Abstract
This study’s purpose was to investigate mortality among individuals with autism spectrum disorders (ASD) ascertained during a 1980s statewide autism prevalence study (n = 305) in relation to controls. Twenty-nine of these individuals (9.5 %) died by the time of follow up, representing a hazard rate ratio of 9.9 (95 % CI 5.7–17.2) in relation to population controls. Death certificates identified respiratory, cardiac, and epileptic events as the most common causes of death. The elevated mortality risk associated with ASD in the study cohort appeared related to the presence of comorbid medical conditions and intellectual disability rather than ASD itself suggesting the importance of coordinated medical care for this high risk sub-population of individuals with ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of mortality in individuals with autism spectrum disorders (ASD) have reported excess mortality associated with autism across intellectual abilities (Mouridsen and Brønnum-Hansen 2008; Shavelle et al. 2001), particularly among females (Gillberg et al. 2010; Isager et al. 1999; Mouridsen and Brønnum-Hansen 2008; Shavelle et al. 2001) and those with severe intellectual disability (Gillberg et al. 2010; Isager et al. 1999; Shavelle et al. 2001). The rising population of individuals identified with ASD (Centers for Disease Control and Prevention 2009; Pinborough-Zimmerman et al. 2012) will be aging into adulthood and necessitates further investigation of morbidity and mortality to inform patient care and public policy.
Reported excess mortality rate ratios found among individuals with autism or ASD in relation to controls have ranged from 0 (Kobayashi et al. 1992) to 5.6 (Gillberg et al. 2010). Isagar et al. 1999 reported a mortality rate twice that expected for Danish individuals with a pervasive developmental disorder. Similarly, 2 years later, Shavelle et al. (2001) reported a standardized mortality ratio (SMR) of 2.4 among a large sample of ambulatory individuals with ASD served by the California Department of Developmental Services. The excess mortality occurred predominantly in persons with severe intellectual disability yet also affected individuals with only mild intellectual disability (Shavelle et al. 2001). Updates were published for both of these studies, in 2008 and 2006 respectively, and reported that the excess mortality risk had remained unchanged (Mouridsen and Brønnum-Hansen 2008; Pickett et al. 2006). Most recently, Gillberg et al. (2010) published the first prospective longitudinal population-based study of mortality in a cohort of Swedish individuals diagnosed with autism (N = 120) in early childhood. ASD diagnosis based on DSM IV criteria was confirmed in the survivors of this cohort in 2006 during the study’s follow up period. Gillberg et al. (2010) found a mortality rate ratio of 5.6 (95 % CI = 2.5–10.5). The authors attributed this particularly high value to the use of a population-based, rather than clinical, sample.
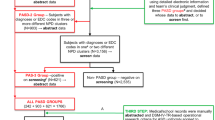
The UCLA-University of Utah Epidemiologic Survey of Autism reported on 241 individuals diagnosed with autism, based on DSM III criteria (Ritvo et al. 1989). Farley et al. (2009) described adult outcomes on 41 high functioning individuals identified in this original study. Data from the original study were also reevaluated using current DSM IV-TR criteria and the Center for Disease Control and Prevention’s (CDC) Autism and Developmental Disabilities Monitoring (ADDM) Network records review methodology (Rice et al. 2007; Van Naarden Braun et al. 2007; Yeargin-Allsopp et al. 2003). During this reassessment, 64 of 108 individuals originally “diagnosed not autistic” during the 1980s autism surveillance study were found to meet DSM IV-TR criteria for an autism spectrum disorder (Miller et al. 2012) using the CDC ADDM methodology. The reclassified group and the original 241 collectively provide a population-based sample of 305 individuals with ASD, who were ascertained during childhood three decades ago. This unique cohort provides an opportunity to study long-term mortality among individuals recognized as having autism during the 1980s (N = 241) and in a subgroup of individuals from the same era who meet ASD criteria exclusively by today’s standard (n = 64).
The aims of this study are to (1) estimate the mortality hazard rate ratios (HRR) for the population-based Utah sample, (2) compare risk factors and HRR between sample subgroups using different types of controls, and (3) report patterns in their causes of death.
