. Behaviours of treated fish were compared with those of the control fish (fish in natural seawater).
Experiment with clumps of harvested dried P. lima cells
Ten fish were introduced into two 4 L glass tanks containing natural seawater. P. lima cultures were gently filtered on Whatman GF/C filters. The cells were scraped off the filters and set aside to dry. Clumps of the dried mass were then presented to the fish.
Feeding fish with fish diet amidst live P. lima cells
Ten fish were held separately in two 6 L tanks, each containing 2 L of natural seawater and 2 L of P. lima culture (with ca. 4.5 × 103 cells mL−1). The fish were fed on a commercial fish diet during 6 weeks. Before the diet was introduced into the tanks, the tank water was moderately stirred to bring the P. lima cells into suspension. Fish behaviours were monitored and catalogued during the study period. Any dead fish was removed and recorded. The medium in which the fish were held was changed once, at the end of the third week, with about the same concentration of P. lima cells.
Feeding fish with P. lima-contained Artemia
Artemia nauplii, hereinafter referred-to as brine shrimp, were obtained after Artemia cysts were treated for hatching. The cysts were incubated at 27 to 29°C in a mixture of filtered seawater and distilled-water, with salinity at 20‰. Hatching occurred within 24 hours. Only metanauplii were employed in these experiments since they fed readily on the alga (Ajuzie 2002, 2007). Prorocentrum lima was fed to the brine shrimps. After one hour the brine shrimps were harvested on a sieve and fed to fish in two study tanks, each with 10 fish. Optimally fed brine shrimps ingested P. lima cells within the first hour of their initial contacts with the cells (Ajuzie 2002, 2007). These experiments lasted six weeks.
Histological examinations of fish tissues
At the end of the six weeks study period, two fish were collected each from among those fed on: (i) P. lima-contaminated brine shrimp, and (ii) a commercial fish diet amidst cells of P. lima. Another group of four fish were captured from the control tanks. All captured fish were treated for histological examinations of the gill, liver, kidney, stomach and intestinal tissues, using standard methods. The fish were killed by a quick partial cut at the junction between the head and the trunk, and preserved rapidly so as to prevent the occurrence of any post-mortem artefacts (Roberts 1978; Speare and Ferguson 1989).
Results
Fish behaviour
Fish that were placed in P. lima cell-free culture medium and in cultures with living P. lima suffered stress. They became frenzied during their initial contacts with the media, behaving quite differently from the control fish that did not show any sign of stress. Immediately after the fish were brought into contact with the cell-free medium, they repeatedly jumped out of the medium into the air, and exhibited pronounced opercular movements, window creepings and window pushings near the surface, surface swims with opened mouth, and fast left-right turns. These behaviours were highly pronounced during the first two hours in the cell-free medium. The hyperactivity subsided only after about this time. During initial contacts, fish that were in tanks with live cells of P. lima also exhibited jumps, pronounced opercular heaves, and surface swims with opened mouth. However, the hyperactivities subsided after the cells had settled to the bottom and the water became “clear”.
Fish that were offered dried clumps of P. lima, rejected them. They approached the clumps, sometimes touching them with their snout, but never ingested them. Fish that hastily picked a descending clump of P. lima immediately spat it out. Fish in tanks with cells of P. lima fed on the commercial diet presented to them. Similarly, those that were fed contaminated brine shrimp accepted the prey. However, fish in the latter groups stopped feeding during the third week, and progressively became less active from then on. The loss of activity persisted until fish death occurred. They also exhibited surface swims with open mouth, which gradually changed into surface swims with permanently opened mouth. The latter situation signalled the commencement of non-feeding (total abstinence from food) by the fish. But before these stages of surface swims with permanently opened mouth and non-feeding, test fish were not able to aim properly at food items. Attempts to capture food particles ended with misses. All treated fish exhibited bursts of uncoordinated swims, head-standing, loss of righting reflex, rolling over, spiral swims and loss of feeding coordination before dying.
