Abstract
This article presents an overview of the Finnish Prenatal Study of Autism and Autism Spectrum Disorders (FIPS-A), a new study designed to examine the relationship between prenatal serologic factors, mediating and moderating developmental antecedents, and risk of autism spectrum disorders (ASD). The FIPS-A is based on register linkages between births from 1987 to 2005 ascertained from the Finnish Medical Birth Register (FMBR) and other national registers on treatment for this group of disorders. All subjects were members of the Finnish Maternity Cohort (FMC), which consists of virtually all births in Finland from 1983 to the present, and which includes archived maternal serum samples. This study also capitalizes on other registry information, such as systematically collected data on pregnancy, prenatal and neonatal complications and manual data collection from well-child clinics providing developmental data from birth to the age of 7 years. In this paper, we describe the methods used in the FIPS-A study, including a description of the national registers, available data and case ascertainment procedures. Finally, we discuss implications of the data for future work on uncovering putative aetiologies of ASD and key strengths and limitations of the design.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, a substantial increase in the prevalence of ASD has been reported (Atladottir et al. 2007; Baron-Cohen et al. 2009; Parner et al. 2008). The rise of prevalence of ASD has been attributed to changes in diagnostic criteria (Rice et al. 2007), changes in surveillance (Rice et al. 2007) or methodological differences between studies (Gardener et al. 2009). We cannot, however, rule out the possibility that the true prevalence of ASD has increased in part because of environmental factors or other reasons yet to be discovered. Increased adverse events in the prenatal and neonatal periods among individuals with ASD have been consistently reported in prior studies (Kolevzon et al. 2007) The interpretation of findings from several previous studies is, however, hampered by several factors including (a) the lack of prospective assessment of in utero risk factors validated by maternal biomarkers; (b) limited ascertainment of cases followed into the age of risk for ASD, (c) data on anthropometric or developmental measures collected at only one or two points in time, and often after the diagnosis of ASD has been made, (d) use of historical controls, which introduces the potential for bias, and (e) small to modest sample sizes resulting in a lack of statistical power to detect significant associations.
The FIPS-A, a new study designed to capitalize on access to a large and representative sample of pregnancies from a national birth cohort with prospectively collected and archived prenatal serum specimens, should offer several strengths over previous studies of autism. The Finnish registries provide comprehensive data on pregnancy events and birth complications as well as diagnoses of both hospital admissions and outpatient treatment, allowing for more complete case ascertainment than in some other studies. The archived sera will be analyzed for biomarkers of potential environmental risk factors, and the extensive data on pregnancy and birth complications will permit the investigation of the relationships between prenatal and neonatal events in the pathogenesis of this disorder. Comprehensive data available from child health clinics will allow for characterization of the growth trajectory, including head circumference, in relation to risk of autism and to perinatal events. In this article, we describe the methods designed for the FIPS-A, including a description of the national registers, available data, case ascertainment procedures, and demographic information on the sample. Finally, we discuss key strengths and limitations of the design, and implications of the data.
Materials and Methods
In Finland, a diagnosis of ASD is based on multidisciplinary assessment and the diagnostic assessment typically includes several visits at the outpatient or inpatient clinic. The public health care system includes primary health care, district and central hospitals as well as university hospitals. Primary health care doctors, who are either general practitioners or pediatricians, are gatekeepers for referral to the more specialized services. Most cases suspected by primary practitioners of having ASD are referred to one of the university or central hospitals, in which specialist care is available. The assessment is led by a child neurologist or child psychiatrist. The assessment procedure includes evaluation of the child’s development including cognitive, communication and social skills as well as differential diagnostic procedures if indicated (e.g. several laboratory tests, metabolic screening, chromosomal analysis, brain imaging, and neurophysiologic examinations). The majority of ASD cases are diagnosed and treated within the public health care system and free of charge. All diagnoses are based on the International Statistical Classification of Diseases (ICD); ICD-8 in 1969–1986, ICD-9 in 1987–1995 and ICD-10 since 1996. Diagnostic information in the registers is based on clinical diagnoses and all diagnoses are routinely registered in the Finnish Hospital Discharge Register (FHDR) by healthcare personnel. Usually registration takes place immediately after any diagnoses are given by the attending clinician. As described below (see section Diagnostic validation), the validity of Finnish register-based diagnoses of childhood autism has been studied and found to be excellent according to the Autism Diagnostic Interview—Revised (ADI-R) (Lampi et al. 2010).