Methods
Participants
Participants were ascertained during the Utah/UCLA population-based autism prevalence study which attempted to identify all possible cases of diagnosed or undiagnosed autism between the ages of 3 and 25 throughout the state of Utah. Participants were recruited through an extensive, 4-year media campaign (1982–1986) across the state, using solicited referrals from all practitioners, agencies, and parent groups known to serve individuals with developmental disabilities, as well as from screening records at residential facilities, group homes, and state hospitals. A total of 489 children were ascertained, 379 (78 %) of whom completed all aspects of the study. Those who did not complete the study either moved out of state or lost contact with the study team (n = 33), chose not to participate (n = 30), or were older than 25 years (n = 47). Of those who completed the study, 241 were “Diagnosed Autistic” (referred to hereafter as the Original DSM III group) and 138 were “Diagnosed Not Autistic” according to the study’s case definition based on DSM-III criteria.
From 2006 to 2011, the records of 138 individuals originally “Diagnosed Not Autistic” in the above study were reviewed using CDC ADDM case definition methodology (Rice et al. 2007; Van Naarden Braun et al. 2007; Yeargin-Allsopp et al. 2003) based on DSM-IV-TR criteria. Of these 138 records, 108 met ADDM criteria for chart abstraction. Sixty-four of these individuals originally “Diagnosed Not Autistic” met ASD case definition based on CDC methodology (Miller et al. 2012). This group of 64 individuals is referred to hereafter as the Reclassified DSM-IV-TR group.
The mortality study included all participants (n = 305) of the Utah/UCLA study who were either in the Original DSM III group (n = 241) or the Reclassified DSM-IV-TR group (n = 64). The age range at the time of follow up for the living members of this collective group is 27–54 years of age and the follow up duration ranged from 20 to 29 years. Table 1 describes participant characteristics.
The Utah Population Database
The Utah Population Database (UPDB), a population-based medical research resource, holds all information from all Utah death certificates, including date of death through December 31, 2011. On these death certificates, causes of death were identified using the International Classification of Disease Revision 9 (ICD 9) prior to 1999 and ICD 10 from 1999 onward. The UPDB provides the basis for selecting controls and their mortality.
For participants, medical comorbidity and IQ were determined from a review of the records available at the time of the initial study ascertainment. Age at ascertainment was defined by the date recorded on the signed releases of information present in the cases’ original record. If this was unavailable, the completion date recorded for the developmental inventory was used.
Statistical Methods for Survival Analysis
The survival analysis was based on Cox proportional hazards regressions (hereafter Cox models). These models analyze the hazard rate of mortality, the dependent variable, based on survival time from the time of ascertainment of individuals identified as having an ASD to the time of death or right censorship. The hazard rate ratio (HRR) provides an estimate of the excess risk associated with the identified exposure (i.e. ASD case status) as a constant effect over a specific time period (i.e. interval since case ascertainment) while adjusting for selected covariates (i.e. birth risk factors) (Symons and Moore 2002). Cox models assume a proportional hazard assumption (i.e. the effects of covariates do not vary with time). This assumption was tested and was satisfied in the results reported here. Three distinct control groups were used: siblings, first cousins, and individuals from the population. For population controls, 15 were age-gender matched to each ASD individual. These controls were selected without replacement with their follow-up date (where they were required to be alive) equaling or exceeding the time after the ascertainment date year of the matched case. For sibling and cousin controls, all eligible siblings and first cousins identified in UPDB were selected so gender and birthdates were included as covariates in the Cox models that rely on these comparisons. The survival time was measured as the time between when the cases (and their matched controls) were ascertained and the time of death or the time last known that they were alive. These later cases were right-censored at this date.
Several variables affect the risk for ASD as well as mortality. Accordingly, several key confounders were also introduced into the models including mother’s age at the time of the subject’s birth, birth weight, gestational age, and whether the mother/father died during the follow-up period (this is the time-varying covariate). Not all of these potential confounders are available for all subjects and thus sample sizes are reduced for various models. These additional covariates were obtained from birth and death certificate data contained within the UPDB.
In some instances for models that use sibling and cousin controls, there were no controls available for individual cases. This occurred when the UPDB could not identify siblings or cousins or these controls did not have complete data on all the requisite variables. When matched controls were unavailable for a given case, that case and their controls were excluded from the analysis. The final sample sizes were: (1) population based controls—193 cases and 2,466 controls, (2) sibling controls—173 cases and 532 controls, and (3) first cousin controls—166 cases and 2,478 controls.