Mortalities
No fish that was exposed to the cell-free culture medium died, even after staying a full week in the medium without feeding. Similarly, no dead fish was seen among those brought into contacts with live cells of P. lima, but which were not fed during the week-long investigation. Mortalities were, however, recorded among fish that were fed P. lima-contaminated brine shrimp and among fish that were kept in tanks with living P. lima cells and fed a commercial diet (Table 1). Seventy five percent of fish fed P. lima-contaminated brine shrimp died by the end of the six weeks study. They started dying during the fourth week. The majority of them died during the fifth week. Fish in this treatment steadily emaciated as they refrained from feeding, particularly from the third week. Fish that were fed the commercial diet in tanks with live cells of P. lima, ingested P. lima along with the diet (Plate 1). Ninety percent mortality was recorded for this group during the six week experimental period. Fish started dying during the third week. At the end of the experimental period, only two fish were remaining. In the control tanks, only 5% mortality was recorded during the six weeks study period.
Histological examinations
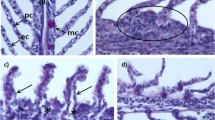
The alimentary tract of fish fed in tanks with live P. lima showed they ingested the alga as well as the fish diet (Plate 1). Kidney, stomach and intestinal tissues of fish directly or indirectly exposed to P. lima (treated fish) showed no histopathological alteration(s). However, gill and liver tissues of treated fish were highly impacted. The gill and liver tissues of the control fish were normal (Table 2; Plates 2, 3, and 4).
Discussion
Fish behaviour
The general behaviour of treated fish was different from that of control fish. Both the cell-free medium and medium with living P. lima caused fish brought into contact with them to be stressed and to behave abnormally. Several workers attest to fact that toxic dinoflagellates induce abnormal behaviours in fish brought into contact with them. For example, juveniles of the red sea bream, Pagrus major, and the Japanese anchovy, Engraulis japonica, fed on Gonyaulax excavata (=Alexandrium tamarense)-contaminated-zooplankton lost their equilibrium and swam on their sides, upside down or in circles (White et al. 1989). When pure ciguatoxins-1 & 2 and brevetoxin-1 were added to water containing the mosquito fish Gambusia affinis they exhibited pronounced opercular movement and uncoordinated swimming (Lewis 1992). When young tilapine cichlids were fed brine shrimp containing Gambierdiscus toxicus cells, they displayed behavioural abnormalities that ranged from spiral swimming to loss of equilibrium Kelly et al. 1992). Similarly, juvenile green back flounder, Rhombosolea taparina, exhibited rapid bursts of uncontrolled swimming, heaving of the operculum/mouth, small/rapid convulsive movements of the whole body and loss of orientation when exposed to the dinoflagellate Alexandrium minutum (Lush et al. 1998). The present work corroborates these findings and suggest that P. lima is toxic to juveniles of the European sea bass. It has even been suggested that OA is genotoxic and induces DNA-adduct formation in fish embryos (Huynh et al. 1998). Torigoe et al. (1988) suggested that P. lima produces neurotoxins. The neurotoxins may have been responsible for the witnessed neurological disorders among the treated fish, and which involved exaggerated reflexes, loss of feeding coordination and signs of asphyxiation.
Fish that were in contact with live cells of P. lima accepted the commercial fish diet presented to them during the first two weeks, but during the third week they became non-feeders. Fish ingested P. lima cells along with the commercial fish diet. The effects of the ingested P. lima appeared not to have been immediate, so the fish continued to feed on the introduced fish diet. But from the third week, when the toxins of P. lima must have started working in the fish, they stopped feeding and progressively became less active until they died. It is, therefore, suggested that a chronic exposure of juvenile fish to P. lima would impact their feeding behaviour.
It was observed that P. lima exudes a strong smell. This strong smell, it is believed, influenced the initial reactions of fish to the flakes of P. lima cells presented to them. Fish do have a strong sense of smell and are attracted to feed with agreeable aroma (Ajuzie and Appelbaum 1993). Apart from its repelling smell, there is also the possible that its taste is unattractive to fish, for fish spat out clumps of P. lima. Behavioural or gustatory rejection of toxic dinoflagellates has also been observed with fish larvae fed on a paralytic shellfish toxin species (see Yamamori et al. 1988; Robineau et al. 1991).
The disagreeable odour and taste of P. lima might be of some significance to its autecology. Grazers heeding to the apparent warning messages associated with its smell and taste will refrain from preying on it. Occurrence of blooms of the toxic dinoflagellate Karenia brevis is due to grazer-avoidance (Prince et al. 2006). However, all grazers may not heed to these seemingly warning features of P. lima, but might consume it to their detriment. For example, Artemia grazes continuously on P. lima until it is killed by the ingested cells (Ajuzie 2007).