Nationwide Registers
The National Institute for Health and Welfare (THL) is a Finnish research and development institute with a mission to promote the well-being and health of the population, preventing diseases and social problems and developing social and health services. To this end, THL compiles and maintains several nationwide registers with comprehensive databases of health and welfare statistics concerning various topics, including psychiatric and other medical diagnoses, data from birth registers and congenital malformations, and prenatal serologic archives. The registers used in the FIPS-A include the FHDR, the Finnish Medical Birth Register (FMBR), the Finnish Register of Congenital Malformations (FRCM), and the register of the Finnish Maternity Cohort (FMC). These registers are described below in detail.
The Finnish Hospital Discharge Register
The FHDR contains the personal identity code and hospital identification code, date of admission and discharge and primary diagnosis at discharge, together with three possible subsidiary diagnoses. The register was established in the early 1960s and since 1967, it has covered all hospitals in Finland; computerized data with complete personal identity codes are available from 1969 to the present. Diagnostic information in the register is based on clinical diagnoses and the ICD, made by the attending clinician. In Finland, all medical diagnoses, both somatic and psychiatric, are registered in the FHDR by healthcare personnel. The FHDR now covers all somatic and psychiatric hospitals, as well as inpatient wards of local health centers, military wards, prison hospitals, and private hospitals. Since 1998, the FHDR has also covered all outpatient care in public hospitals. In the FIPS-A, the purpose of the FHDR is the identification of ASD cases.
The Finnish Medical Birth Register
The FMBR includes comprehensive and standardized data on pregnancy, the prenatal period, and the neonatal period up to age 7 days on all births in Finland. The FMBR was established in 1987 and the computerized register is also maintained by THL, with the primary purpose of collecting statistical data for research, development and provision of maternity care, obstetrics services, and the care of newborn infants. For each infant, a standard form utilized throughout Finland is required to be filled in by the delivering obstetrician in the Finnish maternity clinics where all care is received, up to 7 days after delivery. In the rare cases of home deliveries, the form is filled in by the midwife or the physician who conducted the delivery. The definitions and variables included in this register are based on the ICD-classifications. The data are entered into local electronic databases, and submitted to THL by the delivery hospitals. These data are checked, and any missing data or information suspected of being incorrect are confirmed by contacting the treating hospitals, and then are corrected in the database. Extensive review of the virtually complete data, including cross-checking with the Finnish Population Register, indicate that less than 0.1% of the births are missing (Teperi 1993; Gissler and Shelley 2002). Data contained in the FMBR are organized into the following categories: demographic characteristics, reproductive history, maternal health-related behaviours, complications during pregnancy, and perinatal/neonatal events, and are available from 1987 to the present. The register includes personal identity codes of mothers and live born children that can be used to link the subjects across all of the databases in the study. In the FIPS-A, the purpose of the FMBR is the identification of controls. Other select variables used from the FMBR are presented in Table 1.
The Finnish Register of Congenital Malformations
The FRCM contains national-level data on congenital chromosomal and structural anomalies detected in stillborn and live born infants and foetuses until the age of 1 year, from 1963 onwards. Data on congenital anomalies detected later, after the age of 1 year, are also collected, but not included in the published statistics. The register includes personal identity codes of mothers and live born children. Data on congenital anomalies are received from hospitals, health care professionals and cytogenetic laboratories as well as from other registers, such as the FMBR or the FHDR. Diagnoses obtained from these data sources are confirmed by contacting the hospitals concerned. The main purpose of the FRCM is to study the prevalence and type of congenital anomalies for the early identification of any new environmental factors that potentially cause foetal defects and for the prevention of anomalies by influencing these factors.