Ethics
This mortality study was approved by the Institutional Review Board for the University of Utah and the Resource for Genetic and Epidemiologic Research Review Committee, which controls access to the UPDB.
Results
Mortality Rate
Twenty-nine of 305 individuals (9.5 %) had died by the end of 2011, 20 of whom were male and nine were female comprising 8.8 and 11.6 % of each gender group respectively. Of these, 226 had matching birth certificate data. Of those with available birth certificate data, 193 had complete covariates data available and were subsequently matched by birth year and gender to controls for the analysis. There were no significant differences between those with matching birth certificates and those without in regards to mean IQ and gender. However, the mean age at ascertainment of those without matching birth certificates was significantly higher (12.8 vs. 9.7, 95 % CI 1.7–4.5) than those with matching birth certificates. The hazard rate ratio (HRR) for the combined ASD group is HRR = 9.9 (95 % CI 5.7–17.2) when using population controls, HRR = 8.1 (95 % CI 4.3–15.1) with cousin controls, and HRR = 7.8 (95 % CI 2.8–22.0) with sibling controls (Table 2). After adjusting for cofounders, these excess mortality risks remain significant and are of similar magnitude. To demonstrate how excess mortality varied by gender, we reanalyzed the sample using population controls and show that the HRR = 20.7 (95 % CI 6.2–69.2) for females and HRR = 7.9 for males (95 % CI 4.2–15.0).
Causes of Death
The causes for each of the 29 deaths in the full, combined ASD sample (N = 305) are listed in Table 3. Respiratory, cardiac, and epileptic events were the most prominent categories noted, collectively reported in 12 cases. Epilepsy was listed among the causes of death in six of 29 cases. Lethal respiratory events also occurred frequently (nine cases) and included pneumonia, sleep apnea, and respiratory arrest associated with status epilepticus. Five cases of cardiovascular death occurred and included the following causes: myocarditis, arrhythmia, congestive heart failure, and congenital heart disease.
Associated Medical Disorders
Table 3 also lists the medical disorders described in the records ascertained at the onset of the study. The two individuals with Sanfilippo Syndrome were siblings. A seizure history was noted in 13 individuals and an additional two cases had an abnormal EEG with epileptiform or spike wave discharges, collectively affecting over 50 % of those who had died. Other frequent neurological conditions included cerebral palsy (six cases) and deafness (four cases).
Discussion
The current study examines mortality among individuals ascertained three decades ago during a population-based autism prevalence study, identified either at that time with DSM-III autism or reclassified as having an ASD using current, DSM-IV-TR case definition. This group faced a mortality rate relative risk of 9.9 when compared to population controls. To our knowledge, no previous autism mortality studies have used sibling and cousin controls or included covariates that affect life expectancy (i.e. parents’ lifespan) or overlap with autism risk factors beyond gender (maternal age, birth weight, and gestational age) (Bilder et al. 2009; Larsson et al. 2006). When these covariates were added to the analysis, the mortality risk associated with ASD increased based on population controls while results based on sibling and cousin comparison groups showed no increase when these covariates were included. In addition, mortality risk associated with ASD decreased modestly with sibling and cousin controls compared to population controls. This suggests that additional shared familial factors, both environmental and genetic, may contribute to increased mortality risk in individuals with ASD. The HRR of 9.9 exceeds those previously reported in the literature. Shavelle et al. (2001) reported a mortality rate relative risk of 2.4 among individuals with ASD in California. However, several differences between the California and Utah studies exist that may explain the discrepant findings. The California study ascertained individuals through a single source (California Department of Developmental Services) while the Utah study used an epidemiologically-based sample ascertained from multiple sources (Shavelle et al. 2001; Ritvo et al. 1989). In addition to requiring participants to be ambulatory, the California study excluded those with several comorbid medical conditions that could also contribute to increased mortality such as cerebral palsy, Down Syndrome, and Tuberous Sclerosis. Interestingly, the only other published ASD mortality rate that was measured in a population-based sample (Sweden) reported the previously highest mortality rate relative risk of 5.6. (Gillberg et al. 2010).
