Abstract
Parents (N = 19) of children with autism spectrum disorders (ASD) and adult controls (N = 17) underwent positron emission tomography (PET) using [18F]setoperone to image cortical serotonin type-2 (5-HT2) receptors. The 5-HT2 binding potentials (BPs) were calculated by ratioing [18F]setoperone intensity in regions of interest (ROI) to cerebellar intensity. Cortical 5-HT2 BPs were significantly lower in parents compared to controls and platelet 5-HT levels were significantly negatively correlated with cortical 5-HT2 BP in parents. Lower cortical 5-HT2 receptor density in parents of children with ASD is consistent with reports of diminished 5-HT2 expression and functioning in individuals with ASD. Further research should examine the relationship of reduced 5-HT2 receptor expression to underlying causation and to clinical and neurochemical correlates of autistic behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Autism Spectrum Disorders (ASD), including Autistic Disorder, Asperger’s Disorder and Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) (American Psychiatric Press 1994), are characterized by difficulties in three core areas: social interaction; communication; and repetitive behaviors or circumscribed interests (Rapin 1997). Based on twin and family studies, it appears that ASD’s are largely genetically determined (Bailey et al. 1995; Bolton et al. 1994; Rutter 1999).
Given the complexity of susceptibility factors thought to underlie ASD, examining the determinants of specific behavioral and biological alterations associated with the disorder may be a productive strategy for future research. This focus on specific traits, biomarkers, endophenotypes or intermediate phenotypes should provide measures that are genetically transmitted more simply than the DSM-IV-defined disorder and may point to specific systems playing key roles in autism expression (Gottesman and Gould 2003; Happé et al. 2006; Leboyer et al. 1998; McBride et al. 1996; Skuse 2001; Szatmari et al. 2007).
Parents of individuals with ASD can share specific behavioural and cognitive characteristics with their children. Previously noted findings in parents (on a group basis) include social difficulties, communication impairments and stereotyped, rigid behaviours, as well as cognitive differences (Folstein et al. 1999; Happé 2001; Lainhart et al. 2002; Losh and Piven 2007; Murphy et al. 2000; Piven et al. 1997; Ruser et al. 2008; Stone et al. 2008). It has been suggested that these characteristics constitute a broader phenotype of autism which may represent an expression of the genetic liability for ASD. Several studies have reported biological differences in ASD parents versus controls, including increased whole blood serotonin Leboyer 1999); decreased plasma levels of reelin (Fatemi et al. 2002); diminished size of the left hippocampus (Rojas et al. 2004); altered local gray matter volumes (Peterson et al. 2006) and decreased cortical activation (Baron-Cohen et al. 2006). The biological findings are in need of replication and a structural imaging study not finding differences between parents and controls has been reported (Palmen et al. 2005).
There has been a longstanding interest in the neurotransmitter/neurohormone 5-hydroxytryptamine (5-HT) in autism as a potential intermediate phenotype. Initial interest was stimulated with the early report of increased group mean platelet 5-HT levels in autism (Schain and Freedman 1961). The basic finding has been frequently replicated and platelet hyperserotonemia is now a well-characterized trait in ASD (Anderson 2002; Mulder et al. 2004). However, the mechanism of 5-HT elevation remains unclear. Additional factors contributing to the continuing interest in 5-HT include the compound’s critical role in early neurodevelopment (Janusonis et al. 2004; Polleux and Lauder 2004; Whitaker-Azmitia 2001), the report of reduced 5-HT synthesis in the brains of children with autism (Chandana et al. 2005), and the wide use of 5-HT selective reuptake inhibitors (SSRIs) for treating symptoms associated with autism (Kolevzon et al. 2006).