The Finnish Maternity Cohort and the Prenatal Serology Laboratory
The FMC consists of virtually all pregnancies in Finland since 1983. Mandatory testing for systemic infections in this population is obtained, and the serum are stored in the Prenatal Serology Laboratory (PSL), a repository established in 1983 with the purpose of preserving for research these samples in all pregnant women in Finland. The samples are drawn at municipal maternity care units during the first trimester of pregnancy, following informed consent, for screening of congenital infections. After the screening, 1–3 mL volume of serum from each pregnancy is stored at −25°C in polypropylene cryo vials at the PSL (Koskela et al. 2000). In 2010, the repository contained more than 1.6 million samples from over 810,000 women (about 98% of all pregnancies in the country) and new blood samples are continuously added into the serum bank. The FMC is being used in the FIPS-A for measurement of specific maternal biomarkers, including those for infections, immune function, toxins, and hormones of all subjects in the study. The validity of the analysis of maternal serum specimens within the FMC serum bank, especially in the analysis of the markers proposed in the FIPS-A, has been established in nearly 100 previous publications. The serologic assays will be performed at the PSL in the city of Oulu, Finland.
Child Health Clinics
The Finnish child health clinic system is a population-based, municipal service for every child below school age (i.e. until the age of 7 years). The Finnish child health clinic services are part of the primary health care guided by the Ministry of Social Affairs and Health. They are seen as a continuation of maternal health care, with the general aim of promoting the health of the child and the entire family. Practically all Finnish children visit the child health clinic once a month during the first year of life, and once a year after that until school age. Public health nurses collaborating with physicians are the primary caregivers monitoring children’s physical, cognitive and social development as well as implementing the national vaccination programme.
Overview of the Design
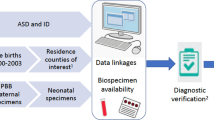
The FIPS-A has been authorized by the Ministry of Social Affairs and Health of Finland with the approval from the THL. The FIPS-A is based on a nested case–control design, which relies upon linkages between the nationwide registers and child health clinics to identify cases and controls (Fig. 1), obtain maternal serum specimens for assays, evaluate pregnancy and birth complications, assess developmental antecedents and risk of autism, and examine the inter-relationships between these domains. Case–control design was selected as it is an efficient approach for the investigation of risk factors for disease occurrence at the population level and is especially useful when it is prohibitively expensive to collect complete covariate information (such as from serologic analyses) for the whole cohort. The sampling frame, defined so that all members of the at-risk cohort were within the age of risk of autism includes more than 1.2 million offspring, consisting of all births in Finland (approximately 60,000 births per year) between the years 1987–2005. These births were identified from the FMBR.
Case Identification
A total of 5,019 ASD cases were identified during the study period. Cases were identified from the FHDR using the ICD codes 299x (ICD-9) or F84x (ICD-10). The majority of cases were diagnosed with ICD-10, while only 19 cases were diagnosed with ICD-9. The most recently registered diagnosis was used for identification. Table 2 reports the distribution of ASD subtypes.
Diagnostic Validation
The validity of the diagnoses of childhood autism that are registered in the FHDR has previously been studied and a full description of the validation study is available (Lampi et al. 2010). In order to validate registry-based diagnoses, 80 cases with diagnoses of childhood autism according to the FHDR were re-assessed using the Autism Diagnostic Interview-Revised (ADI-R). Among these 80 cases, 77 (96%) met the criteria for autism according to the ADI-R. These data strongly support the validity of Finnish registry-based diagnoses of infantile/childhood autism, but do not ensure validation for other diagnostic groups.
Control Inclusion and Exclusion Criteria
Controls are defined as offspring from the FMBR who are without ASD or severe/profound mental retardation according to the FHDR. Each case was matched with four controls on date of birth (±30 days), sex, and place of birth, yielding 25,000 controls. Date of birth was included as a matching factor in order to control for secular changes in prevalence of exposures and ASD outcomes, and to control for potential confounding by season of birth. Matching on sex was conducted in order to ensure that there are adequate numbers of female cases who meet matching criteria given the male predominance of autism. If the birth place was a very municipality and a control could not be found, the first option was to match by birth hospital; if a control was still not found, controls were identified by regional hospital district. Matching for birth place ensures that the controls for each case are representative of the population at risk, even though all Finnish permanent residents are covered by public health care services and national health insurance despite their background, e.g. residence. Matching on birth place not only ensures representativeness but also controls for unknown potential confounders that vary by community and region.
