Abstract
This study examined the differential contribution of pre- and perinatal risks in narrowly versus broadly defined autism spectrum disorder (ASD) and across core symptom domains, IQ and co-morbid problems. Children with a DSM-IV diagnosis of autistic disorder (AD) (n = 121) or pervasive developmental disorder not otherwise specified (PDD-NOS) (n = 75) were compared to a typical control sample (n = 311). Diagnoses were based on extensive assessments between 12 and 49 months of age (M = 33.3, SD = 6.4) and re-evaluated at 43–98 months (M = 68.1, SD = 10.7) in 70 % of the cases. Compared with controls, cases with ASD were more likely to be firstborn and show a suboptimal condition after birth. Case mothers reported more infections and more stress during pregnancy. Although the ASD subgroups showed mostly overlapping risks, cases with PDD-NOS differed from those with AD by higher exposure to smoking during pregnancy (SDP) and by a negative association of smoking with IQ, regardless of confounders. SDP appears to contribute more to broadly defined (PDD-NOS) than to narrowly defined ASD (AD). Findings suggest differences in etiological contributors between ASD phenotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impairments in social reciprocity, communication deficits and restricted or repetitive behaviors and interests. Although ASDs are predominantly genetically determined (Freitag 2007; Lichtenstein et al. 2010), previous studies have shown that adverse pre- and perinatal conditions may also contribute, including increased parental age, maternal infections, fetal hypoxia, prematurity and low birth weight (see Gardener et al. 2009, 2011; Kolevzon et al. 2007 for reviews). These factors are not purely environmental, but may be viewed as correlating and interacting with the genetic susceptibility to ASD (Bolton et al. 1997; Ronald et al. 2010b; Rutter and Silberg 2002; Thapar and Rutter 2009; Zwaigenbaum et al. 2002). In studies on pre- and perinatal risk factors in ASD, the differential role of these risks across ASD phenotypes has received little attention. ASDs represent a heterogeneous set of disorders and it is likely that the phenotypic heterogeneity of ASDs partly reflects etiological heterogeneity (Bill and Geschwind 2009; Constantino 2011). This study was undertaken to improve our understanding of clinical and etiological heterogeneity of ASD by examining the relation of pre- and perinatal factors to psychopathology along the whole range of severity in children with an ASD diagnosis, and in narrowly versus broadly defined ASD subgroups.
For defining ASD phenotypes there is growing consensus that dimensional approaches should be taken to measure disorders. In the current DSM-IV-TR taxonomy, the distinction between the categories autistic disorder (AD), Asperger syndrome (AS) and pervasive developmental disorder not otherwise specified (PDD-NOS) is disputed due to its associations with severity, language level or intelligence rather than with the core symptom domains (Snow and Lecavalier 2011; Witwer and Lecavalier 2008). Differentiation between ASDs based on quality of affected symptom domains is further hampered by a high degree of co-occurrence between core domains, with severity of impairment in one domain often predicting impairment in the others (Constantino and Todd 2008; Gotham et al. 2007). Furthermore, the validity of AS and PDD-NOS as diagnostic entities is questionable since not all three symptom domains need to be affected in these diagnoses. PDD-NOS is mostly criticized for its heterogeneity, since the criteria can also be met when all three domains are affected but less severely (Towbin et al. 2005; Walker et al. 2004). These considerations have led to propose new diagnostic criteria for ASD in the DSM-5 by merging the various ASD subcategories into one overall quantitative autistic spectrum (www.dsm5.org).
However, with regard to the relationship of risk factors with autistic and associated psychopathology, there is support for both quantitative (i.e. dimensional) and qualitative (i.e. categorical) risk models. On the one hand, several studies have found that similar genetic and/or environmental factors impact on quantitative variation in autistic traits irrespective of the core symptom domains (Constantino 2011; Lecavalier et al. 2009; Spiker et al. 2002). The role of heritability as well as the associations with pre- and perinatal risks have further been shown to extend into broader phenotypes that may even fall outside the ASD classification (Bolton et al. 1997; Juul-Dam et al. 2001; Robinson et al. 2011a; Zwaigenbaum et al. 2002). On the other hand, it has been found that genetic influences were largely specific for one part of the key domains (Happe and Ronald 2008; Robinson et al. 2011b) and that the associations with pre- and perinatal factors may differ between ASD subtypes (Glasson et al. 2004; Kalkbrenner et al. 2012). We thus investigated to what extend pre- and perinatal risks are associated with autistic symptom domains along the whole range of severity in ASD and in narrowly defined autism versus broadly defined autism.
In addition to investigating the associations of pre- and perinatal risks to core autistic domains it is very relevant to also investigate their associations with related domains, such as IQ and accompanying behavioral problems. These problems occur in 40–70 % of children with ASD (Charman et al. 2011; Gadow et al. 2004; Simonoff et al. 2008) and contribute to the phenotypic expression of ASD (Georgiades et al. 2011; Hoekstra et al. 2010; Leyfer et al. 2006; Matson et al. 2011; Matson et al. 2010; Reiersen et al. 2008; Ronald et al. 2010a; Yerys et al. 2009). Moreover, some of these associated problems, in particular ADHD related problems, have shown etiological overlap with ASD at the genetic level (Reiersen and Todorov 2011; Rommelse et al. 2010) and with regard to involvement of pre- and perinatal risks, including smoking during pregnancy (SDP), asphyxia and low birth weight (Huizink and Mulder 2006; Newcombe et al. 2007; Saigal et al. 2003; Samara et al. 2008). The simultaneous exploration of autistic and co-morbid features in relation to pre- and perinatal risks may thus shed light on domain specific associations with risk factors in ASD.