From a descriptive perspective, many similarities exist between the current sample and this Swedish sample in regards to birth years (1957–1984 vs. 1962–1984), follow-up duration (21–29 years vs. 20–29 years), and proportion with intellectual disability (69 vs. 80 %). The current, full sample, however, is much larger (305 vs. 120) and includes 64 individuals who did not meet DSM III autism criteria but did meet DSM-IV-TR case definition. Although the Reclassified DSM-IV-TR group had a lower average IQ than the original autism cohort, the combined group still contained a higher percentage of normal IQ cases than the Swedish study. The proportion (27/29, 93 %) of comorbid ID among deceased individuals with ASD was higher than the proportion (192/305, 63 %) of comorbid ID among the combined ASD group. This increased risk in the ID subset is consistent with some (Gillberg et al. 2010; Shavelle et al. 2001) but not all (Mouridsen and Brønnum-Hansen 2008) prior autism mortality studies. When examining the full case group, mortality rate increased with the severity of ID as 79 % of deceased cases had an IQ below 50 compared to 42 % of the combined cohort. Only two of the 29 deceased individuals had an IQ measured within the normal range while 27 % of the full cohort had a normal IQ. The differences between the Swedish and Utah studies do not raise any obvious explanations for the markedly higher mortality rate relative risk among the Utah sample compared to its Swedish counterpart. Because mortality rate relative risk is intrinsically linked to background population mortality rates, differences between Swedish and Utah mortality rates may account, at least in part, for the discrepant findings. A 2003 comparison between Utah and the United States (US) is available and shows Utah with a lower rate (8.10 per 1,000 individuals) than the US (8.44/1,000). Sweden’s 2003 mortality rate (10.58/1,000) exceeds that of both Utah and the US (CIA World Factbook 2011a, b; Utah Department of Health 2005).
The current study found a notable difference in HRR between male (9.9) and female (20.7) ASD cases; however, the difference between the genders’ crude mortality rates was relatively modest: 8.8 % for males and 11.7 % for females. The higher HRR associated with female ASD cases likely reflects the lower mortality rates in the female (65.92 per 100,000 person years) compared to male (85.72 per 100,000 person years) Utah population controls. Table 4 provides a comparison between gender groups of ASD mortality cases. Prior ASD studies that found higher crude morality rates in the female case groups also reported higher rates of intellectual disability and/or epilepsy associated with female gender (Gillberg et al. 2010; Mouridsen and Brønnum-Hansen 2008; Shavelle et al. 2001). In contrast, the rate of these comorbid conditions in the Utah cohort was comparable between males and females, likely contributing to the similarity in their mortality rates.
Unlike previous studies (Gillberg et al. 2010; Isager et al. 1999; Mouridsen and Brønnum-Hansen 2008; Shavelle et al. 2001), the Utah sample reported relatively few deaths, 3 of 29 or 10 %, resulting from unnatural causes such as accidents and suicide. One individual died from choking; two individuals died from an adverse event related to medication. In contrast, Mouridsen and Brønnum-Hansen (2008) reported on 5 of 26 individuals who had died from unnatural causes. These deaths appeared to cluster around levels of intellectual functioning. Accidents were associated with ID, and suicides, with normal IQ. The relatively few deaths due to unnatural causes in the Utah cohort may reflect the near absence of normal IQ among those who had died. Only two individuals among the deceased had normal intellectual functioning. One is accounted for above with the unnatural cause of death listed as related to medication. The other individual’s cause of death was listed as an arrhythmia. In the case of arrhythmia, records available at the time of ascertainment (about 10 years before death) contained no mention of an underlying cardiac abnormality and included a treatment history indicating several psychotropic medication trials. This individual also had a remarkably strong family history of completed suicides. This information raises the possibility that this death may have been categorized as unnatural (such as from a medication reaction or intentional/unintentional overdose) if more information were available regarding the events surrounding death.
A significant limitation of this study is its reliance on death certificates as the sole source of data for cause of death. The literature surrounding the accuracy of death certificate data varies substantially and appears dependent on disease studied. A study from the Mayo Lung Project examining lung cancer deaths found death certificates to have a sensitivity and specificity of 90 and 99 %, respectively (Doria-Rose and Marcus 2009). Yet, when studying deceased individuals with Down Syndrome, Baird and Sadovnick (1990) found the underlying cause of death was identified appropriately in only 52.4 % of the cases. While there exists no published study on death certificate accuracy in individuals with autism, there is reason to assume the Utah cohort shares more similarities with that of Down Syndrome than of the Mayo Lung Project. In Utah’s deceased cohort, nearly all had intellectual disabilities, and five cases had an identified, underlying genetic disorder which includes two cases with Down syndrome. For cause of death, two cases had “mental retardation”, one had “microcephaly”, and another had “ill defined and unspecified cause of mortality” as the single item listed. Baird and Sadovnick (1990) suggested that in situations where the individual has an obvious underlying syndrome, those completing the death certificate may feel less urgency to distinguish a specific cause of death. The results from this study suggest this may extend to individuals in general with intellectual disabilities and/or autism spectrum disorders.