Maternal Sera
Although archived maternal sera are available in the serum bank of the FMC on 99% of all live births in Finland, an additional linkage is conducted between the specific register of the FMC and the identified cases to ensure that cases included in the study have available maternal sera. Maternal serum specimens corresponding to pregnancies of cases and controls will be assayed for biomarkers of several prenatal exposures that have been implicated in autism such as infectious, immune, hormonal, and toxic insults with the goal of examining relationships between these exposures and autism risk and evaluating whether any associations are mediated by relationships of these factors with other prenatal and neonatal events, and child developmental measures (see next section). Effect modification of prenatal biomarkers by sex will also be examined.
Child Health Records
There are approximately 350 municipalities in Finland providing health services for children and families. The FHDR includes information on the place of birth for the cases. The place of birth for the controls was already known as it was a matching criterion in the study. Personal identity codes are always included in all register databases as well as in child health records. Child health clinics in each municipality were contacted for manual data abstraction; letters for permission were sent to the chief physicians on site, in order to identify the staff responsible for archiving developmental data at these clinics. A list containing personal identity codes for the cases and controls was sent to each municipality based on information of the place of birth. Archived data are being collected manually either by public health nurses on site or by research nurses, who travel to the site. Health records containing information on motor, cognitive and socio-emotional developmental milestones (e.g. crawling, tracking and reaching for objects, smiling, rolling); growth trajectories including height, weight and head circumference; and vaccination history are requested for each child. Additionally, records of observations made by public health nurses during regular visits to the child health clinics are collected from selected sites.
Discussion
The FIPS-A design offers the promise of prevention, early identification, and facilitation of translational research to uncover pathogenic mechanisms by which prenatal and other early life exposures alter foetal brain development and may lead to autism spectrum disorders. We will examine the relationship between several developmental factors from the 10th week of pregnancy to the age of 7 years and the outcome of ASD. The design offers the potential to investigate several risk factors of potential relevance to autism from previous studies as well as new risk factors based on analysis of serum biomarkers. These include, for example, birth weight, small for gestational age status, parental age, childhood head circumference, other pregnancy and other neonatal complications, and biomarkers of infections, immune activation, hormonal abnormalities, and toxic exposures. The large sample size improves precision and statistical power for estimating the relationship between prenatal, perinatal and childhood antecedents and risk of ASD. Figure 2 depicts the detectable odds ratios by prevalence of exposure for different levels of correlation. The case–control correlation in exposure (ϕ) is expected to be between 0.1 and 0.3, based on previous studies. This correlation is caused by non-independence between subjects after being matched on specific factors. There is excellent power to detect small effect sizes for exposures with prevalence of at least .05 (Dupont 1988). The inclusion of virtually all births in this national cohort and the availability of comprehensive national registers, which cover over 99% of the entire Finnish population, provide data which are unlikely to be biased by selective ascertainment, loss to follow-up or reporting differences (e.g. recall bias). The registers include both hospital and outpatient diagnoses; thus, we expect both mildly and severely affected individuals to be included in the study.
Limitations
Some limitations of the study design should be mentioned. We did not directly verify cases of ASD. Thus, it is likely that some misclassification errors may exist. While validation of diagnoses of childhood autism has been made, validation studies for other ASD have not yet been undertaken. In addition, there has been no cross-validation of these autism diagnoses to any specific geographic locality. Finnish registers account for those born as well as resident in Finland. With regard to limitations of the serologic data, it should be noted that maternal sera are available at only one time point (first trimester) during pregnancy. However, no other birth cohort studies on autism of this magnitude with prenatal sera exist. Moreover, it has recently been suggested that early pregnancy, including the period of embryogenesis may represent a critical gestational window in the etiopathogenesis of autism (Ploeger et al. 2010).