An interesting behaviour of fish that were in contact with the cell-free medium and cells of P. lima was jumping. Fish repeatedly jumped out of the different media into the air. It may have been that enough oxygen was not passing over the gills of fish due to the high viscosity of the cell-free medium, even though the media holding the fish were aerated. Jenkinson and Arzul (1998) observed that Gymnodinium mikimotoi (=Karenia mikimotoi) could thicken the water with mucus, causing fish to need more oxygen to fuel water pumping over their gills, than can be extracted from the same water. In the current work, the culture medium of P. lima was observed to be viscous and gummy. It could have also been that OA produced by P. lima (Rausch de Traubenberg and Morlaix 1995; Bravo et al. 2001) caused tissue irritation in the treated fish. The viscous and gummy culture medium was also observed to cause human skin irritation. Sueoka and Fujiki (1998) even reported that OA is a potent skin-tumour promoter.
Sea bass juveniles display a high level of social interactions (Ajuzie 1998). Fish of the same age do not grow at the same rate (Houvenaghel and Huet 1987, 1989; Ajuzie 1998). Similarly, fish used in these experiments, though of same age, were not of same size. Under normal conditions, as in the control tanks, bigger intracohort siblings exhibit various dominant traits against the smaller subordinates (Ajuzie 1998). But the treated fish were somewhat passive and exhibited no agonistic behaviour as has been witnessed and described for this species in Ajuzie (1998).
Fish mortality
No mortalities were recorded among fish exposed to P. lima cell-free medium, even though fish were not fed during the one-week experimental period. This was the same for fish that were in tanks with live P. lima, but which were not fed. The implication is that fish might survive toxic blooms by not feeding. Therefore, withholding feeding of trapped fish, as in pens and cages, during harmful algal blooms could prevent mass mortalities of the farmed fish. This is in agreement with the findings of Rensel (1995). Rensel observed that one of the most effective and least costly mitigation practices for finfish aquaculture is to withhold feeding immediately prior to, and during minor harmful algal bloom episodes, arguing that this reduces the digestive demand for oxygen that is still needed for other physiological functions.
Mortalities were recorded in tanks holding treated fish. From the third week, fish fed contaminated Artemia nauplii and those that were fed the commercial diet in tanks holding living P. lima cells progressively emaciated till they died. Fish emaciation and death occurred after periods of non-feeding. For example, during the first two weeks, fish in tanks harbouring P. lima continuously fed on the introduced commercial fish feed. During feeding, they also ingested the re-suspended cells of P. lima. During the third week, however, the rate of feeding was reduced and some fish even stopped feeding. Histological examinations of gills and liver of treated fish revealed diseased and degenerated tissues.
Failures in gill respiratory and osmoregulatory functions may have contributed to fish death. Gill swellings and the subsequent separation of lamellar epithelia from the gill vessels could have affected gaseous exchange to the extent that death resulted, since the secondary lamellae of gills are the principal respiratory tissues (Laurent 1984; Ajuzie and Houvenaghel 2003). Lifting of the gill lamellar epithelium will impair oxygen transfer as a result of the increased distance between water and secondary lamellar capillaries. Also, gill swellings will reduce water space between adjacent lamellae, and thus the amount of oxygen available to the gill. The secondary lamellar structure is thus, optimized for exchange with the environmental medium with short diffusion distance (Pärt et al. 1982; Ajuzie and Houvenaghel 2003).
Secreted mucus appeared to have overwhelmingly covered the respiratory epithelium of the primary and secondary gill lamellae causing the aorta blood to become hypoxic. Hypoxia is capable of inducing a cascade of events that can disrupt the normal metabolic systems of fish and cause their death (Yang and Albright 1992; Ajuzie and Houvenaghel 2003). Since P. lima has no noticeable spines, it might be that OA and DTX produced by the PL2V P. lima strain (Pillet et al. 1995; Barbier et al. 1999; Bravo et al. 2001) caused the damage observed on the gills of affected fish. Fish could absorb biotoxins through the gills (Hughes and Perry 1976; Colin et al. 1979; Pärt et al. 1982; Haya et al. 1990). However, further work is needed to ascertain the role(s) of OA and DTXs in organ pathologies observed in fish exposed to P. lima cells.