The critical role of the 5-HT2 receptor in cortical function (Jakab and Goldman-Rakic 1998) provides a theoretical basis for focusing on this receptor and accumulating evidence supports the idea that this system may be altered in autism. In 1989, McBride and colleagues reported that central and peripheral 5-HT2 receptor functioning appeared reduced in autism. A diminished 5-HT2-mediated neuroendocrine response was observed and was paralleled by lower platelet 5-HT2 receptor binding and reduced 5-HT2-mediated platelet aggregation in individuals with autism (McBride et al. 1989). Platelet 5-HT2 binding alterations in relatives of individuals with autism have been reported by Cook et al. (1993); moreover, in both studies 5-HT2 receptor measures were inversely related to platelet 5-HT levels. More recently, Murphy et al. (2006) reported lower 5-HT2A receptor density centrally by in vivo SPECT imaging in eight adults with Asperger’s syndrome. It is worth noting that there have been several negative candidate gene studies of the 5-HT2A receptor gene in ASD (Cho et al. 2008; Herault et al. 1996; Veenstra-Vander-Weele et al. 2002). However, the studies have been limited in size and have not fully studied the genetic variation.
We performed a neuroimaging study examining brain 5-HT2 receptor binding density in parents of two or more children with ASD. Parents from such multiplex families are assumed to have higher genetic loading for ASD and should offer advantages when examining potential intermediate phenotypes associated with the disorder. We hypothesized that parents of probands with ASD would have diminished central 5-HT2 receptor binding density compared to controls. While there are several neuroimaging studies of parents of children with ASD (mentioned above), only the study of Murphy and colleagues (2006) focused on serotonin.
Methods
Subjects
The parents of the children with ASD were volunteers from families participating in our autism research program; controls were recruited as volunteers by advertising at the Hamilton Health Sciences Centre. Potential participants were informed of the study (approved by the Institutional Review Board of McMaster University) and, after giving signed consent, completed an unstructured interview with one of the investigators (JG). The interview was used to exclude a history of neurological and/ or psychiatric disorder during the 6 month period predating the study; subjects with histories of medication and/or substance use that could affect 5-HT levels were similarly excluded.
The “parent” group consisted of 19 parents from 11 multiplex families and was comprised of 8 females and 11 males (2 parents were Hispanic, 2 Asian and 15 Caucasian). The families were unrelated and the marriages were non-consanguineous. The control group consisted of 9 females and 8 males (1 Asian and 16 Caucasian). The mean (±SD) ages of the parent and control groups were similar (44.8 ± 6.2 and 43.6 ± 8.4 years, respectively; F = 0.22, p = 0.65) and there was no significant sex difference between the groups (Fisher’s exact test, p = 0.74).
Proband Assessment
The probands (the children of the parents) did not participate in this investigation; however, they had been diagnosed with ASD following the completion of the Autism Diagnostic Observation Schedule (ADOS) (Gotham et al. 2007) and the Autism Diagnostic Interview Revised (ADI-R) (Lord et al. 1994) with their parent(s) as informant(s). There were 23 affected children with ASD (mean age of 13.1 years, range 5–25 years). Twenty-one children met ADI-R criteria for autism and two met criteria for ASD by having a score one point below the autism ADI-R algorithm cut-off in one domain. The mean IQ of the probands based on the Leiter scale (Levine 1986) was 64.2 ± 31.1; mean adaptive behavior scores in socialization, communication and daily living based on the Vineland Scales (Sparrow et al. 1984) were between 47 and 55.
Parent and Control Psychological Assessment
The parents and controls were assessed with the Family History Interview for Developmental Disorders-shortened version (Folstein and Rutter 1991), as modified by Bolton et al. (1994) and MacLean et al. (1999). The shortened version includes those questions that probe symptoms of the BAP (Bolton et al. 1994), as well as screening questions about other psychiatric disorders. An algorithm constructs a three-factor definition of the BAP corresponding to each domain of the autistic triad. Parents with one social, communication or repetitive activities impairment are considered to have the “broad” variant of BAP and those with two types of impairment, the “narrow” variant.
Measurement of Central 5-HT2 Receptor Density
The [18F]setoperone positron emission tomography (PET) studies were performed at the Hamilton Health Sciences PET Centre between 2 and 6 pm, over a period of 3 years (1998–2001). Prior studies of animals and humans have shown that [18F]setoperone PET is a reliable method for selectively measuring 5-HT2 receptor density in the human cortex (Blin et al. 1990). Because a significant portion of [18F]setoperone in the subcortex is bound to dopamine-type-2 (D2) receptors, subcortical BP’s were not included in the analyses.
