Abstract
We explored possible cognitive, behavioral, emotional, and physiological risk markers for sleep disturbance in children with autism spectrum disorders. Data from 1,583 children in the Autism Treatment Network were analyzed. Approximately 45 potential predictors were analyzed using hierarchical regression modeling. As medication could confound findings, it was included in the analyses as a covariate. Results revealed that anxiety, autism symptom severity, sensory sensitivities, and GI problems were associated with sleep disturbance. IQ positively predicted sleep disturbance, and children with Asperger’s Disorder were more vulnerable than others. The amount of variance in sleep outcomes explained by predictor variables was modest (i.e., R 2 from .104 to .201). Predictor variables were evaluated in the context of a bidirectional theoretical framework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of insomnia in children with autism spectrum disorders (ASDs) is reported to range from 40 to 80 % (Johnson et al. 2009), and investigators have been evaluating possible risk markers for insomnia. Areas of interest include physiological, behavioral, and the emotional characteristics. Over the recent past, investigators have examined the relationship between intellectual functioning, overall development, and sleep with mixed results. Several studies showed significant associations between level of intellectual functioning and sleep (Williams et al. 2004; Gabriels et al. 2005; Bruni et al. 2007; Giannotti et al. 2008; Taylor et al. 2012), while the results of other studies did not reveal a relationship (Patzold et al. 1998; Diomedi et al. 1999; Krakowiak et al. 2008; Mayes and Calhoun 2009; Autism Treatment Network 2010). A few studies showed that age was a predictor of sleep problems (Giannotti et al. 2008; Mayes and Calhoun 2009; ATN 2010) and that sleep problems vary with age (Goldman et al. 2012).
Some researchers have found that children with neurodevelopmental disorders (including epilepsy) are at risk for insomnia (Williams et al. 2004; Liu et al. 2006; Kotagal 2007; Giannotti et al. 2008; Mayes and Calhoun 2009; Touchette et al. 2009), and that disturbed sleep could also be the result of medical conditions (Williams et al. 2004; Reid et al. 2009). Insomnia has been associated with gastrointestinal problems, acid reflux, allergies to milk, and colic (Owens and Witmans 2004; Williams et al.; Liu et al. 2006; Touchette et al. 2009; ATN 2010).
Investigators have evaluated autism symptom severity to determine whether more severe ASD predicted sleep problems (Patzold et al. 1998; Ehlers et al. 1999; Tani et al. 2003; Gabriels et al. 2005; Allik et al. 2006; Goldman et al. 2009; Mayes and Calhoun 2009). The majority of studies showed that children with more severe symptoms of ASD were at risk.
Level of adaptive functioning was also evaluated for its predictive value. One study showed no association (Krakowiak et al. 2008); while the other revealed that lower adaptive functioning was associated with disturbed sleep (Taylor et al. 2012). Child internalizing behavior has also been implicated (Reid, et al. 2009; Patzold et al. 1998; Tani et al. 2003; Ivanenko et al. 2004; Limoges et al. 2005; Dahl & Harvey 2007; Allik et al. 2006; Malow et al. 2006; Mayes and Calhoun 2009). Similarly, externalizing behavior has been of interest; disturbed sleep was associated with hyperactivity, disruptive behavior, and aggression (Mindell 1993; Patzold et al. 1998; Owens and Witmans 2004; Malow et al. 2006; McGrew et al. 2007; Meltzer and Mindell 2008; Mayes and Calhoun 2009; Goldman et al. 2009).
To summarize, level of intelligence, age, medical conditions, and neurodevelopmental disorder were all associated with sleep difficulties. A strong relationship between internalizing and externalizing behavior and sleep disturbance was also established. Children with ASDs may be at risk for sleep problems in part because of the unique behavioral characteristics of autism.
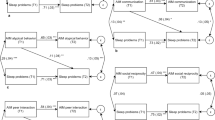
In a recent review of the literature Hollway and Aman (2011a, b) proposed a theoretical model for predicting sleep disturbance in children with ASD. This bidirectional theoretical framework was posited based on past research. The model illustrated that intellectual functioning was a moderator of autism symptom severity and ultimately of insomnia. Core symptoms of autism served as vulnerability factors and predisposed children to insomnia when presented with environmental stressors. The model maintained that each of the three autism core symptoms created an additive effect that increased maladaptive coping mechanisms such as internalizing behavior, externalizing behavior, and overarousal by decreasing likelihood of sleep. The effects of comorbid medical conditions were also depicted as predictors of sleep disturbance. A bidirectional component was also portrayed, as studies have shown that lack of sleep may exacerbate maladaptive responding. See Fig. 1.
Bidirectional theoretical framework for insomnia in children with ASDs. Note Figure reproduced from "Sleep Correlates of Pervasive Developmental Disorders: A review of the literature," by Hollway and Aman (2011b)
Although, a number of variables were revealed to be related to disturbed sleep in children with autism, the results were often mixed and inconclusive. Therefore, further exploration of these variables in a much larger sample was conducted to replicate findings and provide a foundation for evidence-based interventions. This study had four objectives, namely to determine whether (1) an inverse association existed between disturbed sleep, intellectual functioning, adaptive behavior, age, and parent education, (2) a positive association existed between sleep disturbance, autism symptom severity, and internalizing and externalizing behavior, (3) medical issues such as epilepsy, GI problems, and medication use, were positively associated with sleep disturbance, and (4) multiple components of Hollway and Aman’s model for sleep disturbance would be confirmed by this study.