Prorocentrum lima caused fatal degeneration of hepatic tissues among treated fish. This appeared in the form of liver necrosis and generalized loss of liver tissue architecture. Hydropic degeneration, hypertrophy, and dissociation of the hepatocytes may have led to the dissolution of the parenchymal architecture, loss of tissue integrity and ultimately loss of liver functions. Apart from the classical DSP toxins of OA and DTX, P. lima might produce hepatotoxin(s) as well. Microcystins, known hepatotoxins, induced similar pathological effects in fish liver (Phillips et al. 1985; Kent et al. 1988; Kent 1990; Råbergh et al. 1991; Andersen et al. 1993). Affected fish could have died from haemodynamic shock, resulting from congestion of the liver.
Both the gill and liver poisonings witnessed here, suggest that P. lima strain PL2V is ichthyotoxic. Jones et al. (1982) proposed a hypothesis involving ichthyotoxins when a bloom of Gyrodinium aureolum damaged the gills of farmed salmon and caused their death. Similarly, Lush et al. (1998) suggested that cells of the toxic dinoflagellate Alexandrium minutum are ichthyotoxic when they caused degenerative and mortal changes in the gills of fish. These results are at variance with the observations of Kohler et al. (1989) who reported that no reaction when ocean surgeon Acanthuris bahianus were fed various quantities of P. lima. Perhaps the P. lima employed by Kohler and co-workers was a less toxic strain, compared to the highly toxic PL2V strain employed in this work. Various workers including Lee et al. (1989) and Bravo et al. (2001) have demonstrated that toxicity in P. lima varies with strains. It could also be that the ocean surgeon is more resistant to P. lima than the European sea bass.
Prorocentrum lima may have been involved in fish kills in natural waters, but because of its cryptic nature, it evades sampling during such episodes. During fish kills, sediment, floating structures and materials that include detritus, leaves and wood, as well as other macroplant materials in the vicinity of the event should be sampled and analyzed before ruling out the involvement of P. lima. Fish in contact with P. lima toxins may die from chronic exposure to this toxic alga. This is not the case for the paralytic shellfish-poisoning species Alexandrium minutum or for the ichthyotoxic species Chattonella and Cochlodinium, which actions are acute. Juveniles of the green back flounder, Rhombosolea taparina, exposed to whole cell culture and cell-free medium of A. minutum died within three hours (Lush et al. 1998). While yellowtails, Seriola quinqueradiata, exposed to C. antiqua died within 25–90 minutes (Toyoshima et al. 1985). And juveniles of Leiognathus nuchalis exposed to Cochlodinium species died within 48 hours (Yuki and Yoshimatsu 1989).
Prorocentrum lima is typically benthic or epiphytic (Bomber et al. 1988; Faust 1993a,b). Thus, it was necessary to stir the medium holding the cells so as to bring them into the water column, prior to feeding. This was done to sort of mimic any climatological and/or hydrological conditions like storms, upwellings, destratification and mixing that could disturb the substratum on which P. lima dwells, and, by so doing, bring the cells into the water column. Results from this work show that if any of such events occurs and persists for a while, in the vicinity of P. lima populations, juvenile fish can be seriously impacted, as they might directly or indirectly ingest P. lima during feeding. Plankton filter-feeding and epibenthic micrograzing are largely a non-selective processes through which predators can ingest toxic algae.
In fish farm operations, the physical structures of cages and pens create room for the development of fouling macroalgae and other organisms on which P. lima lives and flourishes as an epiphyte (Lawrence et al. 2000). Prorocentrum lima can also hang or adhere directly on the structures. During storms, any hanging P. lima might be thrown into (re-suspended in) the medium with caged fish. This might be disastrous, especially if the P. lima cells are in high concentrations, and if fish are fed. DSP toxicity outbreaks have been linked to re-suspended P. lima in natural waters (Lawrence et al. 1998). Though P. lima is not likely to be an acceptable food for juveniles of the European sea bass, it might be picked up in and/or with acceptable food items during feeding.