Earlier studies on pregnancy related factors in ASD have mostly compared children with ASD as one group with typical developing children or children with other disabilities (Bolton et al. 1997; Bryson et al. 1988; Buchmayer et al. 2009; Burd et al. 1999; Deb et al. 1997; Hultman et al. 2002; Larsson et al. 2005; Lord et al. 1991; Mann et al. 2010; Piven et al. 1993; Schendel and Bhasin 2008). The increased incidence of a variety of pre- and perinatal risks that was generally found in these case–control studies is difficult to interpret etiologically due to lack of quantitative or qualitative specification of the ASD phenotype. Quantitative risk models for pre- and perinatal etiology in ASD have been addressed by two recent studies, examining pre- and perinatal variables in relation to severity of autistic symptoms in a clinical ASD sample (Wallace et al. 2008) and in a large population based twin sample (Ronald et al. 2010b). In the two studies several prenatal risks were associated with severity of autistic symptoms, namely: maternal hypertension-related conditions in the Wallace et al. (2008) study, and (weakly) with number of cigarettes smoked by the mother, gestation and birth weight in the Ronald et al. (2010b) study. The later study also investigated the separate autistic domains and found no specific associations with prenatal risks (Ronald et al. 2010a, b, c). Categorical risk models relating to ASD diagnosis have been addressed by Juul-Dam et al. (2001) finding increased incidence of several risk factors in AD and PDD-NOS compared to a large population based control group, but no differences between AD and PDD-NOS. Similarly, in the Zwaigenbaum et al. (2002) study with ASD children and their unaffected siblings, ASD subtype taken as co-variable did not influence the associations with pregnancy related risks, suggesting that the different ASD subtypes share the same risk factors. However, the composite measure used in this study for the risk factors might have masked specific effects. Glasson et al. (2004) did find support for differences in pregnancy related risk between the ASD subtypes, with AS children having the fewest complications, followed by PDD-NOS children and lastly the AD children. However, differences between groups like intelligence might have confounded the results. Therefore, it remains to be determined whether differences in prenatal risks exist between ASD phenotypes.
This study focused on the association of pre- and perinatal risk factors to core autistic domains and to related domains, investigating to what extent associations with risk factors are continuously distributed across the whole range of ASDs or differ between the narrowly versus broadly defined autistic phenotypes. For this purpose we first compared the occurrence of pre- and perinatal risk factors in an ASD group and a typical control group matched for age and gender. Secondly, within the whole ASD group and in the narrow (AD) and broad ASD (PDD-NOS) subgroups the same risk factors were examined. If significant differences emerged between groups (i.e. whole ASD vs control groups and broad vs narrow ASD subgroups) with regard to pre- and perinatal factors, associations of these factors to the autistic core domains (social reciprocity, communication and restricted repetitive behavior) and to associated domains (IQ and behavioral problems) were analyzed.
Methods
Participants
The study included 196 children (82.1 % boys) with ASD and 311 healthy control children (81.4 % boys). The ASD sample consisted of all children who received an ASD diagnosis from a sample of 278 children at high-risk for ASD consecutively enrolled in the DIANE study (Diagnosis and Intervention of Autism in the Netherlands) (Oosterling et al. 2010) between October 2003 and April 2007 at Karakter Child and Adolescent Psychiatry University Centre, Nijmegen, the Netherlands. This study, focusing on early detection and intervention of ASD, enrolled children between 12 and 40 months of age with a positive score (≥ 3 items) on the ESAT 14-items questionnaire (Early Screening of Autistic Traits Questionnaire) (Swinkels et al. 2006) and/or for whom there were major concerns regarding social and communicative development. Families of children enrolled in the initial study were contacted to undergo a follow up assessment 2–4 years later. In order to improve diagnostic reliability, given the very young age of the sample, the diagnoses used in the present study are based on the follow up assessment or, in case of non-participation (i.e. 30 % of the cases), on the first assessment. Diagnoses were assigned between 4 and 12 weeks after the first assessment, which was at 43–98 months of age (M = 68.1, SD = 10.7) in 70 % of the sample and at 12–49 months of age (M = 33.3, SD = 6.4) in 30 % of the sample. The total mean age of diagnosis was 56.47 months (SD = 19.0; range = 15–98 months). Ethnically, 88.8 % were Caucasian and 12.2 % were from non-western ethnicity. Measures from the ASD samples used in the present study were based on the first assessment and are summarized in Table 1. A gender-matched group of 311 controls from the same birth cohort (M = 36.1; SD = 8.35) and geographical region was recruited through multiple pre-school day care centres intended for children with a normal development in the ages of 2–4 years. Before inclusion, all control children had been screened with the ESAT and excluded in case of a positive score. The study was approved by the Medical Ethical Committee of the University Medical Centre St Radboud Nijmegen, the Netherlands.
Diagnostic Protocol
The diagnostic protocol included: (a) medical and developmental history; (b) semi-structured parent–child play observation; (c) psychiatric evaluation; (d) the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2001); (e) IQ measured with the Mullen Scales of Early Learning (Mullen 1995) or with the Psychoeducational Profile-Revised (PEP-R; Schopler et al. 1990). For the PEP-R, IQ was calculated as: (developmental age in months/chronological age in months) × 100); (f) the Autism Diagnostic Interview-Revised (ADI-R) (Rutter et al. 2003); and (g) the Child Behavior Checklist—1½–5) (CBCL) (Achenbach and Rescorla 2000), for evaluation of co-morbid behavior problems. The ADOS and ADI-R were administered by psychologists who met standard requirements for research reliability. Based on the total of aforementioned data, at least two highly experienced clinicians, a child psychiatrist and a psychologist, achieved consensus best-estimate clinical diagnostic classifications (BECD; American Psychiatric Association 2000). The classification rules for AD and the broad ASD (PDD-NOS) were based on DSM-IV-TR criteria with a cut-off point for PDD-NOS of three (and maximal five) items including one social interaction item (Buitelaar and van der Gaag 1998).
Measures
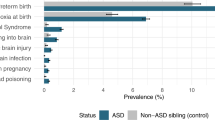
Pre- and perinatal information was retrospectively obtained through a questionnaire that was filled out by the parents before diagnostic assessments. The questionnaire is derived from the Prechtl optimality scales used by (Gillberg and Gillberg 1983), with addition of relevant items like paternal age, intoxications and maternal stress (Table 2). As shown in Table 2, several items were not included in the analyses because of underreport. Different from the Prechtl scales, the critical parental age was further raised to 35 years for the mother and to 40 years for the father, taking account of the mean age of the parents in our sample. For each pre- and perinatal factor, a dichotomous variable was created with code ‘1’ if the risk had occurred and code ‘0’ indicating the absence of the risk factor. Finally, seven dichotomized [no factor present (score 0) and any factor present (score 1)] aggregated factors were created grouping together factors that were related concerning content. The remaining three factors, Maternal Infections, Maternal Stress and being Firstborn, were included as individual factors. Measures for symptom domains consisted of the social, communication and restricted behavior domain scores of the ADI-R and ADOS, the overall IQ score and CBCL internalizing and externalizing T-scores (Fig. 1).