Methods
Study Participants
Data were taken from 1,583 children enrolled in the ATN study. The ATN maintained a large national registry for children with ASDs, and 17 sites were collecting standard data on every child enrolled (Lajonchere et al. 2012). Study participants were enrolled if they met the diagnostic criteria for an ASD based on the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2001) and DSM-IV criteria (American Psychiatric Association 2000). Participants were 2–17 years of age (M = 6.34, SD = 3.5). Eighty-four percent of participants were male (n = 1327) and 16 % were female (n = 256). A breakdown of racial characteristics revealed that 81 % were Caucasian (n = 1236), 7 % were African American (n = 103), 6 % were Asian (n = 94), and 6 % fell in the “Other” category (n = 95). Approximately 10 % of study participants were Hispanic. As for PDD categories, 65 % of study participants had autism (n = 1032), 10 % Asperger’s Disorder (n = 152), and 25 % Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS) (n = 399).
Demographics
Demographic characteristics of the sample appear in Table 1.
Missing data analyses revealed that there were no significant differences between participants with gastrointestinal problems, respiratory problems, and seizures. However, participants with and without missing data differed significantly on two medication categories (melatonin and melatonin agonists, χ2 = 6.619, df = 1, N = 1583, p < .05, phi .065; stimulants, χ2 = 7.787, df = 1, N = 1583, p < .01, phi .070).
Study Design
This was a cross-sectional study, and the results of the analyses reflect one point in time for the variables of interest. The study was approved by Ohio State University’s Institutional Review Board.
Study Measures
Outcome Variables
Children’s Sleep Habits Questionnaire (CSHQ)
The CSHQ is a valid measure of sleep problems with good psychometric properties (Owens et al. 2000). The CSHQ was designed for use in children ages 4–12. It includes 48 items and is rated over the previous week by parents to screen for the most common sleep problems. The Total Sleep Disturbance Score consists of 33 out of 48 items. The CSHQ contains items related to eight sleep domains as follows: (1) bedtime resistance (2) sleep onset latency, (3) sleep duration, (4) anxiety around sleep, (5) night awakenings, (6) sleep disordered breathing, (7) parasomnias and (8) morning waking/daytime sleepiness. The CSHQ was used as the primary outcome for this study.
Predictor Variables
Vineland Adaptive Behavior Scale (VABS) (Sparrow et al. 1984)
The VABS is a parent interview designed to examine functional skills in developmental domains called Communication, Socialization, Daily Living Skills, and Motor Skills. The VABS provides an Adaptive Behavior Composite Score, based on domain scores for children up to age 5 years and 11 months. For older children, the composite score is based on all but the Motor Skills domain. The VABS Composite is an estimate of overall adaptive behavior. Relationships between three adaptive behavior domain scores and sleep disturbance were evaluated in the primary analyses.
Mullen Scales of Early Learning (MSEL) (Mullen 1995)
The MSEL are used to assess cognitive functioning in young children from birth to 5.5 years. The five brief scales measure early cognitive and motor development. The cognitive scores may be summarized into an Early Learning Composite score, and age equivalence scores were derived from the subscale raw scores. The MSEL was used to characterize the ATN study sample, and IQ was also estimated for younger participants.
Stanford-Binet Intelligence Scale: Fifth Edition (SB5) (Roid 2003)
The SB5 was used to characterize the study sample and for its predictive properties.
Demographics, Medical and Medication Histories
Demographics included age, race, gender, ASD diagnosis, regressed autism (developmental regression), and parent education. Medical histories included data on comorbid medical conditions (epilepsy and gastrointestinal problems). Medication histories provided information regarding all types of medicines taken at assessment.
Child Behavior Checklist (CBCL) (Achenbach 1991)
Two parent-rated versions of the CBCL were used. The first was for preschool children, ages 1.5–5 years, and the second for children and adolescents 6–18 years of age. The CBCL for younger children consists of 99 items addressing specific behavioral and emotional problems. Parents rated each behavior on a 3-point Likert-scale (0 = not true, to, 2 = very often true). The scores were summed to supply total scores for seven syndrome scales (Emotionally Reactive, Anxious/Depressed, Somatic Complaints, Withdrawn, Sleep Problems, Attention Problems, Aggressive Behavior) and five DSM-IV-derived scales (Affective Problems, Anxiety Problems, Pervasive Developmental Problems, Attention Deficit Hyperactivity Problems, Oppositional Defiant Problems). The CBCL for older children and adolescents (6–18 years of age) consists of 118 items including two open-ended items. Scoring items is the same as for the younger-child version, but types and number of subscales differ. We analyzed the T-scores of the subscales that had clear counterparts in both versions (i.e., Affective, Anxiety, Attention, and Oppositional DSM-IV subscales; Somatic and Aggressive Syndrome subscales).
Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2001)
The ADOS is a semi-structured diagnostic assessment based on a series of scheduled observations. It consists of a number of social presses, which allow the examiner to code behaviors that are related to ASDs. The ADOS is used to evaluate persons from toddlerhood to adulthood, and it provides a diagnostic classification of ASD. The newly revised diagnostic algorithm totals for each subscale were calculated to increase comparability between modules and were to determine whether autism symptom severity was a predictor of sleep problems (Gotham et al. 2008).
Short Sensory Profile (SSP) (Dunn 1999)
The Short Sensory Profile is a 38-item caregiver questionnaire that measures responses to sensory events in daily life. The items are grouped into three sections: Sensory Processing, Modulation, and Behavioral and Emotional responses. The seven subscales target Tactile Sensitivity, Under-Responsiveness/Seeks Sensation, Auditory Filtering, Visual/Auditory Sensitivity, Low Energy/Weak, Taste/Smell Sensitivity, and Movement Sensitivity. The SSP uses a 5-point Likert-scale and scores indicate more impairment.