[18F]setoperone was prepared from the nitro precursor of setoperone using an adaptation of a method described by Crouzel et al. (1988). The PET imaging was performed with an ECAT 953/31 tomograph (Siemens/CTI) following a bolus injection of 185 MBq of [18F]setoperone in a manner similar to that described by Blin et al. (1990). After infusion of [18F]setoperone, images were obtained for 90 min in a sequence similar to that described by Kapur et al. (1997). Following attenuation correction using a 68Ge rotating pin, images were reconstructed with a Hanning filter at 5 mm FWHM. Regions of interest (ROIs) were drawn for 12 cortical regions in three different planes of the brain and in the cerebellum. Given the very low levels of 5-HT receptors (Pazos et al. 1987) and the very small amount of displaceable 18F-setoperone binding in the cerebellum (Blin et al. 1990), cerebellar activity was used as a reference region for determining non-specific binding and background radiation. It has been previously shown that the ratio (S/C) of cortical (S) activity to cerebellar activity (C), termed the binding potential (BP), is an appropriate index for determining [18F]setoperone binding in the brain (Kapur et al. 1997). Furthermore, Petit-Tabou et al. (1996) have shown that when [18F]setoperone reaches a state of pseudo-equilibrium between 60 and 90 min post injection, the cortex-to-cerebellum ratio is highly correlated with cortical binding density (Bmax/Kd ratio). Cerebellar counts (controlled for age and sex) did not differ significantly across the groups studied (parents 1,916 ± 845, controls 1,427 ± 1,105; t(1) = −1.42, p = 0.34).
The PET system was calibrated approximately every 6 months with variations of around 10% noted. Intra-class correlation coefficients (ICCs) were derived for four scans (two parent and two control) to determine test-retest (1 month apart) and inter-rater reliability for the ROI method of determining BP. ICCs were also calculated for the 12 individual cortical ROIs and for the composite cortical BP (i.e. ROIs combined). Calculated test–retest ICCs for the regional BP’s ranged from 0.80 to 0.98, while an ICC of 0.91 was observed for the composite cortical 5-HT2 BP. The inter-rater ICCs for the regional BP’s ranged from 0.75 to 0.97; the composite BP ICC was 0.92. Cases and controls were intermingled and studied over the same time period in an effort to minimize effects of drift.
Measurement of Whole Blood Serotonin (5-HT)
Blood specimens obtained for the determination of platelet 5-HT were drawn into tubes containing EDTA anticoagulant and initially kept at room temperature. Levels of serotonin are stable for up to 24 h in whole blood samples kept at room temperature (Danaceau et al. 2003). Portions were removed within 1–3 h and stored at −70°C until analyzed. Whole blood 5-HT levels (ng/ml) were determined by high-performance liquid chromatography (HPLC) with fluorometric detection as previously described, with within-assay and assay-to-assay coefficients of variation of 4.6% and 5.9%, respectively (Anderson et al. 1987; Epperson et al. 2001). As over 99% of blood 5-HT is found within the platelet, whole blood measurements reflect the platelet pool of 5-HT.
Statistical Analysis
We used a hierarchical approach to the statistical analysis. In the first set of planned comparisons, individual regional cortical BP’s were combined to obtain a single or composite (mean) measure for the cortex. The composite BP was compared between the parent and control groups; individual regional cortical BP’s were then compared across groups. Due to previously reported age-related changes in 5-HT2 binding (Arranz et al. 1993; Meltzer et al. 1999), marked sex differences in ASD occurrence (Baron-Cohen et al. 2005), and suggested sex differences in human brain 5-HT synthesis (Young et al. 1999), both age and sex were used as covariates in all analyses. However, it should be noted that age and the male-to-female ratio did not differ substantially or significantly between parents and controls (see above). Interactions terms were also added to the model, testing the interaction between age and group and sex and group on BP’s. Relationships between 5-HT2 BP and platelet 5-HT levels were tested by Pearson correlation.