Analyses
Comparison by Diagnostic Grouping
Firstly, the occurrence of pre- and perinatal risk factors was compared between the whole ASD group and control group and secondly between the AD and PDD-NOS subgroups. Comparisons were made on the 7 aggregated factors and 3 individual factors using χ2 tests. The variable parental age was also analyzed as a continuous variable using independent T tests. In case of significant between group differences on the aggregated factors, χ2 tests were conducted on the underlying individual factors. Correction for multiple comparisons was applied according to the Holm–Bonferroni procedure (Holm 1979). To investigate the combined association of the 7 aggregated and 3 individual factors with ASD, cases and control children were further compared using binary logistic regression to calculate odds ratios (ORs) and 95 % confidence intervals (CIs), with values of p < .05 considered to be significant.
Associations with ASD Symptom Domains and Related Domains
To investigate the association of the significant risk factors with distinct symptom domains in ASD we conducted Pearson correlation analyses in the whole ASD sample. For pre- and perinatal factors that significantly differed between the AD and PDD-NOS, associations with the symptom domains were investigated in these subgroups as well. In case of a significant association, we adjusted for possible confounders using analyses of covariance with variables known to be of influence. Differences within pairs of correlation coefficients between ASD subgroups were calculated using Fisher r-to-z transformation. Two sided p values were applied throughout. SPSS for Windows (version 15.0) was used for data analysis.
Results
Comparison by Diagnostic Grouping
Table 3 presents the χ2 tests and ORs for the dichotomized pre- and perinatal risks. When the whole ASD and control sample were compared, significant between group differences emerged on 5 factors: Maternal Infections (χ2 = 6.86; p = .012), Maternal Stress (χ2 = 17.69; p < .0001), Suboptimal Condition Child Post Partum (χ2 = 5.04; p = .030), Prematurity-Low Birth Weight (χ2 = 5.89; p = .019), and being Firstborn (χ2 = 8.41; p = .005). Case mothers were more likely to have experienced at least one infection (OR 5.12; 95 % CI 1.51–17.32), and reported more stress compared to mothers of control children (OR 3.06; 95 % CI 1.72–5.44). Cases were more likely to have shown at least one suboptimal condition at birth (OR 1.55; 95 % CI 1.02–2.37) and to be firstborn (OR 1.75; 95 % CI 1.16–2.63). The factor Prematurity-Low Birth weight was no longer significant in the combined regression model. Examination of the factors underlying Suboptimal Condition Child Post Partum revealed that the effect was jointly carried by Hypoxia (χ2 = 6.89; p = .011), Hypotonia (χ2 = 6.46; p = .016) and Abnormal Movements (χ2 = 15.08; p < .0001). In comparison, the AD and PDD-NOS subgroups showed overlapping frequencies of all risk factors, with the exception of Intoxications (χ2 = 8.57; p = .005) that were more often reported by mothers of children with PDD-NOS (OR 2.51; 95 % CI 1.22–5.16). Examination of underlying factors revealed that the effect was carried by SDP that showed higher occurrence in PDD-NOS than in AD (χ2 = 16.73; p < .0001), which AD cases did not differ from controls with regard to SDP (χ2 = 1.02; p = .337).
Associations of the Significant Risk Factors with Domains of Impairment
In the whole ASD sample several risk factors were associated with ASD core domains, namely: Birth Weight was inversely associated with the ADI Restrictive Repetitive Behavior score (r = −.20; p = .011) and Maternal Infections during pregnancy with a lower score on the ADI Communication domain (r = −.20; p = .011). With regard to related domains, the factor Suboptimal Condition of the child was associated with the CBCL Internalizing scale (r = .20; p = .007) which, at the level of subscales was mainly accounted for by Anxiety-Depression (r = .23; p = .002). Maternal Stress during pregnancy was associated with the Externalizing scale (r = .22; p = .003) which, at the level of subscales was accounted for by Inattention (r = .26; p < .0001) and Aggression (r = .20; p = .006). In the whole ASD sample no association of risk factors with IQ were found.
Examination of the one factor distinguishing between ASD subgroups (Intoxications) revealed that SDP and Quantity of Cigarettes Smoked During Pregnancy were associated with lower IQ (r = −.36; p = .002 and r = −.33; p = .008 respectively) in PDD-NOS but not in AD (r = −.001; p = .993 and r = −.057; p = .560 respectively). The difference between the correlations coefficients remained significant for SDP (Z = −2.44; p = .014) after Fisher r-to-z transformation. Among the factors possibly confounding the association with SDP or IQ, i.e. parental education, Maternal Stress, Inattention and Aggression, only Maternal Education was related to IQ (r = .336; p = .004). Birth Weight, that may act as causal intermediary influenced by SDP, was found to be inversely associated with Inattention in PDD-NOS but not in AD (r = −.337; p = .004 and r = −.054; p = .564 respectively (Z = −1.94; p = .050). The effect of SDP on IQ remained significant after adjusting for maternal education (F(1, 64) = 7.13; p = .010), and for Inattention and Aggression (both not associated with either SDP or IQ) (F(1, 64) = 8.16; p = .006).
