Abstract
Inhibition of return (IOR) reflects slower reaction times to stimuli presented in previously attended locations. In this study, we examined this inhibitory after-effect using two different cue types, eye-gaze and standard peripheral cues, in individuals with Asperger’s syndrome and typically developing individuals. Typically developing participants showed evidence of IOR for both eye-gaze and peripheral cues. In contrast, the Asperger group showed evidence of IOR to previously peripherally cued locations but failed to show IOR for eye-gaze cues. This absence of IOR for eye-gaze cues observed in the participants with Asperger may reflect an attentional impairment in responding to socially relevant information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Efficient interaction with our visual environment requires individual regions of interest within the visual field are selected for further processing while other less relevant regions are ignored. This selection is largely determined by attentional orienting (e.g., Posner 1980). There are two ways in which spatial attention can be oriented in the visual field: the endogenous orienting, referring to the voluntary allocation of attention, and the exogenous orienting, involving an automatic allocation of attention and occurring in response to external salient events (Jonides 1981). Symbolic and centrally presented cues (e.g., an arrow presented at the centre of the screen that indicates the likely target location) have been used to investigate endogenous orienting, whereas peripheral cues (e.g., the abrupt onset of an object in the periphery not providing any information regarding the location of the upcoming target) have been employed in order to study exogenous orienting (Jonides 1981; Posner 1980).
For the past 30 years, abrupt visual onsets occurring in the visual periphery, but not centrally presented symbolic stimuli, were thought to activate an exogenous orienting of attention (Jonides 1981; Posner 1980). However, recent studies have demonstrated that gaze direction—used as a central spatial cue—reflexively triggers attentional shift (for a review, see Frischen et al. 2007a). In these studies a spatial cueing paradigm, first introduced by Posner (1980) and afterwards revisited by Friesen and Kingstone (1998), provides a face unpredictably gazing either left or right, as a cue to orient attention. A target is presented afterwards either in the gazed location or in the opposite location. Participants are typically faster to detect or identify the target when it appears at the gazed location, as compared to when it does in the opposite ungazed location (gaze cueing effect), despite they are instructed to ignore it. This gaze direction effect exhibits some of the reflexive characteristics of peripheral onset cues (Jonides 1981; Müller and Rabbitt 1989; Posner and Cohen 1984). For example, participants are faster to respond to targets appearing congruently to the gaze direction (facilitation effect) even when it is counterpredictive and it is advantageous for participants to redirect attention toward the uncued position (see, e.g., Driver et al. 1999; Friesen et al. 2004). Furthermore, it has recently observed that at sufficiently long SOA (Stimulus Onset Asynchrony; e.g., 2,400 ms) gaze cuing also produces Inhibition Of Return (IOR) effects (Frischen and Tipper 2004; Frischen et al. 2007b), as well as it is found with peripheral onset cues (Posner and Cohen 1984). IOR effect reflects slower reaction time (RT) to stimuli presented in previously cued locations and is thought to bias attentional orienting to novel locations in the environment (Posner 1980; Posner and Cohen 1984).
In a typical IOR paradigm (Posner and Cohen 1984), a sudden visual stimulus (spatial cue) is presented in a peripheral position before the onset of the stimulus target. Subsequent targets appearing at the cued location are processed faster and more efficiently, but this initial facilitation effect reliably turns into inhibition effects at longer SOAs (approximately greater than 300 ms), with a slowing of processing for stimuli occurring in the cued location (Posner and Cohen 1984). The mechanism underlying this effect is considered crucial in healthy cognition and it is assumed to aid the search of new events in the environment by favouring the inspection of new locations, to detriment of recently explored ones (Posner et al. 1985; see also Klein 1988).
Over the last two decades, reflecting the idea that gaze-cueing paradigm tapped into social cognition, several researchers have adapted and applied this paradigm to study social attention in populations with typical and atypical social development (for a review, see Frischen et al. 2007a). Facilitation effects of gaze direction have been observed with almost everyone and most notably with individuals with autism (Chawarska et al. 2003; Kylliainen and Hietenan 2004; Okada et al. 2003; Senju et al. 2004; Swettenham et al. 2003). These results conflict with a common knowledge showing that individuals with autism do not spontaneously engage in joint attention behaviours (Baron-Cohen 1995), such as following someone’s eye gaze (the reason for this discrepancy is not the focus of the present article; however, the interested reader will find possible explanations in Nation and Penny 2008). However, to our knowledge, it has not been studied yet whether IOR triggered by an eye gaze cue is impaired in people with autism spectrum disorders.