Results
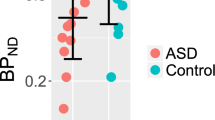
The mean (±SD) parent composite cortical 5-HT2 BP was 2.13 ± 0.34 compared to 2.65 ± 0.75 in the controls (see Fig. 1). Controlling for age and sex, this difference was significant [F(1df) = 6.90, p = 0.013], with a large effect size (0.9; Cohen 1988). Neither of the interaction terms was significant. We observed a trend-level negative correlation (moderate) between cortical 5-HT2 BP and age in the parent group (r = −0.42; p = 0.07), but not in the controls (r = −0.26; p = 0.31).
Comparison of composite cortical 5-HT2 receptor BP in parents (N = 19) of children with autism and controls (N = 17). Group means are indicated by the horizontal bar and the standard deviations by brackets. The group mean composite 5-HT2 BP was significantly lower in the parent group: 2.13 ± 0.34 versus 2.65 ± 0.75; [F(1df) = 7.16, p = 0.010]
We next examined the BPs of specific cortical ROIs using ANCOVA, again with age and sex as covariates. These analyses showed that 5-HT2 BP’s were significantly diminished in all cortical regions in parents as compared to controls—based on a nominal p-value of 0.05 (see Table 1). Overall, the individual cortical ROI BP’s of the parents ranged from 77% to 88% of those seen in the controls. There was no effect of sex on BP and no sex by group or age by group interaction for any of the cortical regions.
There was no significant difference between the platelet 5-HT level of the parents (129 ± 47 ng/ml) and the controls (150 ± 47 ng/ml) (F = 1.87; 2df; p = 0.13). The mean platelet 5-HT level of the parents was negatively correlated with their composite cortical 5-HT2 BP (r = −0.59; p = 0.02). In addition, in eight of the 12 cortical regions the 5-HT2 BP versus platelet 5-HT correlation reached a significance level of 0.05 or less (see Table 2). The calculated r values ranged from −0.52 to −0.65, with the highest correlations seen in the right inferior frontal (r = −0.65, p = 0.01) and right inferior parietal lobes (r = −0.64, p = 0.01). Platelet 5-HT level was not correlated with mean cortical 5-HT2 BP (r = −0.08, p = 0.77) nor with any of the individual cortical regional 5-HT2 BP’s in the controls.
The Family History Interview indicated that 5 of the 19 parents (26%) met criteria for the broader autism phenotype (+BAP; all due to significant difficulty with social interaction). When parents were divided into +BAP and −BAP groups, no significant difference in 5-HT2 BP was observed (composite cortical BP 2.08 ± 0.57 and 2.15 ± 0.25, respectively; p = 0.32]. This comparison is presented for the sake of completeness and is limited by the small number of +BAP parents.
Discussion
We have presented evidence of lower 5-HT2 receptor density throughout the cerebral cortex of parents with two or more children with ASD—a group presumed to have relatively high genetic loading for ASD. This finding is consistent with a previous report of lower central 5-HT2 responsivity in autism (McBride et al. 1989) and with a recent neuroimaging study that observed lower cortical 5-HT2A receptor density in young adults with Asperger’s syndrome using a different imaging methodology (single photon emission computed tomography) and an alternative 5-HT2A receptor ligand (123I-R91150) (Murphy et al. 2006). It is notable that a neuroendocrine challenge paradigm (McBride et al. 1989) and two neuroimaging studies (Murphy 2006 and ourselves), as well as a study of postmortem brain tissue (G. Blatt, personal communication, 2007), have provided converging evidence of lower central 5-HT2 receptor expression or function associated with autism.
In our study, the 5-HT2 BPs measured in the cortical regions of parents were negatively correlated with platelet 5-HT levels. This inverse relationship is consistent with the reported negative correlation of platelet 5-HT levels and 5-HT2-mediated neuroendocrine response in autism (McBride et al. 1989) and with inverse correlations for platelet 5-HT level and platelet 5-HT2 receptor density in individuals with autism or their relatives (Cook et al. 1993; McBride et al. 1989). While the correlations between platelet 5-HT levels and central or platelet 5-HT2 receptor density are intriguing, the nature of this relationship has yet to be determined. Furthermore, we did not show global differences of platelet 5-HT levels between parents and controls. Notwithstanding these limitations, the inverse relationships observed by us and others do suggest the possibility that some 5-HT2 receptor-related factor may play a role in the platelet hyperserotonemia of autism.