References
Ajuzie CC (1998) Aspects of behavior in European sea bass juveniles. Aquac Mag 24(2):37–44
Ajuzie CC (2002) Studies on the harmful algal bloom phenomenon: a first perception and monitoring in Nigerian coastal waters, and the effects of the DSP-causing dinoflagellate Prorocentrum lima (Ehrenberg) Dodge on other aquatic biota. D.Sc. thesis, Université Libre de Bruxelles, Belgium
Ajuzie CC (2007) Palatability and fatality of the dinoflagellate Prorocentrum lima to Artemia salina. J Appl Phycol doi: 10.1007/s10811-007-9164-9
Ajuzie CC, Appelbaum S (1993) Feed attractants for glass eels. Fish Farmer March/April:25–27
Ajuzie CC, Houvenaghel GT (2001) Allelopathic growth inhibition of Prorocentrum micans Ehrenberg by Prorocentrum lima (Ehrenberg) Dodge in laboratory cultures. Can Tech Rep Fish Aquat Sci 2386:54–66
Ajuzie CC, Houvenaghel GT (2003) Prorocentrum lima is toxic to juveniles of the European sea bass Dicentrarchus labrax. Can Tech Rep Fish Aquat Sci 2498:37–45
Andersen RJ, Luu HA, Chen DZX, Holmes CFB, Kent ML, Leblanc L, Taylor FJR, Williams DE (1993) Chemical and biological evidence links microcystins to salmon “netpen liver disease”. Toxicon 31:1315–1323
Arzul G, Bodennec G, Gentien P, Bornens P, Crassous M-P (1998) The effect of dissolved oxygen on the haemolytic property of Gymnodinium ichthyotoxins. In: Reguera B, Blanco J, Fernández ML, Wyatt T (eds) Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO, pp 611–614
Barbier M, Amzil Z, Mondeguer F, Bhaud Y, Soyer-Gobillard M-O, Lassus P (1999) Okadaic acid and PP2A cellular immunolocalization in Prorocentrum lima (Dinophyceae). Phycologia 38:41–46
Bomber JW, Morton SL, Babinchak JA, Norris DR, Morton JG (1988) Epiphytic dinoflagellates of drift algae-another toxigenic community in the ciguatera food chain. Bull Mar Sci 43:204–214
Bravo I, Fernández ML, Ramilo I, Martínez A (2001) Toxin composition of the toxic dinoflagellate Prorocentrum lima isolated from different locations along the Galician coast (NW Spain). Toxicon 39:1537–1545
Colin DA, Kirsch R, Leray C (1979) Haemodynamic effects of adenosine on gills of the trout (Salmo gairdneri). J Comp Physiol 130:325–330
Drenner RW, Mummert JR, de Noyelles F Jr, Kettle D (1984) Selective particle ingestion by a filter-feeding fish and its impact on phytoplankton community structure. Limnol Oceanogr 29:941–948
Drenner RW, Hambright KD, Vinyard GL, Gophen M, Pollingher U (1987) Experimental study of size-selective phytoplankton grazing by a filter-feeding cichlid and the cichlid’s effects on plankton community structure. Limnol Oceanogr 32:1138–1144
Edebo L, Hovgaard P, Hu Y, Li XP (1992) On the presence of diarrheic shellfish toxins in fish. Planta Med 58(Suppl. Issue 1):A583–A584
Faust MA (1993a) Alternate asexual reproduction of Prorocentrum lima in culture. In: Smayda TJ, Shimizu Y (eds) Toxic phytoplankton blooms in the sea. Elsevier, Amsterdam pp 115–120
Faust MA (1993b) Sexuality in a toxic dinoflagellate, Prorocentrum. In: Smayda TJ. Shimizu Y (eds) Toxic phytoplankton blooms in the sea. Elsevier, Amsterdam pp 121–126
Grzebyk D, Denardou A, Berland B, Pouchus YF (1997) Evidence of a new toxin in the red-tide dinoflagellate Prorocentrum lima. J Plankt Res 19:1111–1124
Haya K, Martin JL, Waiwood BA, Burridge LE, Hungerford JM, Zitko V (1990) Identification of paralytic shellfish toxins in mackerel from southwest Bay of Fundy, Canada. In: Graneli E, Sundstrom B, Edler L, Anderson DM (eds) Toxic marine phytoplankton. Elsevier, New York pp 350–355
Heerbrand TC, Lin J (2006) Larviculture of red front shrimp, Caridina gracilirostris (Atyidae, Decapoda). J World Aquacult Soc 37:186–190