Recently, Rinehart et al. (2008) reported normal levels of IOR for individuals with Asperger’s disorder in the context of a typical IOR paradigm with peripheral cues. However, it is not yet known whether similar levels of IOR can be observed across different types of cues.
In the novel IOR paradigm developed by Frischen and Tipper (2004), a centrally presented directional eye-gaze has been used to signal the position of an upcoming target. In this paradigm with healthy participants IOR effect for stimuli presented at gazed location emerged at SOA of 2,400 ms and it was observed with several dependent variables and tasks: for example, in experiments that used manual key-press and eye movement latencies as dependent variables, and in detection and localization tasks. In the current study, we used this gaze-cueing IOR paradigm as an instrument to evaluate important aspects of attention related to social communication and interaction (see Frischen and Tipper 2004). In fact, individuals with autism spectrum disorder (ASD) have impairments in social attention showing a reduced orienting in response to socially relevant information (e.g., eyes and face). This deficit is generally attributed to a specific impairment in social cognition, which in turn is generally referred to a complex set of mental operations, including perceiving, interpreting, and generating responses to the intentions, dispositions, and behaviours of others (Baron-Cohen 1989, 2000; Baron-Cohen et al. 1997; Charman et al. 2001; Stone et al. 1997). The purpose of the present study is to examine IOR as a function of eye gaze within individuals with Asperger’s disorder and typically developing people. A gaze cueing procedure, in which the IOR effect in the cued location is thought to reflect the operation of a specialized social processing, is compared to a standard peripheral cue procedure in which IOR effect is observed in response to a stimulus with no socio-biological significance. Since people with Asperger’s disorder show impairments in social cognitive functions, they should show impairments in IOR effect in response to eye-gaze, which rely on social cognition. Normal levels of IOR should be observed only within typically developing participants. In contrast, no difference between Asperger group and matched comparison group should be observed for peripheral cues, since normal levels of IOR have been reported with peripheral cues in individuals with Asperger’s disorder (Rinehart et al. 2008). This result would strongly suggest that the reason of the impairment in IOR effect with an eye-gaze spatial cue in individuals with Asperger’s disorder is due to their attentional difficulty in responding to socially relevant information.
Method
Participants
Fourteen individuals with Asperger’s disorder (12 males and 2 female; mean age = 10.6 years, SD = 2.7; range 10–18 years) and 14 typically developing participants (12 males and 2 female; 10.4 years, SD = 2.0; range 10–18 years) were included in the study. The Asperger’s group received an IQ valuation through Wechsler Adult Intelligence Scale Revised (WAIS-R) or Wechsler Intelligence Scale for Children-III Edition (WISC-III).
Diagnosis had been made by a child psychiatrist according to established criteria (DSM-IV-TR; American Psychiatric Association 2000) and after an extensive diagnostic evaluation, including a review of prior records (developmental history and child psychiatric and psychological observations). The symptomatic valuation and the severity of the patients’ Asperger syndrome was estimated through Childhood Autism Rating Scale (CARS, Schopler et al. 1998), Autism Diagnostic Observation Schedule (ADOS, Lord et al. 1999), Autism Diagnostic Interview-Revised (ADI-R, Lord et al. 1994) and Australian Scale for Asperger’s Syndrome (ASA, Garnett and Attwood 1998). CARS is a direct observation of child/adolescent behaviour with Pervasive Developmental Disorder (PDD) suspect in a number of contests with different social valence. ADOS is a direct observation of child/adolescent behaviour with PDD suspect in relation to many structured activities with elevated social and relational valence. ADI is an interview to parents on principal steps of social and relational child development. ASA is a questionnaire/interview to parents about presence of Asperger’s syndrome symptoms.
None of the participants had known associated medical disorders at the time of testing, and visual examination was found to be normal. Further, all them had normal cognitive and language development and attended normal schools. None of them met, or had ever met, the diagnostic criteria for autism. All the participants were unmedicated.