Conclusion
The FIPS-A design offers the potential for a considerable advance over previous investigations to develop a fuller understanding of the role of early life determinants of ASD. Unique features of the study design include the use of maternal biomarkers to provide direct measures of prenatal exposures to be investigated as putative risk factors for ASD, and longitudinal data on child development. This study will offer the potential for significant improvement in the investigation of specific prenatal and other early life exposures that are modifiable by straightforward public health measures. These approaches include, but are not limited to: vaccination, improved hygiene, barrier contraceptives and antibiotics to prevent infection, supplementation to correct hormonal deficiencies and measures to reduce exposures to environmental toxins. If these modifiable risk factors are shown to be related to ASD, the identification of new aetiologies has the potential to reduce the risk of this disorder. The FIPS-A also has the potential to elaborate deviations in the developmental trajectory which antedate onset of ASD, and to relate these measures to prenatal and neonatal factors, potentially resulting in an improved understanding of its pathogenic mechanisms. In addition, comprehensive child health records from select sites provide an opportunity for qualitative research in early indicators of ASD.
References
Atladottir, H. O., Parner, E. T., Schendel, D., Dalsgaard, S., Thomsen, P. H., & Thorsen, P. (2007). Time trends in reported diagnoses of childhood neuropsychiatric disorders: Danish cohort study. Archives of Pediatrics and Adolescent Medicine, 161(2), 193–198.
Baron-Cohen, S., Scott, F. J., Allison, C., Williams, J., Bolton, P., Matthews, F. E., et al. (2009). Prevalence of autism-spectrum conditions: UK school-based population study. The British Journal of Psychiatry: The Journal of Mental Science, 194(6), 500–509.
Dupont, W. D. (1988). Power calculations for matched case–control studies. Biometrics, 44(4), 1157–1168.
Gardener, H., Spiegelman, D., & Buka, S. L. (2009). Prenatal risk factors for autism: Comprehensive meta-analysis. The British Journal of Psychiatry: The Journal of Mental Science, 195(1), 7–14.
Gissler, M., & Shelley, J. (2002). Quality of data on subsequent events in a routine medical birth register. Medical Informatics and the Internet in Medicine, 27(1), 33–38.
Kolevzon, A., Gross, R., & Reichenberg, A. (2007). Prenatal and perinatal risk factors for autism: A review and integration of findings. Archives of Pediatrics and Adolescent Medicine, 161(4), 326–333.
Koskela, P., Anttila, T., Bjorge, T., Brunsvig, A., Dillner, J., Hakama, M., et al. (2000). Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. International Journal of Cancer. Journal International Du Cancer, 85(1), 35–39.
Lampi, K. M., Sourander, A., Gissler, M., Niemela, S., Rehnstrom, K., Pulkkinen, E., et al. (2010). Brief report: Validity of Finnish registry-based diagnoses of autism with the ADI-R. Acta Paediatrica (Oslo, Norway: 1992).
Parner, E. T., Schendel, D. E., & Thorsen, P. (2008). Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics and Adolescent Medicine, 162(12), 1150–1156.
Ploeger, A., Raijmakers, M. E., van der Maas, H. L., & Galis, F. (2010). The association between autism and errors in early embryogenesis: What is the causal mechanism? Biological Psychiatry, 67(7), 602–607.
Rice, C. E., Baio, J., Van Naarden Braun, K., Doernberg, N., Meaney, F. J., Kirby, R. S., et al. (2007). A public health collaboration for the surveillance of autism spectrum disorders. Paediatric and Perinatal Epidemiology, 21(2), 179–190.
Teperi, J. (1993). Multi method approach to the assessment of data quality in the Finnish medical birth registry. Journal of Epidemiology and Community Health, 47(3), 242–247.
Acknowledgments
This study was supported by Autism Speaks Foundation, USA. The authors have no potential conflict of interests to be disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lampi, K.M., Banerjee, P.N., Gissler, M. et al. Finnish Prenatal Study of Autism and Autism Spectrum Disorders (FIPS-A): Overview and Design. J Autism Dev Disord 41, 1090–1096 (2011). https://doi.org/10.1007/s10803-010-1132-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-010-1132-6