Ho KC, Hodgkiss IJ (1993) The occurrence and environmental significance of red tides caused by Prorocentrum minimum. Book of Abstracts 6th Int. Conf. on Toxic Phytoplankton, Nantes, France, 18–22 Oct. 1993 p 99
Houvenaghel GT, Huet T (1987) Computer-aided production modeling in fish farming, application to the eel. In: Balchen JG (ed) Automation and data processing in aquaculture (IFAC). Pergamon Press, Oxford pp 105–108
Houvenaghel GT, Huet T (1989) A model for eel growth in aquaculture. In: De Pauw N, Jaspers E, Ackefors H, Wilkins N (eds) Aquaculture a biotechnology in Progress, vol. 1. European Aquaculture Society, Bredene pp 179–184
Hughes GM, Perry SF (1976) Morphometric study of trout gills: a light-microscopic method suitable for the evaluation of pollutant action. J Exp Biol 64:447–468
Huynh C, Pinelli E, Puiseux-Dao S, Boulekbache H, Pfohl-Leszkowicz A (1998) Okadiac acid and DNA aduct formation. In: Reguera B, Blanco J, Fernández ML, Wyatt T (eds) Harmful algae. Grafisant, Spain pp 581–583
Jenkinson IR, Arzul G (1998) Effects of the flagellates, Gymnodinium mikimotoi, Heterosigma akashiwo and Pavlova lutheri, on flow through fish gills. In: Reguera B, Blanco J, Fernández ML, Wyatt T (eds) Harmful algae. Grafisant, Spain pp 425–428
Jones KJ, Ayres P, Bullock AM, Roberts RJ, Tett P (1982) A red tide of Gyrodinium aureolum in sea lochs of the Firth of Clyde and associated mortality of pond-reared salmon. J Mar Biol Ass U.K. 62:771–782
Juell J-E (1995) The behaviour of Atlantic salmon in relation to efficient cage-rearing. Rev Fish Biol Fish 5(3):320–335
Keller M, Selvin RC, Claus A, Guillard RRL (1987) Media for the culture of oceanic ultraplankton. J Phycol 23:633–638
Kelly AM, Kohler CC, Tindall DR (1992) Are crustaceans linked to the ciguatera food chain? Environ Biol Fish 33:275–286
Kent ML (1990) Netpen liver disease (NLD) of salmonid fishes reared in seawater; species susceptibility, recovery, and probable cause. Dis Aquat Org 8:21–28
Kent ML, Myers MS, Hinton DE, Eaton WD, Elston RA (1988) Suspected toxicopathic hepatic necrosis and megalocytosis in pen-reared Atlantic salmon Salmo salar in Puget Sound, Washington, USA. Dis Aquat Org 4:91–100
Kim CS, Bae HM, Yun SJ, Cho YC, Kim HG (2000a) Ichthyotoxicity of a harmful dinoflagellate Cochlodinium polykrikoides: aspect of hematological responses of fish exposed to algal blooms. J Fish Sci Tech 3:111–117
Kim CS, Lee SG, Kim HG (2000b) Biochemical responses of fish exposed to harmful dinoflagellate Cochlodinium polykrikoides. J Exp Mar Biol 254:131–141
Kohler CC, Paleudis GA, Tindall DR (1989) Behavioral abnormalities displayed by ocean surgeon following consumption of ciguatoxigenic dinoflagellates. Proc Assn Is Mar Lab Carib 22:34
Kudela R, Grant P, Probyn T, Figueiras F, Moita T, Trainer V (2005) Harmful algal blooms in coastal upwelling systems. Oceanography 18:184–197
Laurent P (1984) Morphologie et physiology des organes de la respiration aquatique chez les Vertébrés: la branchie. J Physiol Paris 79:98–112
Lawrence JE, Bauder AG, Quilliam MA, Cembella A (1998) Prorocentrum lima: a putative link to diarrhetic shellfish poisoning in Nova Scotia, Canada. In: Reguera B, Blanco J, Fernández ML, Wyatt T (eds) Harmful algae. Grafisant, Spain pp 78–79
Lawrence JE, Grant J, Quilliam MA, Bauder AG, Cembella AD (2000) Colonization and growth of the toxic dinoflagellate Prorocentrum lima and associated fouling macroalgae on mussels in suspended culture. Mar Ecol Prog Ser 201:147–154