The inclusion criteria to participate in the study were the diagnosis of Asperger’s syndrome, no neurological disease, no mental retardation, no co-morbid disorders, no history of drug treatment or cerebral injury and a Total Intelligence Quotient (TIQ) in WISC/WAIS greater than 85. The TIQs of the Asperger group were in the normal range (Full-scale IQ: Mean = 107.63, SD = 9.15; Verbal IQ: Mean = 107.05, SD = 13.16; Performance IQ: Mean = 104.50, SD = 10.53).
Typically developing participants were gender and age-matched with the Asperger group and were selected from a wider group of 120 children recruited from one public school in Rome. The control group participants had no history of cerebral injury or other neurological or psychiatric disorders. All the participants have TIQ greater than 85. The Child Psychiatry and Neurology Institute Ethical Committee approved the study. All parents or legal guardians of children gave written informed consent before testing.
Apparatus
The displays and the stimuli were presented on a high definition CRT 21-inch monitor with a Pentium-based computer system (running at 100 MHz) using a Nvidia Quadro FX 3500 (256 Mb) graphics card. E-Prime software controlled the presentation of the stimuli, timing operations and data collection. Responses were gathered with a standard keyboard.
Stimuli
In the eye-gaze IOR task, a photograph of a face (3° × 3° of visual angle) was the eye gaze cue. The face photograph was manipulated to produce the left-gaze and right-gaze cues by cutting out the pupil/iris area of each eye and pasting it into the left and right corner, respectively, of each eye, by using Adobe PhotoShop 7 software. Thus, only the area within the eyes differed between the cue and straight-gaze stimuli. In the standard IOR paradigm, a central fixation was flanked by two peripheral boxes. The brightening of one of the boxes was used to produce the peripheral cues. The target was an “X” letter, subtending 0.9° × 0.9° of visual angle and it was presented at the centre of the right or left box at an eccentricity of 6° of visual angle.
Procedure
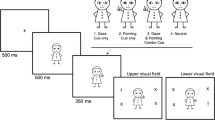
Participants were seated 60 cm directly in front of a computer monitor, in a dimly lit, sound-attenuated room and their heads were held steady with a chin/head rest. Each trial began with a display consisting of a central fixation stimulus flanked by two peripheral boxes. For gaze cue the fixation stimulus was a face photograph with the pupils centred vertically in the eyes. For peripheral cue the fixation stimulus was a cross within a box centred on the screen. This display was presented for 1,000 ms. Then, a cue was presented for 150 ms. The cue was the movement of the eyes, or the brightening of one of the peripheral boxes. Then, a central box was brightened for 1,750 ms (refixation cue). The gaze cue was also followed by the presentation of the face with a direct gaze (please refer to Fig. 1). Finally, 500 ms after the offset of the refixation cue, the target appeared to either the left or right of the screen. Thus, the interval between the onset of the directional cue and the onset of the target (SOA) was 2,400 ms. Participants were instructed to respond by pressing the spacebar as soon as they detected the target. They were also informed that the location signalled by peripheral or central cues did not predict target location, and that they should ignore it, while maintaining central fixation throughout each trial. Each of the two experimental sessions (one for each cue type) was composed of 10 practice trials followed by an experimental block of 45 trials. Five catch trials, in which no target was presented, occurred randomly in each block. For each participant cued location and target location were randomly selected within each block of trials. The cue types (gaze/peripheral) were separated into different blocks and the order of blocks was counterbalanced across participants.
Design
The same two mixed-factor designs was used to compare IOR performance of people with Asperger’s disorder and typically developing people in eye-gaze and peripheral cueing procedures. Validity (valid trials vs. invalid trials) was manipulated within participants and the Group (people with Asperger’s disorder vs. typically developing people) was manipulated between participants.
RTs less than 200 ms were deemed to be anticipations; RTs that exceeded 2.5 standard deviations above the means for each participant were recorded as misses. RT anticipations (e.g., responses given after the cue but before the target) and misses (e.g., fails to respond after a target has appeared) were examined separately for the Asperger’s disorder group and control group.