Procedures
Continuous Sleep Outcome Variables
Children’s Sleep Habits Questionnaire (CSHQ)
Instead of using the CSHQ 33-item Total cut-point of ≥41 which was established to determine good and poor sleepers (Owens et al. 2000), we opted to analyze the CSHQ insomnia subscale and 33-item total scores as continuous variables for increased power. We also computed sleep duration variables and, in the end, we had a total of 12 sleep outcome variables.
Using all subscale scores and the newly computed sleep duration variables from the CSHQ, we calculated correlations between the 12 sleep variables, and then computed squared correlations. The squared correlations were then averaged, and the square root of the mean squared correlations was calculated. This allowed us to determine which CSHQ variables showed the greatest magnitude of association with the others and provided a means for a straightforward process of elimination. Sleep Disordered Breathing and Parasomnias were only mildly related to the insomnia subscales, and these variables were eliminated from subsequent analyses. To be consistent, the items were also dropped from the CSHQ 33-item Total score, and in the end the final primary outcome variable included 23-items, the total of all items listed in the insomnia subscales of the CSHQ. The square root of the squared correlation means were recalculated, and the results are shown in Table 2. Subsequently, we were able to reduce the number of CSHQ outcome variables and retained only those outcomes with the greatest magnitude of association to one another. The two primary outcomes included, the CSHQ 23-item Total score and the Sleep Duration subscale score, and the two secondary outcomes included, Bedtime Resistance and Sleep Anxiety.
Predictor Variables and Covariates
Medication Use
One aim was to determine whether medication use contributed to sleep disturbance or whether it alleviated sleep disturbance, which could be considered an investigational confound. In order to describe pharmacological treatment, we classified all medications reported. When classification was complete, type of medication was entered into the analyses as a covariate to ensure that we were not assessing effects that might be due to medications. We created nine categories of medications: (1) melatonin, melatonin agonists, and antianxiety medications; (2) alpha agonists; (3) antipsychotics; (4) psychostimulants; (5) selective serotonin reuptake inhibitors (SSRIs); (6) cyclic antidepressants; (7) anticonvulsants; (8) antiasthmatics; and (9) antihistamines.
IQ
Our primary analyses included the SB-5 composite scores and the MSEL composite scores integrated as one continuous variable.
Age
The age variable was analyzed both as a continuous variable and after recoding into four age categories. The age categories were <5 years, 5–6 years, 7–12 years, and 13–17 years. Recoding the variable into four separate age categories prior to analysis allowed for the possibility that a non-linear relationship existed between the outcome variables and the age category coefficients. This was important because of the differences shown in sleep patterns within the human lifespan (Acebo, et al. 2005; Carskadon & Acebo 2002; Nixon et al. 2008; Ohayon et al. 2004). The age group coefficients were plotted, which revealed that the coefficients were linear and did not disqualify use of the continuous age variable in the primary analyses.
Data Management
Missing Data Analyses
A missing data analysis was conducted in SPSS to describe the pattern of missing data and to determine whether the data were missing at random. In addition, we ran analyses on the demographic variables between cases with and without missing data, to look for significant differences between groups.
Variable Reduction Procedures
To reduce the number of predictor variables to be evaluated, we ran t tests, χ2 tests, correlations, and analysis of variance (ANOVA) significance tests. All variables significant at ≤.10 were retained for the primary analyses and those ≥.10 were eliminated from analyses. We eliminated at the .10 significance level instead of the .05 level in order to ensure that we did not remove an important predictor from the analyses.
Hierarchical Regression Models
Hierarchical regression models were selected to (a) expose any confounding or spurious relationships, (b) establish causal priority, and (c) correct for capitalization on chance (Cohen et al. 2003; Leech et al. 2005). We added the medication categories in the first block of the hierarchy to account for any confounding effects of medication. Next, we gave priority to predictor variables that had previously been found to contribute to sleep disturbance. The priority of possible predictors was based on the literature review conducted by Hollway and Aman (2011b). In addition, the significance and contribution of each beta weight was reviewed following the initial analysis to ensure the best linear combination of predictors. If necessary, the regressions were conducted again implementing the more efficient model.
Results
Primary Regression Analyses
CSHQ 23-Item Total Score
An HMLR was conducted to determine the best linear combination of the independent variables for predicting the CSHQ 23-item Total Score. The combination of predictors in Table 3 significantly predicted the CSHQ 23-item Total Score [F(12, 1,376) = 27.345, p < .001], with all eight hierarchical blocks significantly contributing to the prediction. Beta weights presented suggested that the CBCL Anxiety Problems scale score contributed the most to the variance in the CSHQ 23-item Total Score and that regressed autism, GI problems, and age, also contributed to the prediction. To a lesser degree, having Asperger’s Disorder, lower scores on the SSP underresponsive and sensation seeking subscale, or the SSP auditory filtering subscale, in addition to having higher scores on the ADOS reciprocal social interactions algorithm, and taking antidepressants, alpha agonists, or melatonin agonists, also contributed to the prediction. R 2 value was .193, indicating that 19.3 % of the variance in the CSHQ 23-item total score was explained by this model, a small-to-moderate effect (Cohen 1988). As medications accounted for about 3 % of the variance, the remaining variables accounted for about 16 % of the variance.