The mechanism of the lower 5-HT2 receptor density in ASD family members is unknown. It is well known that brain 5-HT2 receptors do not follow the classical receptor regulation model: both 5-HT antagonists and agonists have been associated with down-regulation of 5-HT2 receptors, and 5-HT2 receptors do not up-regulate following serotonergic denervation (Eison and Mullins 1996). It should be noted that lower cortical 5-HT2 receptor expression has been reported in schizophrenia (Burnet et al. 1996; Laruelle et al. 1993; Lewis et al. 1999), bipolar disorder (Lopez-Figueroa et al. 2004), depression (Meyer et al. 1999; Minton et al. 2003), and after antidepressant treatment (Yatham et al. 1999). Higher levels have been reported in suicide victims (Mann et al. 2001), in recovered depressed patients (Bhagwagar et al. 2006) and after estrogen treatment (Kugaya et al. 2003).
It is unclear whether the apparent lower 5-HT2 receptor density in cortical regions of parents of children with ASD represents a primary underlying etiological process or whether it represents a secondary phenomenon—as compensation for another more fundamental abnormality in the 5-HT system or elsewhere in the brain. The observed alterations in cortical regions do tend to focus attention on factors involved in cortical development. A longstanding interest in 5-HT’s role in cortical development and in neurodevelopmental disorders has recently increased as patterns of early serotonergic innervation of the cortex have been elaborated (Bonnin et al. 2007; Janusonis et al. 2004). The pattern of decreased variance of 5HT2 BP in parents as compared to controls is intriguing; however, a consideration of the possible causes of differences in the observed variances would be premature in the absence of replication.
The findings of this study provide preliminary evidence of decreased cortical 5HT2 expression in parents of children with ASD and it can be speculated that this might be contributing to previously noted social difficulties in these individuals—particularly in those parents with the BAP. Focusing on the 5-HT system and especially on the 5-HT2 receptor might provide a meaningful intermediate phenotype for biological and genetic studies, and may lead to improved nosology for ASD. Identification of the clinical correlates related to diminished 5HT2 function could lead to greater understanding and improved treatment of ASD and the BAP.
We acknowledge several limitations with the current study. First, the region of interest (ROI) methodology used to define [18F]setoperone activity only included specific predefined regions in the analysis; furthermore, MRI co-registration could have been useful for ROI definition. Second, the S/C ratio (BP) is an indirect estimate of receptor density that is linearly proportional to Bmax/Kd (see Kapur et al. 1997); therefore, the possibility of changes in BP having arisen from changes in Kd (receptor affinity) cannot be ruled out. Third, familial aspects of 5-HT2 receptor expression were not directly examined, as the children (of the parents) did not participate in this study. Fourth, using a more dimensional measure of the broader phenotype—such as, the social responsiveness scale (SRS; Constantino et al. 2003) or the Broader Autism Phenotype Questionnaire (BAPQ; Hurley et al. 2007)—might have been more revealing in correlating phenotype and 5HT2 BP (Duvall et al. 2007). Finally, the requirement of studying drug-free and disorder-free parents (at least by recent history) required us to select an inherently non-representative sample.
References
American Psychiatric Press. (1994). Diagnostic and statistical manual of mental disorders 4th edition (DSM-IV). Washington, DC: American Psychiatric Association.
Anderson, G. M. (2002). Genetics of childhood disorders: XLV. Autism, Part 4: Serotonin in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 1513–1516. doi:10.1097/00004583-200212000-00025.
Anderson, G. M., Feibel, F. C., & Cohen, D. J. (1987). Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultra-filtrate. Life Sciences, 40, 1063–1070. doi:10.1016/0024-3205(87)90568-6.
Arranz, B., Eriksson, A., Mellerup, E., Plenge, P., & Marcusson, J. (1993). Effect of aging on human cortical pre and post synaptic serotonin binding sites. Brain Research, 620, 163–166. doi:10.1016/0006-8993(93)90286-V.