Overall, the mortality risk associated with ASD in the Utah cohort appears more related to the presence of comorbid medical conditions and intellectual disability than the ASD itself. However, both are common among individuals with autism spectrum disorders (Danielsson et al. 2005; Depositario-Cabacar and Zelleke 2010; Gillberg and Coleman 1996; Pellock 2004; Ritvo et al. 1989; Ritvo et al. 1990). Previous studies found a strong association between the presence of epilepsy and risk of death (Gillberg et al. 2010; Isager et al. 1999; Mouridsen and Brønnum-Hansen 2008; Pickett et al. 2011; Shavelle et al. 2001). The Utah sample also found epileptiform activity (seizures, epilepsy, EEG findings) among 52 % of the individuals who had died (15 of 29 cases).
From a clinical perspective, the presence of autism and/or intellectual disabilities complicates the management of epilepsy in regards to medication tolerability and adherence to medication management. Many psychotropic medications (such as stimulants, antipsychotics, and antidepressants) used to manage emotional and behavioral problems in this population lower seizure threshold and can exacerbate seizure activity (Pellock 2004). Several anticonvulsant medications (i.e. phenytoin, levetiracetam, phenobarbital) can cause or worsen underlying behavioral and emotional problems in developmentally delayed individuals (Depositario-Cabacar and Zelleke 2010; Pellock 2004). Compounding this further is the need for regular serum laboratory monitoring for some of the most potent anticonvulsant medications (carbamazepine, divalproex sodium, phenytoin) which can be quite challenging to obtain in adults with severe developmental disabilities. Thus, well-coordinated care among the individual’s caregiver, primary care provider, neurologist, and psychiatrist is essential to minimize mortality and optimize health, functioning, and quality of life.
References
Baird, P. A., & Sadovnick, A. D. (1990). Underlying causes of death in Down syndrome: Accuracy of British Columbia death certificate data. Canadian Journal of Public Health, 81(6), 456–461.
Bilder, D., Pinborough-Zimmerman, J., Miller, J., & McMahon, W. (2009). Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics, 123(5), 1293–1300.
CIA World Factbook. (2011a). Sweden death rate. Retrieved from http://www.cia.gov.
CIA World Factbook. (2011b). United States death rate. Retrieved from http://www.cia.gov.
Centers for Disease Control and Prevention. (2009). Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, United States, 2006. Morbidity and Mortality Weekly Report, 58(10), 1–20.
Danielsson, S., Gillberg, I. C., Billstedt, E., Gillberg, C., & Olsson, I. (2005). Epilepsy in young adults with autism: A prospective population-based follow-up study of 120 individuals diagnosed in childhood. Epilepsia, 46(6), 918–923.
Depositario-Cabacar, D. F., & Zelleke, T. G. (2010). Treatment of epilepsy in children with developmental disabilities. Developmental Disabilities Research Reviews, 16(3), 239–247.
Doria-Rose, V. P., & Marcus, P. M. (2009). Death certificates provide an adequate source of cause of death information when evaluating lung cancer mortality: An example from the Mayo Lung Project. Lung Cancer, 63(2), 295–300.
Farley, M. A., McMahon, W. M., Fombonne, E., Jenson, W. R., Miller, J., Gardner, M., et al. (2009). Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism, 2(2), 109–118.
Gillberg, C., Billstedt, E., Sundh, V., & Gillberg, I. C. (2010). Mortality in autism: A prospective longitudinal community-based study. Journal of Autism and Developmental Disorders, 40(3), 352–357.
Gillberg, C., & Coleman, M. (1996). Autism and medical disorders: A review of the literature. Developmental Medicine and Child Neurology, 38, 191–202.