Results
Reaction Time
RTs data were submitted to a two mixed-factor analysis of variance (ANOVA), with Group as between factor and Validity as within-participants factors. Figure 2 presents RTs performance to eye-gaze cues and peripheral box cues. In the analysis for the eye-gaze cue condition, neither the main effect of Validity (F < 1) nor the main effect of Group (F1,26 = 1.13; p = . 30) were significant. Importantly, the critical Validity × Group interaction was significant (F1,26 = 9.77; p = .004). Planned comparisons showed that RTs were significantly slower on valid trials than on invalid trials (475 vs. 451 ms) only in the control group (F1,26 = 6.02; p = .002). In contrast, RTs were marginally faster on valid trials than on invalid trials (497 vs. 516 ms) in the Asperger group (F1,26 = 3.87; p = .06). The analysis for the peripheral cue condition showed a significant effect of Validity (F1,26 = 10.64; p = .003) with slower responses for valid than invalid trials (552 vs. 512 ms). The main effect of Group was not significant (F1,26 = 1.25; p = .27). Of interest, the interaction was not significant (F < 1): planned comparisons revealed that IOR effect was significant both in Asperger (F1,26 = 4.23; p = .05) and in control group (F1,26 = 6.53; p = .02).
Given the limited number of participants, which did not allow to normal distributions of data, to confirm the robustness of results we compared the IOR effect for the two types of cue (peripheral and eye-gaze) in both groups of participants. For typically developing group, the results have confirmed an IOR effect for both the peripheral cue (χ2 = 90.02; p < .0000001) and the gaze-cue cue (χ2 = 31.29; p < .005); for Asperger group, the effect of IOR has been confirmed only for the peripheral cue (χ2 = 159.29; p < .0000001), while for the gaze-cue cue (χ2 = 73.11; p < .0000001) a significant facilitation effect was observed.
Anticipated Responses
A two-way ANOVA (Group × Type of Cue) revealed a main effect of Group (F1,26 = 4.27; p = .05), indicating that the Asperger group committed significantly more anticipation errors compared to the control group (mean number of anticipations = 1.64 vs. 0.35). No other main effect or interaction was found.
Misses
For the eye-gaze cue condition, a two-way ANOVA similar to that conducted for the RTs data revealed an interaction of Group × Validity (F1,26 = 4.22; p = .05), indicating that the control group was more likely to miss a target on valid trials than on invalid trials (0.93 vs. 0.21); this result is consistent with the IOR effect. In contrast, the Asperger group was more likely to miss a target on invalid trials than on valid trials (1.43 vs. 1). For the peripheral cue condition, a two way ANOVA revealed a main effect of Validity (F1,26 = 6.79; p = .01), with more miss errors for valid trials (1.67 vs. 1.03). Of interest, the interaction was not significant (F < 1).
Discussion
The present study has examined IOR attentional effects related to eye-gaze direction or peripheral cues, either congruent or incongruent with target presentation, in people with and without Asperger’s syndrome. Eye-gaze and peripheral cues represented social and non-social signals, respectively. Impairment in IOR effects for gaze cues was observed only in Asperger group. They failed to show evidence of IOR, but rather they responded faster to targets presented in locations previously signalled by eye-gaze direction.Footnote 1 In contrast, typically developing participants showed the expected effect of IOR for eye-gaze cues, confirming previous results (Frischen et al. 2004, 2007a, b). These findings are consistent with the impairment in social attention generally observed in individuals with autism spectrum disorder (Dawson et al. 1998; Mundy et al. 1986; Sigman et al. 1992) and support the claim that eye gaze represents a special attention stimulus to study social attention (Frischen et al. 2007a, b; Marotta et al. 2011).
On the other hand, peripheral cues, elicited significant levels of IOR in both Asperger and typically developing participants. This result replicated that recently reported by Reinhart et al. (2008) who by means of peripheral cues showed comparable levels of IOR between individuals with autism and the matched comparison group. The inability of eye gaze to trigger an IOR effect in participants with Asperger, together with the evidence of an IOR effect elicited by a peripheral cue highlights a specific attentional impairment of people with Asperger’s disorder in responding to socially relevant information. This view is supported by a growing body of neurological studies showing impaired attentional responses to social stimuli in autism spectrum disorders (ASD). For example, Pelphrey et al. (2005) found that individuals with ASD showed normal activation of the superior temporal sulcus (STS) when viewing gaze shifts. However, STS activity varied depending on the intentions conveyed by the gaze shift in control participants, but this different modulation was not observed in the ASD group. Greene et al. (2011) also found that typically developing individuals showed increased activity in frontoparietal attention networks, visual processing regions, and the striatum, when attention is directed by social cues compared to non-social cues. On the contrary, ASD individuals show increased activity only in the superior parietal lobule. These findings suggest in ASD an impairment of the neural circuitry involved in social orienting.