Bailey, A., Le Couteur, A., Gottesman, I., Bolten, P., Simonoff, E., Yuzda, E., et al. (1995). Autism is a strongly genetic disorder: Evidence from a British twin study. Psychological Medicine, 25, 63–77.
Baron-Cohen, S., Knickmeyer, R. C., & Belmonte, M. K. (2005). Sex differences in the brain: Implications for explaining autism. Science, 310, 819–823. doi:10.1126/science.1115455.
Baron-Cohen, S., Ring, H., Chitnis, X., Wheelwright, S., Gregory, L., et al. (2006). fMRI of parents of children with Asperger’s Syndrome: A pilot study. Brain and Cognition, 61, 122–130. doi:10.1016/j.bandc.2005.12.011.
Bhagwagar, Z., Hinz, R., Taylor, M., Fancy, S., Cowen, P., & Grasby, P. (2006). Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [(11)C]MDL 100, 907. The American Journal of Psychiatry, 163, 1580–1587. doi:10.1176/appi.ajp. 163.9.1580.
Blin, J., Sette, G., Fiorelli, M., Bletry, O., Elghozi, J. L., et al. (1990). A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. Journal of Neurochemistry, 54, 1744–1754. doi:10.1111/j.1471-4159.1990.tb01229.x.
Bolton, P., Macdonald, H., Pickles, A., Rios, P., Goode, S., Crowson, M., et al. (1994). A case–control family history study of autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 35, 877–900. doi:10.1111/j.1469-7610.1994.tb02300.x.
Bonnin, A., Torii, M., Wang, L., Rakic, P., & Levitt, P. (2007). Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nature Neuroscience, 10, 588–597. doi:10.1038/nn1896.
Burnet, P. W., Eastwood, S. L., & Harrison, P. J. (1996). 5-HT1A and 5-HT2A receptor mRNAs and binding densities are differentially altered in schizophrenia. Neuropsychophamacology, 15, 422–455.
Chandana, S. R., Behen, M. E., Juhasz, C., Muzik, O., Rothermel, R. D., Mangner, T. J., et al. (2005). Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. International Journal of Developmental Neuroscience, 23, 171–182. doi:10.1016/j.ijdevneu.2004.08.002.
Cho, I. H., Yoo, H. J., Park, M., Lee, Y. S., & Kim, S. A. (2008). Family-based association study of 5-HTTLPR and the 5-HT2A receptor gene polymorphisms with autism spectrum disorder in Korean trios. Brain Research, 1139, 34–41. doi:10.1016/j.brainres.2007.01.002.
Constantino, J. N., Davis, S. A., et al. (2003). Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders, 33, 427–433. doi:10.1023/A:1025014929212.
Cook, E. H., Jr, Arora, R. C., Anderson, G. M., Berry-Kravis, E. M., Yan, S. Y., Yeoh, H. C., et al. (1993). Platelet serotonin studies in hyperserotonemic relatives of children with autistic disorder. Life Sciences, 52, 2005–2015. doi:10.1016/0024-3205(93)90685-V.
Crouzel, C., Venet, M., Irie, G., Sanz, G., & Boullais, C. (1988). Labeling of a serotonergic ligand with 18-F Setoperone. Journal of Labelled Compounds and Radiopharmaceuticals, 25, 403–414. doi:10.1002/jlcr.2580250407.
Danaceau, J. P., Anderson, G. M., McMahon, W. M., & Crouch, D. J. (2003). A liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. Journal of Analytical Toxicology, 27, 440–444.
Duvall, J. A., Lu, A., et al. (2007). A quantitative trait locus analysis of social responsiveness in multiplex autism families. The American Journal of Psychiatry, 164, 656–662. doi:10.1176/appi.ajp. 164.4.656.
Eison, A. S., & Mullins, U. L. (1996). Regulation of central 5HT2A receptors: A review of in vivo studies. Behavioural Brain Research, 73, 177–181. doi:10.1016/0166-4328(96)00092-7.
Epperson, C. N., Czarkowski, K. A., Ward-O’Brien, D., Weiss, E., Gueorguieva, R., Jatlow, P., et al. (2001). Maternal sertraline treatment and serotonin transport in breastfeeding mother-infant pairs. The American Journal of Psychiatry, 158, 1631–1637. doi:10.1176/appi.ajp. 158.10.1631.