Discussion
This study investigated retrospectively recalled pre- and perinatal risks in young children with an ASD diagnosis, focusing on the whole range of ASD and on the comparison of narrowly (AD) with broadly (PDD-NOS) defined ASD phenotypes. The risk factors were examined in relation to autistic features as well as to co-occurring psychopathology and IQ. Our results indicate that pregnancy related problems are more likely to have occurred in children with an ASD diagnosis than in healthy controls and that most pre- and perinatal risks are equally prevalent in the AD and PDD-NOS subgroups. However, SDP forms a striking exception to this, being more prevalent in PDD-NOS than in AD and being related to a lower IQ in the PDD-NOS subgroup. The effect of SDP remained after controlling for maternal education, inattention and aggression. SDP was further related to lower birth weight in all groups, but lower birth weight was associated with inattention in the PDD-NOS subgroup only. In the whole ASD group there were also some associations of risk factors with autistic domains or co-occurring psychopathology.
To our knowledge, increased occurrence and effect of SDP in PDD-NOS compared to AD has not been reported before. However, in a recent large population based study SDP was found to be related to higher functioning autism and not to AD or to ASD in general (Kalkbrenner et al. 2012). An explanation for the increased occurrence of SDP in PDD-NOS versus AD children, may be some between group differences in parental characteristics such as age, education or maternal stress, i.e. factors known to be related to SDP (Havens et al. 2009; Huizink 2009; Lee et al. 2011). However, no such differences were present in mothers or fathers and the association between SDP and IQ in the PDD-NOS subgroup remained after adjusting for these factors. Alternatively, the broad ASD phenotype (PDD-NOS) may be more strongly related to inferior placenta function resulting from SDP (Knopik 2009). Lower birth weight is a well-established prenatal effect of SDP (Thapar et al. 2009) that has also been associated with lower IQ (Newcombe et al. 2007). If so, it would be expected that SDP affects fetal growth retardation and IQ only in the PDD-NOS but not in the AD group. We found that although SDP was associated with lower birth weight in all groups, SDP only affected IQ in PDD-NOS, suggesting indeed some specific toxic effect of SDP in the PDD-NOS but not AD group. An association of SDP with cognitive deficits has been reported in community based studies (Fried et al. 1992; Julvez et al. 2007; Lambe et al. 2006; Sexton et al. 1990), but see Gilman et al. (2008), possibly explained by interactions between SDP and metabolic genes, with the subjects with poorer detoxification ability showing poorest cognitive abilities in relation to SDP (Morales et al. 2009). A combination of a genetically based poorer detoxification ability and SDP may thus be of particular importance in PDD-NOS, but less so in AD. However, previous findings from genetically informed studies suggest that SDP may also be seen as a proxy for genetic vulnerability transmitted from the smoking mother to her offspring instead of a purely environmental factor (D’Onofrio et al. 2010; Knopik 2009; Knopik et al. 2006; Obel et al. 2011; Thapar and Rutter 2009). Indeed, our results indicated that SDP still predicted lower IQ in PDD-NOS after adjusting for birth weight, an outcome heavily influenced by SDP. If so, this may indicate that the genetic factors underlying AD and PDD-NOS are at least partly different.
Previous studies suggesting that SDP is a proxy for genetic vulnerability also raise the possibility that PDD-NOS may show some etiological overlap with disorders such as ADHD that are also related to genetic factors increasing SDP (Knopik et al. 2005; Obel et al. 2011; Thapar et al. 2009). Although we found no direct association of SDP with ADHD-related symptoms, lower birth weight was specifically associated with Inattention in the PDD-NOS subgroup and not in ASD, suggesting similarities in the origins of PDD-NOS and inattention. In this line, results based on a twin sample have shown that one of the risk alleles increasing the risk for severe ADHD combined subtype in the presence of SDP (Neuman et al. 2007; Todd and Neuman 2007), also increased the risk for high autistic traits among children with ADHD (children with obvious or severe AD had probably been excluded from participation; Reiersen and Todorov 2011). Such results may indeed suggest some etiological overlap between broadly defined ASD and ADHD. However, the current findings are also somewhat inconsistent with the aforementioned studies because exposure to smoking appeared related to increased risk for broadly defined ASD and was not directly related to ADHD. These inconsistencies may come from important differences between the samples under study, such as selection of the sample (ASD vs ADHD in our vs their study) and large age differences (with our study focusing on infants and toddlers in whom the different types of ADHD symptoms may still not have become evident and may be harder to distinguish from mild ASD features (Swinkels et al. 2006), and their study focusing on children and adolescents). Nevertheless, in our and previous studies the exposure to and vulnerability towards SDP seems to appears higher in children with ADHD and ASD traits, suggesting SDP to be an important shared risk factor for both disorders. Testing the hypothesis that ADHD and broadly defined ASD may be related to similar etiological underpinnings, in particular SDP, requires further investigation with full diagnostic data on both ASD and ADHD in genetically informative research designs.
Despite the differences in relation to SDP between PDD-NOS and AD children, the similarities in pre- and perinatal risks were much more obvious. Overall, mothers of children with PDD-NOS and AD alike reported more pregnancy related risks than those of control children: higher frequency of prenatal maternal infection is consistent with earlier findings (Atladottir et al. 2010), possibly related to immune dysfunction impacting on neurodevelopment (Onore et al. 2011); higher frequency of suboptimal child condition at birth also confirms previous findings (Burd et al. 1999; Glasson et al. 2004; Hultman et al. 2002; Larsson et al. 2005), and is possibly related to hypoxia which has also been found in other neuropsychiatric disorders (Kolevzon et al. 2007); higher frequency of being firstborn is consistent with earlier reports (Bilder et al. 2009; Bolton et al. 1997; Gillberg and Gillberg 1983; Glasson et al. 2004; Turner et al. 2011); and finally a higher frequency of maternal stress during pregnancy has also been reported by Ronald et al. (2010c). Prenatal maternal stress has been related to diverse psychopathological conditions in the offspring (Huizink et al. 2004), which is in line with the associations between maternal stress and externalizing problems in our ASD sample. Increased maternal stress may be caused by the higher frequency of prenatal complications in the ASD sample and by increased parental psychopathology which is likely to be more prevalent in ASD families (Yirmiya and Shaked 2005). In any case, our and previous results suggest that, except for SDP, pre- and perinatal risk factors are similarly associated with narrowly versus broadly defined ASD, which supports continuously distributed risk factors across the whole range of ASDs. However, one clear discrepancy was found in the role of SDP in the broadly versus mild ASD phenotypes, possibly pointing to differences in the etiological mechanisms underlying ASD subtypes that may also be related to their differences as regards co-morbid problems or cognitive impairment.