CSHQ Sleep Duration
An HMLR analysis determined the best linear combination of independent variables for predicting CSHQ Sleep Duration subscale score. The combination of predictors in Table 4 significantly predicted the CSHQ Sleep Duration subscale score [F(5, 1,430) = 33.101, p < .001], with all 4 hierarchical blocks significantly contributing to outcome. Beta weights suggest that VABS Communication Domain Standard, CBCL Anxiety Problems subscale, and CBCL Somatic Complaints score contributed the most to the variance in CSHQ Sleep Duration score. To a lesser degree, taking alpha agonists and melatonin agonists contributed to the prediction. The R 2 value indicated that 10.4 % of the variance in the CSHQ Sleep Duration subscale score was explained by this model, a small effect (Cohen 1988). As medicines accounted for about 3 % of the variance, the remaining variables accounted for only about 7 % of the variance.
Secondary Regression Analyses
CSHQ Bedtime Resistance
An HMLR was conducted to determine the best linear combination of variables for predicting Bedtime Resistance. The combination of predictors in Table 5 significantly predicted Bedtime Resistance [F(7, 1,425) = 35.733, p < .001], with all five hierarchical blocks contributing to the prediction. Beta weights suggest that anxiety and age contributed the most to variance in CSHQ Bedtime Resistance scores. Being Asian, having higher scores on the ADOS Reciprocal Social Interactions algorithm, and taking melatonin agonists, also contributed to prediction. In all, 14.9 % of the variance in the Bedtime Resistance subscale score was explained by this model, a small-to-moderate effect (Cohen 1988). As melatonin agonists accounted for less than 1 % of the variance, the remaining predictors accounted for almost all variance in Bedtime Resistance.
CSHQ Sleep Anxiety
The combination of variables in Table 6 significantly predicted the CSHQ Sleep Anxiety score [F(7, 1,416) = 50.848, p < .001]. Beta weights suggest that CBCL Anxiety Problems scores and age contributed the most to the variance, and that taking a melatonin agonist or an SSRI, and having lower scores on the SSP Taste/Smell Sensitivities subscale also contributed to prediction. To a lesser degree GI problems and level of intelligence also contributed. In all, 20.1 % of the variance in the Bedtime Resistance subscale score was explained by this model, a small-to-moderate effect. Melatonin agonists and SSRIs accounted for about 2 % of the variance.
Discussion
Summarizing Predictor Variables
Whereas numerous possible predictors of sleep problems were explored, only a few were confirmed as placing children with ASDs at risk for sleep difficulty. Table 7 summarizes the results.
Predictor Variables and Magnitude of Association
Of the four hierarchical multiple linear regression analyses, the model of best fit explained 20 % of the variance in Sleep Anxiety scores when all predictors were entered into the equation (i.e., R 2 = .201). Each of the other three models in Study 1 explained less variance in sleep outcomes: (a) CSHQ 23-item Total, R 2 = .193; (b) Bedtime Resistance, R 2 = .149; (c) and Sleep Duration, R 2 = .104. This was somewhat disappointing considering the large sample size and the large range of predictors explored. However, this may be the best one can hope for in correlational sleep research. According to Ferguson (2009), all reported R 2s were above the recommended minimum effect size for representing a “practically” significant effect in social science data. In addition, the current study results were found to be comparable with other independent research (Taylor et al. 2012; Giannotti et al. 2008; Schreck et al. 2004).
Conceptualizing Sleep Dysfunction in Children with ASDs
We hypothesized that a number of variables would be positively and negatively associated with sleep disturbance in children with ASDs. Our hypotheses were based on a comprehensive review of the sleep literature and a “Bidirectional Theoretical Framework of Sleep Disturbance” was posited to organize risk markers for sleep disturbance in ASD. In the discussion to follow, we explain how well results of this study fit the hypotheses. Some predictor variables were consistent and fit well within the framework, while other predictor variables were inconsistent with the model presented, and still others remained inconclusive (i.e., neither consistently confirming nor disconfirming the model). Please refer back to Fig. 1 for clarification.
Vulnerability Factors
Level of Intellectual Functioning
One of the more surprising results of this study was that IQ was positively associated with one outcome, Sleep Anxiety scores on the CSHQ. Although, the outcomes of previous research revealed mixed results in general, the model was based on the belief that lower-functioning individuals have more severe symptoms and poorer coping skills and therefore exhibit more sleep problems when compared to higher-functioning individuals. This inconsistency with Hollway and Aman’s (2011b) conceptual model indicates that other factors may be involved. For example, a review of the results shown in Table 3 reveals that the ASD subtype, Asperger’s Disorder, contributed to the variance in CSHQ 23-item Total scores. Considering the DSM-IV-TR diagnostic criteria for Asperger’s Disorder, there should be no clinically significant delays in language acquisition or cognitive development (American Psychiatric Association 2000). Although IQ positively predicted Sleep Anxiety, it did not predict the other three sleep outcomes. Thus, when considering “vulnerability factors” for disturbed sleep, it may be necessary, but not sufficient, to consider level of intellectual functioning alone. ASD subtype may be the conditional factor at work.
Autism Symptom Severity and Sleep: Regressed Type of Autism
Developmental regression contributed to the variance shown in CSHQ 23-item Total scores (see Table 3). These results are consistent with recent literature that reported more sleep problems in children with developmentally regressed autism than non-regressed autism, or typically developing children (Giannotti et al. 2008; Giannotti et al. 2011). In addition, previous investigators have found that children with regressed autism often score lower on intelligence tests and have more severe autism symptoms than children who do not, especially in the area of restricted and repetitive patterns of interest (Meilleur and Fombonne 2009). Therefore, the result appears consistent with Hollway and Aman’s (2011b) hypothesized model in relation to IQ.