Furthermore, our results are similar to those recently reported by Nestor et al. (2010) with patients with schizophrenia, who are generally referred as impaired in social attention behaviour (Sasson et al. 2007). In particular, they found that patients failed to show evidence of an IOR effect for eye-gaze cues, whereas showed normal levels of IOR for peripheral cues. Therefore, taken together, our findings and those of Nestor et al. (2010) indicated that IOR to eye-gaze cues may represent a key instrument to study social attention in populations with typical and atypical social development. Future studies will be important in clarifying and strengthening this conclusion.
Conclusions
For the first time, an eye-gaze cueing paradigm has been used to assess IOR effect in people with Asperger’s syndrome. Of particular relevance is the different behaviour shown by participant with Asperger’s syndrome in the IOR effect when a social (eye-gaze) or a neutral (peripheral) cue was used. This dissociation highlights that people with Asperger’s syndrome demonstrate to have preserved the attentional processes involved in the IOR behaviour, but they present a specific impairment in social attention. Further studies will be needed in order to clarify the strengthening of this conclusion. It will be also relevant specify whether this behaviour pattern is specific of Asperger’s disorder or it is typical of the whole autism spectrum disorders. Another weakness of the present study is the small number of participants. Future studies should address these limitations by both increasing the sample of participants and evaluating social attention in people with high functioning autism.
Notes
While our results suggested an absence of IOR to eye-gaze cues in the Asperger group, the possibility of a delayed IOR response to eye-gaze cues cannot be ruled out, as Asperger individuals might require longer SOA intervals for IOR to eye-gaze cues. Although further research is necessary to shed light upon this issue, the fact that Asperger individuals not only did not show IOR for gaze cues but did show a marginal facilitatory effect makes unlikely that they would show IOR with a longer than 2,400 ms SOA.
References
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, 4th edition (DSM-IV). Washington, DC: American Psychiatric Association.
Baron-Cohen, S. (1989). The autistic child’s theory of mind: A case of specific developmental delay. Journal of Child Psychology and Psychiatry, 30, 285–297.
Baron-Cohen, S. (1995). Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press.
Baron-Cohen, S. (2000). The cognitive neuroscience of autism: Evolutionary approaches. In M. S. Gazzaniga (Ed.), The new cognitive neurosciences (pp. 1249–1257). Cambridge, MA: MIT Press.
Baron-Cohen, S., Baldwin, D. A., & Crowson, M. (1997). Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child Development, 68, 48–57.
Charman, T., Baron-Cohen, S., Swettenham, J., Baird, G., Cox, A., & Drew, A. (2001). Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development, 15, 481–498.
Chawarska, K., Klin, A., & Volkmar, F. (2003). Automatic attention cueing through eye movement in 2-year-old children with autism. Child Development, 74(4), 1108–1122.
Dawson, G., Meltzoff, A. N., Osterling, J., Rinaldi, J., & Brown, E. (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28, 479–485.
Driver, J., Davis, G., Ricciardelli, P., Kidd, P., Maxwell, E., & Baron-Cohen, S. (1999). Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6(5), 509–540.
Friesen, C. K., & Kingstone, A. (1998). The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin and Review, 5(3), 490–495.
Friesen, C. K., Ristic, J., & Kingstone, A. (2004). Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance, 30(2), 319–329.
Frischen, A., Bayliss, A. P., & Tipper, S. P. (2007a). Gaze cueing of attention: visual attention, social cognition and individual differences. Psychological Bulletin, 133(4), 694–724.
Frischen, A., Smilek, J. D., & Tipper, S. P. (2007b). Inhibition of return in response to gaze cues: The roles of time course and fixation cue. Visual Cognition, 15, 881–895.
Frischen, A., & Tipper, S. P. (2004). Orienting attention via observed gaze shifts evokes longer term inhibitory effects: Implications for social interactions, attention, and memory. Journal of Experimental Psychology: General, 133, 516–533.
Garnett, M. S., & Attwood, A. J. (1998). The Australian Scale for Asperger’s syndrome. In T. Attwood (Ed.), Asperger’s syndrome. A guide for parents and professionals (pp. 17–19). London: Kingsley.
Greene, D. J., Colich, N., Iacoboni, M., Zaidel, E., Bookheimer, S. Y., & Dapretto, M. (2011). Atypical neural networks for social orienting in autism spectrum disorders. NeuroImage, 56(1), 354–362.