The findings in the current study have to be interpreted in the context of our research design and accompanying limitations. First, the validity of specific ASD diagnoses under 3 years of age is undermined by the repeatedly reported low inter-rater reliability in distinguishing between ASDs at that age (Klin et al. 2000; Rondeau et al. 2011; van Daalen et al. 2009). However, in our study only less than 10 % of the diagnoses were assigned before 2 years of age, an age from which a more reliable diagnosis is possible (Chawarska et al. 2007). Besides, more than 70 % of the diagnoses were re-evaluated after several years and all diagnoses were the result of an extensive structured assessment including standardized interviews and observations. Another concern is our reliance on retrospective parent report for pre- and perinatal problems. However, in previous studies parent report about gestational age, hypertension, mode of delivery, birth weight and maternal SDP shows good agreement with medical records, whereas this is not the case for parent report of alcohol use, length of labor and Apgar scores (Buka et al. 2004; Troude et al. 2008; Walton et al. 2000). The reliability of maternal recall of smoking several years postpartum has further been shown to be good (Heath et al. 2003; Jacobson et al. 2002). As for maternal infections in pregnancy, research suggests that there is a tendency towards underreport after birth, but also that medical records generally only report the more severe infections whereas mild infections might also lead to complications (Collier et al. 2009). Nevertheless, recall bias in mothers of referred children who had concerns about their child’s development may have been of influence on reporting higher levels of pregnancy related complications retrospectively than mothers of control children, although it is unlikely that recall bias can explain the differences we found in exposure to SDP between PDD-NOS and AD. Unfortunately, Apgar scores and information on special care post partum were not included in the analyses because of underreport, whereas these items are important indicators of perinatal risk (Gardener et al. 2011). However, 5 other indicators of postnatal condition were included that are a constituent part of the Apgar score and are also related to special care. It should further be noted that the aggregated factors Maternal Disease, Labor/parturition and Suboptimal Condition Postpartum are composed of a set of heterogeneous risks as regards underlying pathology (e.g. hypoxia is a potential cause of brain injury, while hypotonia and abnormal movements may be indicators that pre- or perinatal brain injury has occurred). Many risk factors did not distinguish ASD cases from controls, and earlier findings in autism of increased occurrence of older parental age, bleeding during pregnancy and various maternal diseases were not observed. This may be explained by the moderate size of our sample in combination with the relative low occurrence of several of these risk factors. Another potential limitation is that measuring autistic and related features in the control group would have allowed the exploration of subclinical child characteristics in relation to risk factors. Finally, the associations that we found in our samples do probably only exist against a certain genetic background, which certainly warrants further investigation using genetically informative designs.
Conclusions
Several pre- and perinatal risks tend to be associated with increased risk for ASD in general. One risk factor, i.e. SDP, may exert a role during the early development of the broadly defined ASD phenotype and an insignificant role in the narrowly defined ASD phenotype. This may point to potential prenatal etiological distinctions between the narrowly versus broadly defined ASD phenotype that seem related to associated psychopathology. Such partial differences between AD and PDD-NOS are in line with recent findings suggesting that application of the DSM-5 diagnostic rules results in fewer children with milder (but still substantial) symptoms or with PDD-NOS being diagnosed with ASD compared to those on the DSM-IV (Gibbs et al. 2012; Matson et al. 2012a, b).
References
Achenbach, T. M., & Rescorla, L. A. (2000). Manual for ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association.
Atladottir, H. O., Thorsen, P., Ostergaard, L., Schendel, D. E., Lemcke, S., Abdallah, M., et al. (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(12), 1423–1430.
Bilder, D., Pinborough-Zimmerman, J., Miller, J., & McMahon, W. (2009). Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics, 123(5), 1293–1300.
Bill, B. R., & Geschwind, D. H. (2009). Genetic advances in autism: Heterogeneity and convergence on shared pathways. Current Opinion in Genetics & Development, 19(3), 271–278.
Bolton, P. F., Murphy, M., Macdonald, H., Whitlock, B., Pickles, A., & Rutter, M. (1997). Obstetric complications in autism: Consequences or causes of the condition? Journal of the American Academy of Child and Adolescent Psychiatry, 36(2), 272–281.
Bryson, S. E., Smith, I. M., & Eastwood, D. (1988). Obstetrical suboptimality in autistic children. Journal of the American Academy of Child and Adolescent Psychiatry, 27(4), 418–422.
Buchmayer, S., Johansson, S., Johansson, A., Hultman, C. M., Sparen, P., & Cnattingius, S. (2009). Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics, 124(5), e817–e825.
Buitelaar, J. K., & van der Gaag, R. J. (1998). Diagnostic rules for children with PDD-NOS and multiple complex developmental disorder. Journal of Child Psychology and Psychiatry, 39(6), 911–919.
Buka, S. L., Goldstein, J. M., Spartos, E., & Tsuang, M. T. (2004). The retrospective measurement of prenatal and perinatal events: Accuracy of maternal recall. Schizophrenia Research, 71(2–3), 417–426.
Burd, L., Severud, R., Kerbeshian, J., & Klug, M. G. (1999). Prenatal and perinatal risk factors for autism. Journal of Perinatal Medicine, 27(6), 441–450.
Charman, T., Pickles, A., Simonoff, E., Chandler, S., Loucas, T., & Baird, G. (2011). IQ in children with autism spectrum disorders: Data from the Special Needs and Autism Project (SNAP). Psychological Medicine, 41(3), 619–627.
Chawarska, K., Klin, A., Paul, R., & Volkmar, F. (2007). Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry, 48(2), 128–138.
Collier, S. A., Rasmussen, S. A., Feldkamp, M. L., & Honein, M. A. (2009). Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Research. Part A, Clinical and Molecular Teratology, 85(3), 193–201.
Constantino, J. N. (2011). The quantitative nature of autistic social impairment. Pediatric Research, 69(5 Pt 2), 55R–62R.