Autism Symptom Severity and Sleep: Communication Deficits
An inverse association was found between VABS Communication Domain and CSHQ Sleep Duration scores. Consistent with the results of this study, a recent investigation found a positive association between TST, the number of hours a child napped, and VABS Communication scores (Taylor et al. 2012). It has been established that a relationship exists between sleep, maturational cognitive processes, and adaptive skills (Dan and Boyd 2006) and that persons with developmental disabilities have altered sleep patterns compared to their typically developing peers (Diomedi et al. 1999; Harvey and Kennedy 2002; Bruni et al. 2007; Giannotti et al. 2008; Johnson et al. 2009; Giannotti et al. 2011). This may have implications for children who are not sleeping well and are challenged daily to acquire better communication skills. Neither the VABS Daily Living Domain scores nor the Social Behavioral Domain scores contributed to the variance in sleep outcomes. We hypothesized that autism symptom severity would predict sleep outcomes and results showed that VABS Communication Domain scores, measuring one of the core autism symptom domains, predicted sleep dysfunction. This is consistent with Hollway and Aman’s framework (2011b).
Autism Symptom Severity and Sleep: Reciprocal Social Interaction
Reciprocal social interaction as measured by the ADOS contributed to the variance in CSHQ 23-item Total scores and Bedtime Resistance. Poor Reciprocal Social Interaction was correlated with problem behavior at bedtime. Deficits in reciprocal social interactions were associated with higher CSHQ 23-item Total scores and Bedtime Resistance scores. Thus the results are consistent with the hypothesized model.
Autism Symptom Severity and Sleep: Sensory Sensitivities
Lower scores on the Taste/Smell Sensitivities subscale of the SSP (i.e., more impairment) were associated with increased sleep anxiety. An inverse relationship was also found between Underresponsive/Sensory Seeking subscale scores, Auditory Filtering subscale scores, and CSHQ 23-item Total scores. To clarify, children who were underresponsive, sensory seeking, and who displayed auditory filtering problems, had more trouble sleeping. Therefore, this result is consistent with the Hollway and Aman (2011b) model. Future investigations targeting physiological arousal, sensory sensitivities, and sensory seeking behavior in relation to sleep dysfunction may provide a service to the field. For example, it has been hypothesized that children who are underresponsive and who have sensory seeking behavior may miss the environmental cues that entrain the sleep/wake cycle, causing delayed and disrupted sleep (Maski et al. 2011). By contrast, children who are sensitive to certain tastes, smells, or textures, may become anxious and defiant when confronted with everyday activities such as teeth brushing just prior to bedtime, causing Sleep Onset Delays (Stein et al. 2011).
Autism Symptom Severity and Sleep: ASD Subtype
Two of the core symptoms of autism (i.e., communication deficits, social skills deficits), in addition to sensory sensitivities, and underresponsiveness to the environment, contributed to the variance in sleep outcomes in our study results. However, Restricted and Repetitive Behavior scores on the ADOS did not significantly predict sleep outcomes. More research is necessary to confirm whether one or more of the symptom clusters within “Autism Spectrum Disorder” predicts disturbed sleep.
Age and Sleep
There was an inverse association between age and sleep outcomes in three out of four analyses (i.e., CSHQ 23-item Total, Bedtime Resistance, Sleep Anxiety). Although three of the studies reviewed by Hollway and Aman (2011b) assessed age-related sleep disturbance, just one showed that age was associated with sleep problems (Giannotti et al. 2008). Therefore, age was not included as a predictor in the conceptual model and a revision may be necessary to include age as a vulnerability factor. A more recent study showed that sleep problems change with age, and that parents of younger children reported more bedtime resistance, sleep anxiety, and night awakenings (Goldman et al. 2012). Continued study will help.
Environmental Stressors
Stimulus Changes, Fear Evoking Stimuli, and Sleep
Hollway and Aman’s (2011b) model posits three components to sleep dysfunction (i.e., Vulnerability Factors, Environmental Stressors, and Maladaptive Coping Strategies). When confronted with certain environmental stressors, children with ASDs are hypothesized to resort to inadequate coping strategies that interfere with sleep. This aspect of the model was not tested. Nevertheless, new information was revealed that may necessitate expansion of this theory. Three types of sensory problems contributed to the variance in sleep outcomes: (1) Taste/Smell Sensitivities, (2) Underresponsiveness/Sensory Seeking, and (3) Auditory filtering. Consistent with this finding, investigators have shown that children with ASDs present with both sensory underresponsivity and overresponsivity (Lane et al. 2010). On the one hand, environmental stimuli may cause discomfort that interferes with sleep and, on the other, the underresponsive child may miss important environmental cues necessary for an uninterrupted sleep/wake cycle (Maski et al. 2011). These contrasting sensory profiles contribute to the complexity of establishing risk markers for sleep dysfunction in children with ASDs.
Maladaptive Coping Strategies
Internalizing Behavior and Sleep
Anxiety was consistently represented in all four analyses. The individual contribution of CBCL Anxiety Problems to the variance in Sleep Anxiety scores established it as the strongest predictor (β = .378). Anxiety problems also contributed to the variance in CSHQ 23-item Total scores (β = .283), Bedtime Resistance (β = .267), and Sleep Duration scores (β = .141). The fact that anxiety was the strongest predictor of disturbed sleep is not surprising, as other investigators have reported associations between sleep disturbance and internalizing behavior (Patzold et al. 1998; Limoges et al. 2005).