Jonides, J. (1981). Voluntary versus automatic control over the mind’s eye’s movement. In J. Long & A. Baddeley (Eds.), Attention and performance IX (pp. 187–203). Hillsdale, NJ: Lawrence Erlbaum Associates Inc.
Klein, R. M. (1988). Inhibitory tagging system facilitates visual search. Nature, 334, 430–431.
Kylliainen, A., & Hietanen, J. K. (2004). Attention orienting by another’s gaze direction in children with autism. Journal of Child Psychology and Psychiatry, 45, 435–444.
Lord, C., Rutter, M., DiLavore, P., & Risi, S. (1999). Autism diagnostic observation schedule—WPS edition. Los Angeles, CA: Western Psychological Services.
Lord, C., Rutter, M., & LeCouteur, A. (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685.
Marotta, A., Lupiáñez, J., Martella, D., Casagrande, M. (2011). Eye gaze versus arrows as spatial cue: Two qualitatively different modes of attentional selection. Journal of Experimental Psychology: Human Perception and Performance. doi:10.1037/a0023959.
Müller, H. J., & Rabbitt, P. M. A. (1989). Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance, 15, 315–330.
Mundy, P., Sigman, M., Ungerer, J., & Sherman, T. (1986). Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry and Allied Disciplines, 27, 657–669.
Nation, K., & Penny, S. (2008). Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology, 20, 79–97.
Nestor, P. G., Klein, K., Pomplun, M., Niznikiewicz, M., & McCarley, R. W. (2010). Gaze cueing of attention in schizophrenia: Individual differences in neuropsychological functioning and symptoms. Journal of Clinical and Experimental Neuropsychology, 32(3), 281–288.
Okada, T., Sato, W., Murai, T., Kubota, Y., & Toichi, M. (2003). Eye gaze triggers visuospatial attentional shift in individuals with autism. Psychologia, 46, 246–254.
Pelphrey, K. A., Morris, J. P., & McCarthy, G. (2005). Neural basis of eye gaze processing deficits in autism. Brain, 128, 1038–1048.
Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32A, 3–25.
Posner, M. I., & Cohen, Y. A. (1984). Components of visual orienting. In H. Bouma & D. G. Bouwhuis (Eds.), Attention and performance X (pp. 531–556). Hove, UK: Lawrence Erlbaum Associates.
Posner, M. I., Rafal, R. D., Choate, L. S., & Vaughan, J. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2, 211–228.
Rinehart, N. J., Bradshaw, J. L., Moss, S. A., Brereton, A. V., & Tonge, B. J. (2008). Brief report: Inhibition of return in young people with autism and Asperger’s disorder. Autism, 12(3), 249–260.
Sasson, N., Tsuchiya, N., Hurley, R., Couture, S. M., Penn, D. L., Adolphs, R., et al. (2007). Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia, 45, 2580–2588.
Schopler, E., Reichler, R., & Rochen Renner, B. (1998). The childhood autism rating scale (CARS). Los Angeles, Ca: Western Psychological Service.
Senju, A., Tojo, Y., Dairoku, H., & Hasegawa, T. (2004). Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry, 45, 445–458.
Sigman, M., Kasari, C., Kwon, J., & Yirmiya, N. (1992). Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Development, 63, 796–807.
Stone, W. L., Ousley, O. P., & Littleford, C. D. (1997). Motor imitation in young children with autism: What’s the object? Journal of Abnormal Child Psychology, 25, 475–485.
Swettenham, J., Condie, S., Campbell, R., Milne, E., & Coleman, M. (2003). Does the perception of moving eyes trigger reflexive visual orienting in autism? Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 358, 325–334.
Acknowledgments
We would like to thank all the children, parents, teachers, and the primary school “Istituto Ugo Bartolomei” and the secondary school “Istituto Giuseppe Sinopoli. We would also like to thank all the children with Asperger’s disorder and their parents, the Dr. Flavia Caretto and the “Gruppo Asperger” Association, with particular regard to Miss. Laura Imbimbo and Miss. Adina Adami.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marotta, A., Pasini, A., Ruggiero, S. et al. Inhibition of Return in Response to Eye Gaze and Peripheral Cues in Young People with Asperger’s Syndrome. J Autism Dev Disord 43, 917–923 (2013). https://doi.org/10.1007/s10803-012-1636-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1636-3