Constantino, J. N., & Todd, R. D. (2008). Genetic epidemiology of pervasive developmental disorders. In: J. J. Hudziac (Ed.), Developmental psychopatholgy and wellness: Genetic and environmental influences. American Psychiatric Publishing, Inc: Arlington.
Deb, S., Prasad, K. B., Seth, H., & Eagles, J. M. (1997). A comparison of obstetric and neonatal complications between children with autistic disorder and their siblings. Journal of Intellectual Disability Research, 41(Pt 1), 81–86.
D’Onofrio, B. M., Singh, A. L., Iliadou, A., Lambe, M., Hultman, C. M., Neiderhiser, J. M., et al. (2010). A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development, 81(1), 80–100.
Freitag, C. M. (2007). The genetics of autistic disorders and its clinical relevance: A review of the literature. Molecular psychiatry, 12(1), 2–22.
Fried, P. A., O’Connell, C. M., & Watkinson, B. (1992). 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. Journal of Developmental and Behavioral Pediatrics, 13(6), 383–391.
Gadow, K. D., DeVincent, C. J., Pomeroy, J., & Azizian, A. (2004). Psychiatric symptoms in preschool children with PDD and clinic and comparison samples. Journal of Autism and Developmental Disorders, 34(4), 379–393.
Gardener, H., Spiegelman, D., & Buka, S. L. (2009). Prenatal risk factors for autism: Comprehensive meta-analysis. British Journal of Psychiatry, 195(1), 7–14.
Gardener, H., Spiegelman, D., & Buka, S. L. (2011). Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics, 128(2), 344–355.
Georgiades, S., Szatmari, P., Duku, E., Zwaigenbaum, L., Bryson, S., Roberts, W., et al. (2011). Phenotypic overlap between core diagnostic features and emotional/behavioral problems in preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 41(10), 1321–1329.
Gibbs, V., Aldridge, F., Chandler, F., Witzlsperger, E., & Smith, K. (2012). Brief report: An exploratory study comparing diagnostic outcomes for autism spectrum disorders under DSM-IV-TR with the proposed DSM-5 revision. Journal of Autism and Developmental Disorders, 42(8), 1750–1756.
Gillberg, C., & Gillberg, I. C. (1983). Infantile autism: A total population study of reduced optimality in the pre-, peri-, and neonatal period. Journal of Autism and Developmental Disorders, 13(2), 153–166.
Gilman, S. E., Gardener, H., & Buka, S. L. (2008). Maternal smoking during pregnancy and children’s cognitive and physical development: A causal risk factor? American Journal of Epidemiology, 168(5), 522–531.
Glasson, E. J., Bower, C., Petterson, B., de Klerk, N., Chaney, G., & Hallmayer, J. F. (2004). Perinatal factors and the development of autism: A population study. Archives of General Psychiatry, 61(6), 618–627.
Gotham, K., Risi, S., Pickles, A., & Lord, C. (2007). The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627.
Happe, F., & Ronald, A. (2008). The ‘fractionable autism triad’: A review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychology Review, 18(4), 287–304.
Havens, J. R., Simmons, L. A., Shannon, L. M., & Hansen, W. F. (2009). Factors associated with substance use during pregnancy: Results from a national sample. Drug and Alcohol Dependence, 99(1–3), 89–95.
Heath, A. C., Knopik, V. S., Madden, P. A., Neuman, R. J., Lynskey, M. J., Slutske, W. S., et al. (2003). Accuracy of mothers’ retrospective reports of smoking during pregnancy: Comparison with twin sister informant ratings. Twin Research, 6(4), 297–301.
Hoekstra, R. A., Happe, F., Baron-Cohen, S., & Ronald, A. (2010). Limited genetic covariance between autistic traits and intelligence: Findings from a longitudinal twin study. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 153B(5), 994–1007.
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6, 65–70.
Huizink, A. C. (2009). Moderate use of alcohol, tobacco and cannabis during pregnancy: New approaches and update on research findings. Reproductive Toxicology, 28(2), 143–151.
Huizink, A. C., & Mulder, E. J. (2006). Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews, 30(1), 24–41.
Huizink, A. C., Mulder, E. J., & Buitelaar, J. K. (2004). Prenatal stress and risk for psychopathology: Specific effects or induction of general susceptibility? Psychological Bulletin, 130(1), 115–142.
Hultman, C. M., Sparen, P., & Cnattingius, S. (2002). Perinatal risk factors for infantile autism. Epidemiology, 13(4), 417–423.
Jacobson, S. W., Chiodo, L. M., Sokol, R. J., & Jacobson, J. L. (2002). Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics, 109(5), 815–825.
Julvez, J., Ribas-Fito, N., Torrent, M., Forns, M., Garcia-Esteban, R., & Sunyer, J. (2007). Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. International Journal of Epidemiology, 36(4), 825–832.
Juul-Dam, N., Townsend, J., & Courchesne, E. (2001). Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics, 107(4), E63.
Kalkbrenner, A. E., Braun, J. M., Durkin, M. S., Maenner, M. J., Cunniff, C., Lee, L. C., et al. (2012). Maternal smoking during pregnancy and the prevalence of autism spectrum disorders using data from the autism and developmental disabilities monitoring network. Environmental Health Perspectives, 120(7), 1042–1048.
Klin, A., Lang, J., Cicchetti, D. V., & Volkmar, F. R. (2000). Brief report: Interrater reliability of clinical diagnosis and DSM-IV criteria for autistic disorder: Results of the DSM-IV autism field trial. Journal of Autism and Developmental Disorders, 30(2), 163–167.
Knopik, V. S. (2009). Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology, 34(1), 1–36.
Knopik, V. S., Heath, A. C., Jacob, T., Slutske, W. S., Bucholz, K. K., Madden, P. A., et al. (2006). Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine, 36(10), 1461–1471.
Knopik, V. S., Sparrow, E. P., Madden, P. A., Bucholz, K. K., Hudziak, J. J., Reich, W., et al. (2005). Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: A female twin study. Psychological Medicine, 35(5), 625–635.