One aim of this study was to sort out whether anxiety or depression was the bigger predictor of insomnia in children with ASDs. However, it was impossible to evaluate the predictive properties of the CBCL Affective subscale (assessing depression), as the presence of sleep items within the scale would have led to circularity (i.e., poor sleep predicts poor sleep). Therefore, we reluctantly excluded the Affective subscale in our analyses. Nevertheless, the results are consistent with Hollway and Aman’s (2011b) conceptual model, that internalizing behavior is a predictor of disturbed sleep. More research is necessary, using depression scales that are not contaminated with sleep items, in order definitively to establish whether anxiety or depression is the more salient in predicting sleep problems.
CBCL Somatic Complaints contributed to the variance in CSHQ Sleep Duration Scores. Somatic complaints often manifest themselves in conjunction with anxiety and depression (Hughes et al. 2008; Haftgoli et al. 2010).
Externalizing Behavior: Aggression
Although not reported in this manuscript, we have data in preparation that support this component of the model and will be described in a future manuscript.
Physical Conditions and Medication Use
Comorbid Medical Conditions: Gastrointestinal Problems
Gastrointestinal problems including constipation and diarrhea, predicted CSHQ 23-itemTotal scores, and Sleep Anxiety. These results are consistent with recent literature which indicated that GI problems were related to disturbed sleep (ATN 2010; Williams et al. 2004; Liu et al. 2006). Consistent with the Hollway and Aman (2011b) theoretical model, the discomfort associated with GI problems may contribute to sleep disturbance.
Comorbid Medical Conditions: Epilepsy
Epilepsy did not predict sleep dysfunction in this study, and this result was therefore inconclusive with the hypothesis presented in the conceptual model.
Medication Use
Previous investigations in children that included medication use as a predictor of sleep are uncommon (Liu et al.; Mayes and Calhoun 2009), and within these investigations the effects of medication were evaluated as an undifferentiated whole (i.e., all medications included in one categorical variable). We tried to mitigate the possible confounds introduced by medication use and any effects that may have been offsetting. Some drugs may worsen sleep in some children while improving it in others. Regardless, we were able to set this variance aside and also to demonstrate that it had rather minor overall effects. As such, the strategy seemed successful.
Study Results and Treatment Implications
The current study findings suggest that the very symptoms that elicit a diagnosis of ASD (i.e., impaired communication, impaired reciprocal social interactions) are risk factors for sleep disturbance. In addition, these characteristics influence how a child responds to the environment. Certain situations will magnify these problems. When this occurs, children may cope by engaging in internalizing and externalizing behaviors. Clinicians and researchers alike should strive to identify ways for decreasing the anxiety that may be related to these events. For example some strategies that might enhance the sleep profile are (a) behavioral training, (b) social skills training, (c) teaching coping skills, and (d) exposure therapy. It may also be necessary to turn to pharmacological treatment for alleviating anxiety related issues.
Study Strengths and Limitations
Among study strengths were the following. First, we were fortunate to have access such a large set of data for this project. Second, the ATN Registry includes children who were recruited for no other reason than that they had an ASD, and the sample appears to be quite representative of the general ASD population. Third, the study sample was diagnosed by DSM-IV interview and by the ADOS which was administered by research-reliable clinicians, and hence the sample was well characterized for the presence of ASD. Fourth, we had access to such a rich set of predictor variables.
Conversely, the ATN Registry data, though a rich source of patient information, had considerable missing data. Missing data can be problematic if they are not missing at random. For this reason, we conducted extra checks using Little’s Chi Square which suggested that the data were missing completely at random (Little 1988). The finding that epilepsy was not associated with sleep disturbance may be spurious, as the ATN did not provide comprehensive information on the type, frequency, and activity level of seizures. Another problem may be that multiple IQ tests were used to assess intelligence, and combining these into one index could have increased error. Importantly, sleep outcome measures were all obtained by parent report and were subjective rather than objective measures such as use of actigraphy. Hence, the problem of source variance (all classifications based on parent report) may have inflated some associations that were found. Finally we were unable to test the hypothesis that depression would be associated with sleep disturbance, as the CBCL Affective Problems subscale contained three sleep items which would have confounded the results.
Future Directions
First, there is a need to focus on any association of anxiety with sleep disturbance, as anxiety was shown to be the strongest predictor in this investigation. Second, any association of depression with sleep disturbance also needs further evaluation given its history of relationship with sleep problems. Furthermore, we as investigators must be careful to ensure that manifestations of sleep quality not appear in our measures of anxiety or depression, as this will only lead to circularity. Third, longitudinal studies are sorely needed to enable us to evaluate possible causal factors that may affect sleep variables. Fourth, there appear to be two types of sensory processing phenotypes that disrupt sleep (i.e., children with taste/smell over-sensitivities and children who are under-responsive and sensory seeking). Investigations into the mechanism of sleep disturbance in these sensory-processing types may provide important clues for interventions that may differ with phenotype. Fifth, the “Bidirectional Theoretical Model” used here provides a framework for conceptualizing and understanding sleep disturbance in ASDs (Hollway and Aman 2011a, b). It may be worth persisting with the model, with appropriate revisions and corrections, as we add to our knowledge of ASD sleep function and dysfunction. Finally, future researchers should consider targeting the correlates of sleep disturbance by investigating possible interventions for anxiety, depression, and sensory sensitivities, while being cautious to ensure that any affective indices are not “contaminated” with markers of sleep.
Conclusion
This study evaluated many compelling risk markers for sleep disturbance in children with ASDs. As a potential confound, medication use explained approximately 3–4 % of the variance. Anxiety was the strongest predictor of sleep disturbance. In addition, the core symptoms of autism, developmental regression, ASD subtype (i.e., Asperger’s Disorder), sensory sensitivities, GI problems, and age predicted some indices of sleep quality. The data were evaluated in light of a model including risk factors for disturbed sleep. Although we have identified some correlates of sleep dysfunction in ASD, this preliminary work has also grappled with some methodological barriers. We have made significant progress, but we still have a long way to go.