Isager, T., Mouridsen, S. E., & Rich, B. (1999). Mortality and causes of death in pervasive developmental disorders. Autism, 3, 7–16.
Kobayashi, R., Murata, T., & Yoshinaga, K. (1992). A follow-up study of 201 children with autism in Kyushu and Yamaguchi areas, Japan. Journal of Autism and Developmental Disorders, 22(3), 395–411.
Larsson, H. J., Eaton, W. W., Madsen, K. M., Vestergaard, M., Olesen, A. V., Agerbo, E., et al. (2006). Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology, 161(10), 916–925.
Miller, J. S., Bilder, D., Farley, M., Coon, H., Pinborough-Zimmerman, J., Jenson, W., et al. (2012). Autism spectrum disorder reclassified: A second look at the 1980’s Utah/UCLA Autism Epidemiologic Study. Journal of Autism and Developmental Disorders. doi:10.1007/s10803-012-1566-0.
Mouridsen, S. E., & Brønnum-Hansen, H. (2008). Mortality and causes of death in autism spectrum disorders: An update. Autism, 12(4), 403–414.
Pellock, J. (2004). Understanding co-morbidities affecting children with epilepsy. Neurology, 62(supplement 2), S17–S23.
Pickett, J. A., Paculdo, D. R., Shavelle, R. M., & Strauss, D. J. (2006). 1998–2002 Update on “Causes of Death in Autism”. Journal of Autism and Developmental Disorders, 36, 287–288.
Pickett, J., Xiu, E., Tuchman, R., Dawson, G., & Lajonchere, C. (2011). Mortality in individuals with autism, with and without epilepsy. Journal of Child Neurology, 26(8), 932–939.
Pinborough-Zimmerman, J., Bakian, A., Fombonne, E., Bilder, D., Taylor, J., & McMahon, W. M. (2012). Changes in the administrative prevalence of autism spectrum disorders: Contribution of special education and health data from 2002–2008. Journal of Autism and Developmental Disorders, 42, 521–530.
Rice, C. E., Baio, J., Van Naarden Braun, K., Doernberg, N., Meaney, F. J., Kirby, R. S., et al. (2007). A public health collaboration for the surveillance of autism spectrum disorders. Paediatric and Perinatal Epidemiology, 21(2), 179–190.
Ritvo, E. R., Freeman, B. J., Pingree, C., Mason-Brothers, A., Jorde, L., Jenson, W. R., et al. (1989). The UCLA-University of Utah epidemiologic survey of autism: Prevalence. American Journal of Psychiatry, 146(2), 194–199.
Ritvo, E. R., Mason-Brothers, A., Freeman, B. J., Pingree, C., Jenson, W. R., McMahon, W. M., et al. (1990). The UCLA-University of Utah epidemiologic survey of autism: The etiologic role of rare diseases. American Journal of Psychiatry, 147(12), 1614–1621.
Shavelle, R. M., Strauss, D. J., & Pickett, J. (2001). Causes of death in autism. Journal of Autism and Developmental Disorders, 31, 569–576.
Symons, M. J., & Moore, D. T. (2002). Hazard rate ratio and prospective epidemiological studies. Journal of Clinical Epidemiology, 55(9), 893–899.
Utah Department of Health. (2005). Utah health status update: Adjusting Utah’s state health ranking for age [PDF]. Retrieved from http://health.utah.gov.
Van Naarden Braun, K., Pettygrove, S., Daniels, J., Miller, L., Nicholas, J., Baio, J., et al. (2007). Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. Surveillance Summaries: Morbidity and Mortality Weekly Report, 56(1), 29–40.
Yeargin-Allsopp, M., Rice, C., Karapurkar, T., Doernberg, N., Boyle, C., & Murphy, C. (2003). Prevalence of autism in a US metropolitan area. JAMA. Journal of the American Medical Association, 289(1), 49–55.
Acknowledgments
This research was supported by Autism Speaks Grant #5955. We thank the Pedigree and Population Resource (funded by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance, and support of the Utah Population Database (UPDB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bilder, D., Botts, E.L., Smith, K.R. et al. Excess Mortality and Causes of Death in Autism Spectrum Disorders: A Follow up of the 1980s Utah/UCLA Autism Epidemiologic Study. J Autism Dev Disord 43, 1196–1204 (2013). https://doi.org/10.1007/s10803-012-1664-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1664-z