Kolevzon, A., Gross, R., & Reichenberg, A. (2007). Prenatal and perinatal risk factors for autism: A review and integration of findings. Archives of Pediatrics and Adolescent Medicine, 161(4), 326–333.
Lambe, M., Hultman, C., Torrang, A., Maccabe, J., & Cnattingius, S. (2006). Maternal smoking during pregnancy and school performance at age 15. Epidemiology, 17(5), 524–530.
Larsson, H. J., Eaton, W. W., Madsen, K. M., Vestergaard, M., Olesen, A. V., Agerbo, E., et al. (2005). Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology, 161(10), 916–925. (discussion 926–918).
Lecavalier, L., Gadow, K. D., DeVincent, C. J., Houts, C., & Edwards, M. C. (2009). Deconstructing the PDD clinical phenotype: Internal validity of the DSM-IV. Journal of Child Psychology and Psychiatry, 50(10), 1246–1254.
Lee, B. K., Gardner, R. M., Dal, H., Svensson, A., Galanti, M. R., Rai, D., et al. (2011). Brief report: Maternal smoking during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(9), 2000–2005.
Leyfer, O. T., Folstein, S. E., Bacalman, S., Davis, N. O., Dinh, E., Morgan, J., et al. (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36(7), 849–861.
Lichtenstein, P., Carlstrom, E., Rastam, M., Gillberg, C., & Anckarsater, H. (2010). The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. American Journal of Psychiatry, 167(11), 1357–1363.
Lord, C., Mulloy, C., Wendelboe, M., & Schopler, E. (1991). Pre- and perinatal factors in high-functioning females and males with autism. Journal of Autism and Developmental Disorders, 21(2), 197–209.
Lord, C., Rutter, M., DiLavore, P., & Risi, S. (2001). Autism diagnostic observation schedule (ADOS) manual. Los Angeles, CA: Western Psychological Services.
Mann, J. R., McDermott, S., Bao, H., Hardin, J., & Gregg, A. (2010). Pre-eclampsia, birth weight, and autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(5), 548–554.
Matson, J. L., Hattier, M. A., & Williams, L. W., (2012a). How does relaxing the algorithm for autism affect DSM-V prevalence rates? Journal of Autism and Developmental Disorders, 42(8), 1549–1556.
Matson, J. L., Kozlowski, A. M., Hattier, M. A., Horovitz, M., & Sipes, M., (2012b). DSM-IV vs DSM-5 diagnostic criteria for toddlers with autism. Developmental Neurorehabilitation, 15(3), 185–190.
Matson, J. L., Mahan, S., Fodstad, J. C., Worley, J. A., Neal, D., & Sipes, M. (2011). Effects of symptoms of co-morbid psychopathology on challenging behaviours among infants and toddlers with autistic disorder and PDD-NOS as assessed with the Baby and Infant Screen for Children with aUtIsm Traits (BISCUIT). Developmental neurorehabilitation, 14(3), 129–139.
Matson, J. L., Worley, J. A., Neal, D., Mahan, S., & Fodstad, J. C. (2010). The effects of inattention/impulsivity and ASD symptom severity on social skills in toddlers. Developmental Neurorehabilitation, 13(6), 408–412.
Morales, E., Sunyer, J., Julvez, J., Castro-Giner, F., Estivill, X., Torrent, M., et al. (2009). GSTM1 polymorphisms modify the effect of maternal smoking during pregnancy on cognitive functioning in preschoolers. International Journal of Epidemiology, 38(3), 690–697.
Mullen, E. (1995). Mullen scales of early learning (AGS Edition ed). Circle Pines, MN: American Guidance Service.
Neuman, R. J., Lobos, E., Reich, W., Henderson, C. A., Sun, L. W., & Todd, R. D. (2007). Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry, 61(12), 1320–1328.
Newcombe, R., Milne, B. J., Caspi, A., Poulton, R., & Moffitt, T. E. (2007). Birthweight predicts IQ: Fact or artefact? Twin research and human genetics, 10(4), 581–586.
Obel, C., Olsen, J., Henriksen, T. B., Rodriguez, A., Jarvelin, M. R., Moilanen, I., et al. (2011). Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?—Findings from a sibling design. International Journal of Epidemiology, 40(2), 338–345.
Onore, C., Careaga, M., & Ashwood, P. (2011). The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity, 26(3), 383–392.
Oosterling, I. J., Wensing, M., Swinkels, S. H., van der Gaag, R. J., Visser, J. C., Woudenberg, T., et al. (2010). Advancing early detection of autism spectrum disorder by applying an integrated two-stage screening approach. Journal of Child Psychology and Psychiatry, 51(3), 250–258.
Piven, J., Simon, J., Chase, G. A., Wzorek, M., Landa, R., Gayle, J., et al. (1993). The etiology of autism: Pre-, peri- and neonatal factors. Journal of the American Academy of Child and Adolescent Psychiatry, 32(6), 1256–1263.
Reiersen, A. M., Constantino, J. N., & Todd, R. D. (2008). Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 47(6), 662–672.
Reiersen, A. M., & Todorov, A. A. (2011). Association between DRD4 genotype and autistic symptoms in DSM-IV ADHD. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 20(1), 15–21.
Robinson, E. B., Koenen, K. C., McCormick, M. C., Munir, K., Hallett, V., Happe, F., et al. (2011a). Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5 %, 2.5 %, and 1 %). Archives of General Psychiatry, 68(11), 1113–1121.
Robinson, E. B., Koenen, K. C., McCormick, M. C., Munir, K., Hallett, V., Happe, F., et al. (2011b). A multivariate twin study of autistic traits in 12-year-olds: Testing the fractionable autism triad hypothesis. Behavior Genetics, 42(2), 245–255.
Rommelse, N. N., Franke, B., Geurts, H. M., Hartman, C. A., & Buitelaar, J. K. (2010). Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. European Child and Adolescent Psychiatry, 19(3), 281–295.
Ronald, A., Edelson, L. R., Asherson, P., & Saudino, K. J. (2010a). Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: Findings from a community twin study of 2-year-olds. Journal of Abnormal Child Psychology, 38(2), 185–196.