References
Acebo, C., Sadeh, A., Seifer, R., Tzischinsky, O., Hafer, A., & Carskadon, M. A. (2005). Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep, 28, 1568–1577.
Achenbach, T. M. (1991). Manual for the child behavior checklist 4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry.
Allik, H., Larsson, J., & Smedje, H. (2006). Insomnia in school-age children with Asperger’s syndrome or high-functioning autism. BMC Psychiatry, 28, 6–18.
American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders, (4th ed., text revision). Washington, DC: American Psychiatric Association.
Autism Treatment Network Collaboration. (2010). Autism treatment network patient registry annual report. Autism Speaks Foundation (Unpublished annual report).
Bruni, O., Ferri, R., Vittiorie, E., Novelli, L., Vignati, M., & Porfirio, M. C. (2007). Sleep architecture and NREM alterations in children and adolescents with Asperger’s Syndrome. Sleep, 30, 1577–1585.
Carskadon, M. A., & Acebo, C. (2002). Regulation of sleepiness in adolescents: Update, insights, and speculation. Sleep, 25, 606–614.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). London: Lawrence Erlbaum Associates.
Cohen, J., Cohen, P., West, S. G., & Aiken, L. S. (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ: Lawrence Earlbaum Associates, Inc., Publishers.
Dan, B., & Boyd, S. G. (2006). A neurophysiological perspective on sleep and its maturation. Developmental Medicine and Child Neurology, 48, 773–779.
Dahl, R. E., & Harvey, A. G. (2007). Sleep in children and adolescents with behavioral and emotional disorders. Sleep Medicine Clinics, 2, 501–511.
Diomedi, M., Curatolo, P., Scalise, A., Placidi, F., Caretto, F., & Gigli, G. L. (1999). Sleep abnormalities in mentally retarded autistic subjects: Down’s Syndrome with mental retardation, and normal subjects. Brain and Development, 21, 548–553.
Dunn, W. (1999). The sensory profile manual. San Antonio: The Psychological Corporation.
Ehlers, S., Gillberg, C., & Wing, L. (1999). A screening questionnaire for Asperger Syndrome and other high-functioning autism spectrum disorders in school age children. Journal of Autism and Developmental Disorders, 29, 129–141.
Ferguson, C. J. (2009). An effect size primer: A guide for clinicians and researchers. Professional Psychology: Research and Practice, 40, 532–545.
Gabriels, R. L., Cuccaro, M. L., Hill, D. E., Ivers, B. J., & Goldson, E. (2005). Repetitive behaviors in autism: Relationships with associated clinical features. Research in Developmental Disabilities, 26, 169–181.
Giannotti, F., Cortesi, F., Cerquiglini, A., Miraglia, D., Vagnoni, C., Sebastiani, T., et al. (2008). An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. Journal of Autism and Developmental Disorders, 38, 1888–1897.
Giannotti, F., Cortesi, F., Cerquiglini, A., Vagnoni, C., & Valente, D. (2011). Sleep in children with Autism with and without autistic regression. Journal of Sleep Research, 20, 338–347.
Goldman, S. E., Richdale, A. L., Clemmons, T., & Malow, B. A. (2012). Parental sleep concerns in autism spectrum disorders: Variations from childhood to adolescence. Journal of Autism and Developmental Disorders, 42, 531–538.
Goldman, S. E., Surdyka, K., Cuevas, R., Adkins, K., Wang, L., & Malow, B. A. (2009). Defining the sleep phenotype in children with autism. Developmental Neuropsychology, 43, 560–573.
Gotham, K., Risi, S., Dawson, G., Tager-Flusberg, H., Joseph, R., Carter, A., et al. (2008). A replication of the autism diagnostic observation schedule (ADOS) revised algorithms. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 642–651.
Haftgoli, N., Favrat, B., Verdon, F., Vaucher, P., Bischoff, T., Burnand, B., et al. (2010). Patients presenting with somatic complaints in general practice: Depression, anxiety and somatoform disorders are frequent and associated with psychosocial stressors. BMC Family Practice, 11, 67.
Harvey, M. T., & Kennedy, C. H. (2002). Polysomnographic phenotypes in developmental disabilities. International Journal of Developmental Neuroscience, 20, 443–448.
Hollway, J. A., & Aman, M. G. (2011a). Pharmacological treatment of sleep disturbance in children with developmental disabilities. Research in Developmental Disabilities, 32, 939–962.
Hollway, J. A., & Aman, M. G. (2011b). Correlates of sleep disturbance in children with pervasive developmental disorders. Research in Developmental Disabilities, 32, 1399–1421.
Hughes, A. A., Lourea-Waddell, B., & Kendall, P. C. (2008). Somatic complaints in children with anxiety disorders and their unique prediction of poorer academic performance. Child Psychiatry and Human Development, 39, 211–220.
Ivanenko, A., Crabtree, V. M., & Gozal, D. (2004). Sleep in children with psychiatric disorders. Pediatric Clinics of North America, 51, 51–68.
Johnson, K. P., Giannotti, F., & Cortesi, G. (2009). Sleep patterns in autism spectrum disorders. Child and Adolescent Psychiatric Clinics of North America, 18, 917–928.
Kotagal, S. (2007). Sleep in children at risk. Sleep Medicine Clinics, 2, 477–490.
Krakowiak, P., Goodlin-Jones, B., Hertz-Picciotto, I., Croen, L. A., & Hansen, R. L. (2008). Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research, 17, 197–206.