Lee J-S, Igarashi T, Fraga S, Dahl E, Hovgaard P, Yasumoto T (1989) Determination of diarrhetic shellfish toxins in various dinoflagellate species. J Appl Phycol 1:147–152
Lewis RJ (1992) Ciguatoxins are potent ichthyotoxins. Toxicon 30:207–211
Linden T, Al Houari D (1993) Hydroacoustic monitoring of fish in Aquaculture—a method for automatic feeding control by detection of fish behaviour. ICES Statutory Meeting 1993. C.M. 1993/F:45/Session/T
Lu S, Hodgkiss IJ (2004) Harmful algal bloom causative collected from Hong Kong waters. Hydrobiologia 512:231–238
Lu J, Takeuchi T, Satoh H (2004) Ingestion and assimilation of three species of freshwater algae by larval tilapia Oreochromis niloticus. Aquaculture 238:437–449
Lush GJ, Hallegraeff GM (1996) High toxicity of the red tide dinoflagellate Alexandrium minutum to the brine shrimp Artemia salina. In: Yasumoto T, Oshima Y, Fukuyo Y (eds) Harmful and toxic algal blooms. UNESCO, Paris pp 389–392
Lush GJ, Hallegraeff GM, Munday BL (1998) Histopathological effects in juvenile greenback flounder Rhombosolea taparina exposed to the toxic dinoflagellate Alexandrium minutum. In: Reguera B, Blanco J, Fernández ML, Wyatt T (eds) Harmful algae. Grafisant, Spain pp 609–610
Martínez-Fernández E, Acosta-Salmon H, Rangel-Dávalos C (2004) Ingestion and digestion of 10 species of microalgae by winged pearl oyster Pteria sterna (Gould, 1851) larvae. Aquaculture 230:417–423
Noga EJ (1998) Toxic algae, fish kills and fish disease. Fish Pathol 33:337–342
Okaichi T (1989) Red tide problems in Seto Inland Sea, Japan. In: Okaichi T, Anderson DM, Nemoto T (eds) Red tides: biology, environmental science and toxicology. Elsevier, Amsterdam pp 137–142
Pärt P, Tuurala H, Soivio A (1982) Oxygen transfer, gill resistance and structural changes in rainbow trout (Salmo gairdneri, Richardson) gills perfused with vasoactive agents. Comp Biochem Physiol 71C:7–13
Phillips MJ, Roberts RJ, Stewart JA, Codd GA (1985) The toxicity of the cyanobacterium Microcystis aeruginosa to rainbow trout, Salmo gairdneri Richardson. J Fish Dis 8:339–344
Pillet S, Pereira A, Braekman JC, Houvenaghel G (1995) Patterns in long-term accumulation of okadaic acid and DTX1 in blue mussels, Mytilus edulis, experimentally fed with DSP containing alga Prorocentrum lima. In: Lassus P, Arzul G, Erard-Le Denn E, Gentien P, Marcaillou-Le Baut C (eds) Harmful marine algal blooms. Lavoisier Intercept Ltd, Paris pp 487–492
Prince EK, Lettieri L, McCurdy KJ, Kubanek J (2006) Fitness consequences for copepods feeding on a red tide dinoflagellate: deciphering the effects of nutritional value, toxicity, and feeding behavior. Oecologia 147:479–488
Rabbani MM, Atiq-Ur-Rehman, Harms CE (1990) Mass mortality of fishes caused by dinoflagellate bloom in Gwadar Bay, Southwestern Pakistan. In: Granéli E, Sundström B, Edler L, Anderson DM (eds) Toxic marine phytoplankton. Elsevier, Amsterdam pp 209–212
Råbergh CMI, Bylund G, Eriksson JE (1991) Histopathological effects of microcystin-LR, a cyclic peptide toxin from the cyanobacterium (blue-green alga) Microcystis aeruginosa, on common carp (Cyprinus carpio L.). Aquat Toxicol 20:131–146
Rangel I (2002) Taxonomia fitoplanctónica e mares vermelhas em Angola.10as Jornadas Técnico Cientificas do Instituto de Investigaçao Marinha. Luanda, Angola
Rausch de Traubenberg CR, Morlaix M (1995) Evidence of okadaic acid release into extracellular medium in cultures of Prorocentrum lima (Ehrenberg) Dodge. In: Lassus P, Arzul G, Erard-Le Denn E, Gentien P, Marcaillou-Le Baut C (eds) Harmful marine algal blooms. Lavoisier Intercept Ltd, Paris pp 493–498