Ronald, A., Happe, F., Dworzynski, K., Bolton, P., & Plomin, R. (2010b). Exploring the relation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Development, 81(1), 166–182.
Ronald, A., Pennell, C. E., & Whitehouse, A. J. (2010c). Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Frontiers in Psychology, 1, 223.
Rondeau, E., Klein, L. S., Masse, A., Bodeau, N., Cohen, D., & Guile, J. M. (2011). Is pervasive developmental disorder not otherwise specified less stable than autistic disorder? A meta-analysis. Journal of Autism and Developmental Disorders, 41(9), 1267–1276.
Rutter, M., Le Couteur, A., & Lord, C. (2003). ADI-R: Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services.
Rutter, M., & Silberg, J. (2002). Gene-environment interplay in relation to emotional and behavioral disturbance. Annual Review of Psychology, 53, 463–490.
Saigal, S., Pinelli, J., Hoult, L., Kim, M. M., & Boyle, M. (2003). Psychopathology and social competencies of adolescents who were extremely low birth weight. Pediatrics, 111(5 Pt 1), 969–975.
Samara, M., Marlow, N., & Wolke, D. (2008). Pervasive behavior problems at 6 years of age in a total-population sample of children born at ≤25 weeks of gestation. Pediatrics, 122(3), 562–573.
Schendel, D., & Bhasin, T. K. (2008). Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics, 121(6), 1155–1164.
Schopler, E., Reichler, R. J., Bashford, A., Lansing, M. D., & Marcus, L. M. (1990). The psychoeducational profile revised (PEP-R). Austin: Pro-Ed.
Sexton, M., Fox, N. L., & Hebel, J. R. (1990). Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. International Journal of Epidemiology, 19(1), 72–77.
Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–929.
Snow, A. V., & Lecavalier, L. (2011). Comparing autism, PDD-NOS, and other developmental disabilities on parent-reported behavior problems: Little evidence for ASD subtype validity. Journal of Autism and Developmental Disorders, 41(3), 302–310.
Spiker, D., Lotspeich, L. J., Dimiceli, S., Myers, R. M., & Risch, N. (2002). Behavioral phenotypic variation in autism multiplex families: Evidence for a continuous severity gradient. American Journal of Medical Genetics, 114(2), 129–136.
Swinkels, S. H., Dietz, C., van Daalen, E., Kerkhof, I. H., van Engeland, H., & Buitelaar, J. K. (2006). Screening for autistic spectrum in children aged 14 to 15 months. I: The development of the Early Screening of Autistic Traits Questionnaire (ESAT). Journal of Autism and Developmental Disorders, 36(6), 723–732.
Thapar, A., Rice, F., Hay, D., Boivin, J., Langley, K., van den Bree, M., et al. (2009). Prenatal smoking might not cause attention-deficit/hyperactivity disorder: Evidence from a novel design. Biological Psychiatry, 66(8), 722–727.
Thapar, A., & Rutter, M. (2009). Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. British Journal of Psychiatry, 195(2), 100–101.
Todd, R. D., & Neuman, R. J. (2007). Gene-environment interactions in the development of combined type ADHD: Evidence for a synapse-based model. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 144B(8), 971–975.
Towbin, K. E., Pradella, A., Gorrindo, T., Pine, D. S., & Leibenluft, E. (2005). Autism spectrum traits in children with mood and anxiety disorders. Journal of child and adolescent psychopharmacology, 15(3), 452–464.
Troude, P., L’Helias, L. F., Raison-Boulley, A. M., Castel, C., Pichon, C., Bouyer, J., et al. (2008). Perinatal factors reported by mothers: Do they agree with medical records? European Journal of Epidemiology, 23(8), 557–564.
Turner, T., Pihur, V., & Chakravarti, A. (2011). Quantifying and modeling birth order effects in autism. PLoS ONE, 6(10), e26418.
van Daalen, E., Kemner, C., Dietz, C., Swinkels, S. H., Buitelaar, J. K., & van Engeland, H. (2009). Inter-rater reliability and stability of diagnoses of autism spectrum disorder in children identified through screening at a very young age. European Child and Adolescent Psychiatry, 18(11), 663–674.
Walker, D. R., Thompson, A., Zwaigenbaum, L., Goldberg, J., Bryson, S. E., Mahoney, W. J., et al. (2004). Specifying PDD-NOS: A comparison of PDD-NOS, Asperger syndrome, and autism. Journal of the American Academy of Child and Adolescent Psychiatry, 43(2), 172–180.
Wallace, A. E., Anderson, G. M., & Dubrow, R. (2008). Obstetric and parental psychiatric variables as potential predictors of autism severity. Journal of Autism and Developmental Disorders, 38(8), 1542–1554.
Walton, K. A., Murray, L. J., Gallagher, A. M., Cran, G. W., Savage, M. J., & Boreham, C. (2000). Parental recall of birthweight: A good proxy for recorded birthweight? European Journal of Epidemiology, 16(9), 793–796.
Witwer, A. N., & Lecavalier, L. (2008). Examining the validity of autism spectrum disorder subtypes. Journal of Autism and Developmental Disorders, 38(9), 1611–1624.
Yerys, B. E., Wallace, G. L., Sokoloff, J. L., Shook, D. A., James, J. D., & Kenworthy, L. (2009). Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research, 2(6), 322–333.
Yirmiya, N., & Shaked, M. (2005). Psychiatric disorders in parents of children with autism: A meta-analysis. Journal of Child Psychology and Psychiatry, 46(1), 69–83.
Zwaigenbaum, L., Szatmari, P., Jones, M. B., Bryson, S. E., MacLean, J. E., Mahoney, W. J., et al. (2002). Pregnancy and birth complications in autism and liability to the broader autism phenotype. Journal of the American Academy of Child and Adolescent Psychiatry, 41(5), 572–579.
Acknowledgments
We are grateful to all the children and parents who participated in this study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Visser, J.C., Rommelse, N., Vink, L. et al. Narrowly Versus Broadly Defined Autism Spectrum Disorders: Differences in Pre- and Perinatal Risk Factors. J Autism Dev Disord 43, 1505–1516 (2013). https://doi.org/10.1007/s10803-012-1678-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1678-6