Lajonchere, C., Jones, N., Coury, D. L., & Perrin, J. M. (2012). Leadership in health care, research, and quality improvement for children and adolescents with autism spectrum disorders: Autism Treatment Network and Autism Intervention Research Network on Physical Health. Pediatrics, 130, S62–S68.
Lane, A. E., Young, R. L., & Baker, A. E. Z. (2010). Sensory processing subtypes in autism: Association with adaptive behavior. Journal of Autism and Developmental Disorder, 40, 112–122.
Leech, N. L., Barrett, K. C., & Morgan, G. A. (2005). SPSS for intermediate statistics: Use and interpretation (2nd ed.). Mahwah, NJ: Lawrence Earlbaum Associates, Inc., Publishers.
Limoges, E., Laurent, M., Bolduc, C., Berthiaume, C., & Godout, R. (2005). Atypical sleep architecture and the autism phenotype. Brain, 128, 1049–1061.
Little, R. J. (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83, 1198–1202.
Liu, X., Hubbard, J. A., Fabes, R. A., & Adam, J. B. (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37, 179–191.
Lord, C., Rutter, M., DiLavore, P., & Risi, S. (2001). Autism diagnostic observation schedule (ADOS) manual. Los Angeles, CA: Western Psychological Services.
Malow, B. A., Marzec, M. L., McCrew, S. G., Wang, L., Henderson, L. M., & Stone, W. L. (2006). Characterizing sleep in child with autism spectrum disorders: A multidimensional approach. Sleep, 29, 1563–1571.
Maski, K. P., Jeste, S. S., & Spence, S. J. (2011). Common neurological co-morbidities in autism spectrum disorders. Current Opinion in Pediatrics, 23, 609–615.
Mayes, S. D., & Calhoun, S. (2009). Variables related to sleep problems in children with autism. Research in Autism Spectrum Disorder, 3, 931–941.
McGrew, S., Malow, B. A., Henderson, L., Wang, Lily., Song, Y., & Stone, W. L. (2007). Developmental and behavioral questionnaire for autism spectrum disorders. Pediatric Neurology, 37, 108–116.
Meilleur, A. A. S., & Fombonne, E. (2009). Regression of language and non-language skills in pervasive developmental disorders. Journal of Intellectual Disability Research, 15, 115–124.
Meltzer, L. J., & Mindell, J. A. (2008). Behavioral sleep disorders in children and adolescents. Sleep Medicine Clinics, 3, 269–279.
Mindell, J. A. (1993). Sleep Disorders in Children. Health Psychology, 12(2), 151–162.
Mullen, E. M. (1995). Mullen scales of early learning (AGS ed.). Circle Pines, MN: American Guidance Service Inc.
Nixon, G. M., Thompson, J. M. D., Han, D. Y., Becroft, D. M., Clark, P. M., Robinson, E., et al. (2008). Short sleep duration in middle childhood: Risk factors and consequences. Sleep, 31, 71–78.
Ohayon, M. M., Carskadon, M. A., Guilleminault, C., & Vitiello, M. V. (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep, 27, 1255–1273.
Owens, J. A., Spirito, A., & Mcguinn, M. (2000). The children’s sleep habits questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23, 1043–1051.
Owens, J. A., & Witmans, M. (2004). Sleep problems. Current Problems in Pediatric and Adolescent Health Care, 34, 155–179.
Patzold, L. M., Richdale, A. L., & Tonge, B. J. (1998). An investigation into sleep characteristics of children with autism and Asperger’s Disorder. Journal of Pediatric Child Health, 34, 528–533.
Reid, G. J., Huntley, E. D., & Lewin, D. S. (2009). Insomnias of childhood and adolescence. Child and adolescent Clinics of North America, 18, 979–1000.
Roid, G. H. (2003). Stanford-Binet Intelligence Scales (5th ed.). Itasca, IL: Riverside Publishing.
Schreck, K. A., Mulick, J. A., & Smith, A. F. (2004). Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities, 25, 57–66.
Sparrow, S. S., Balla, D. A., & Cicchetti, D. V. (1984). Vineland adaptive behavior scales: Survey form manual. Circle Pines: American Guidance Service.
Stein, L. I., Polido, J. C., Mailoux, Z., Coleman, G. G., & Cermack, S. A. (2011). Oral care and sensory sensitivities in children with autism spectrum disorders. Special Care Dentistry, 31, 103–110.
Tani, P., Lindberg, N., Nieminen-von Wendt, L., Appleberg, B., et al. (2003). Insomnia is a frequent finding in adults with Asperger’s Disorder. BMC Psychiatry, 3, 1–10.
Taylor, M. A., Schreck, K. A., & Mulik, J. A. (2012). Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Research in Developmental Disabilities, 33, 1408–1417.
Touchette, E., Petit, D., Tremblay, R. E., & Montplaisir, J. Y. (2009). Risk factors and consequences of early childhood dysomnia: New perspectives. Sleep Medicine Reviews, 13, 355–361.
Williams, P. G., Sears, L. L., & Allard, A. M. (2004). Sleep problems in children with autism. Journal of Sleep Research, 13, 265–268.
Acknowledgments
We gratefully acknowledge the Autism Treatment Network and their study participants for making these data available for study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript was prepared from a dissertation that was conducted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of I/DD Psychology at the Ohio State University.
Rights and permissions
About this article
Cite this article
Hollway, J.A., Aman, M.G. & Butter, E. Correlates and Risk Markers for Sleep Disturbance in Participants of the Autism Treatment Network. J Autism Dev Disord 43, 2830–2843 (2013). https://doi.org/10.1007/s10803-013-1830-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-013-1830-y