Rensel JE (1995) Management of finfish aquaculture resources. In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual on harmful marine microalgae. IOC Manuals and Guides No. 33. UNESCO. 463–474
Roberts RJ (1978) The pathophysiology and systematic pathology of theleosts. In: Roberts RJ (ed) Fish pathology. Bailliere Tindall, London pp 55–91
Robineau B, Gagné JA, Fortier L, Cembella AD (1991) Potential impact of a toxic dinoflagellate (Alexandrium excavatum) bloom on survival of fish and crustacean larvae. Mar Biol 108:293–301
Runge J, Bragford B, Cattet M, Haya K, Paranjape M, Pauley K, Robineau B, Roy S, Stasko A, Turriff N (1992) Transfer of phycotoxins in the pelagic foodweb. Can Tech Rep Fish Aqua Sci 1893:73–80
Speare DJ, Ferguson HW (1989) Fixation artifacts in rainbow trout (Salmo gairdneri) gills: a morphometric evaluation. Can J Fish Aquat Sci 46:780–785
Steidinger KA (1993) Some taxonomic and biologic aspects of toxic dinoflagellates. In: Falconer IR (ed) Algal toxins in seafood and drinking water. Academic Press, London pp 1–28
Sueoka E, Fujiki H (1998) Carcinogenesis of okadaic acid class tumor promoters derived from marine natural products. In: Reguera B, Blanco J, Fernández ML, Wyatt T (eds) Harmful algae. Grafisant, Spain pp 573–576
Takayama H, Adachi R (1984) Gymnodinium nagasakiense sp. nov., a red-tide forming dinoflagellate in the adjacent waters of Japan. Bull Plankton Soc Japan 31:7–14
Tang KW, Dam HG (2001) Phytoplankton inhibition of copepod egg hatching: test of an exudates hypothesis. Mar Ecol Prog Ser 209:197–202
Torigoe K, Murata M, Yasumoto T, Iwashita T (1988) Prorocentrolide, a toxic nitrogenous macrocyle from a marine dinoflagellate, Prorocentrum lima. J Am Chem Soc 110:7876–7877
Toyoshima T, Ozaki HS, Shimada M, Okaichi T, Murakami TH (1985) Ultrastructural alterations on chloride cells of the yellowtail Seriola quinqueradiata, following exposure to the red tide species Chattonella antiqua. Mar Biol 88:101–108
White AW (1981a) Sensitivity of marine fishes to toxins from the red-tide dinoflagellate Gonyaulax excavata and implications for fish kills. Mar Biol 65:255–260
White AW (1981b) Marine zooplankton can accumulate and retain dinoflagellate toxins and cause fish kills. Limnol Oceanogr 26:103–109
White AW, Fukuhara O, Anraku M (1989) Mortality of fish larvae from eating toxic dinoflagellates or zooplankton containing dinoflagellate toxins. In: Okaichi T, Anderson DM, Nemoto T (eds) Red tides: biology, environmental science, and toxicology. Elsevier, Amsterdam pp 395–398
Yamamori K, Nakamura M, Matsui T, Hara TJ (1988) Gustatory responses to tetrodotoxin and saxitoxin in fish: a possible mechanism for avoiding marine toxins. Can J Fish Aquat Sci 45:2182–2186
Yang CZ, Albright LJ (1992) Effects of the harmful diatom, Chaetoceros concavicornis on respiration of rainbow trout Oncorhynchus mykiss. Dis Aquat Org 14:105–114
Yuki K, Yoshimatsu S (1989) Two fish-killing species of Cochlodinium from Harima Nada, Seto Inland Sea, Japan. In: Okaichi T, Anderson DM, Nemoto T (eds) Red tides: biology, environmental science and toxicology. Elsevier, Amsterdam pp 451–454
Acknowledgements
Support for this study came from “Fondation David & Alice Van Buuren”, ULB, and “Fondation de Meurs-François”, ULB. I thank G. Houvenaghel for his invaluable suggestions and support during the course of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajuzie, C.C. Toxic Prorocentrum lima induces abnormal behaviour in juvenile sea bass. J Appl Phycol 20, 19–27 (2008). https://doi.org/10.1007/s10811-007-9176-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9176-5