Fatemi, S. H., Stary, J. M., & Egan, E. A. (2002). Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cellular and Molecular Neurobiology, 22, 139–152. doi:10.1023/A:1019857620251.
Folstein, S. & Rutter, M. (1991). Family history interview for developmental disorders of cognition and social functioning (available from authors).
Folstein, S. E., Santangelo, S. L., Gilman, S. E., Piven, J., Landa, R., Lainhart, J., et al. (1999). Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 40, 1117–1128. doi:10.1017/S0021963099004461.
Gotham, K., Risi, S., Pickles, A., & Lord, C. (2007). The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37, 613–627. doi:10.1007/s10803-006-0280-1.
Gottesman, I. I., & Gould, T. D. (2003). The enodphontype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry, 160, 636–645. doi:10.1176/appi.ajp. 160.4.636.
Happe, F., Briskman, J., & Frith, U. (2001). Exploring the cognitive phenotype of autism: Weak “central coherence” in parents and siblings of children with autism: I. Experimental tests. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 42, 299–307. doi:10.1017/S0021963001006916.
Happe, F., Ronald, A., & Plomin, R. (2006). Time to give up on a single explanation for autism. Nature Neuroscience, 9, 1218–1220. doi:10.1038/nn1770.
Herault, J., Petit, E., Martineau, J., Cherpi, C., Perrot, A., Barthelemy, C., et al. (1996). Serotonin and autism: Biochemical and molecular biology features. Psychiatry Research, 65, 33–43. doi:10.1016/0165-1781(96)02882-X.
Hurley, R. S., Losh, M., et al. (2007). The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders, 37, 1679–1690. doi:10.1007/s10803-006-0299-3.
Jakab, R. L., & Goldman-Rakic, P. S. (1998). 5-Hydroxytryptamine serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in the pyramidal cell apical dendrites. Proceedings of the National Academy of Sciences of the USA, 95, 735–740. doi:10.1073/pnas.95.2.735.
Janusonis, S., Gluncic, V., & Rakic, P. (2004). Early serotonergic projections to Cajal-Retzius cells: Relevance for cortical development. The Journal of Neuroscience, 24, 1652–1659. doi:10.1523/JNEUROSCI.4651-03.2004.
Kapur, S., Jones, C., DaSilva, J., Wilson, A., & Houle, S. (1997). Reliability of a simple non-invasive method for the evaluation of 5-HT2 receptors using (18F) setoperone PET imaging. Nuclear Medicine Communications, 18, 395–399. doi:10.1097/00006231-199705000-00002.
Kolevzon, A., Mathewson, K. A., & Hollander, E. (2006). Selective serotonin reuptake inhibitors in autism: A review of efficacy and tolerability. The Journal of Clinical Psychiatry, 67, 407–414.
Kugaya, A., Epperson, C. N., Zoghbi, S., van Dyck, C. H., Hou, Y., Fujita, M., et al. (2003). Increase in prefrontal cortex serotonin2A receptors following estrogen treatment in postmenopausal women. The American Journal of Psychiatry, 160, 522–524. doi:10.1176/appi.ajp. 160.8.1522.
Lainhart, J. E., Ozonoff, S., Coon, H., Krasny, L., Dinh, E., Nice, J., et al. (2002). Autism, regression, and the broader autism phenotype. American Journal of Medical Genetics, 113, 231–237. doi:10.1002/ajmg.10615.
Laruelle, M., Abi-Dargham, A., Casanova, M. F., Toti, R., Weinberger, D., & Kleinman, J. (1993). Selective abnormalities of prefrontal serotonergic receptors in schizophrenia: A postmortem study. Archives of General Psychiatry, 50, 810–818.
Leboyer, M., Bellivier, F., Nosten-Bertrand, M., Jouvent, R., Pauls, D., Mallet, J., et al. (1998). Psychiatric genetics: Search for phenotypes. Trends in Neurosciences, 21, 102–105. doi:10.1016/S0166-2236(97)01187-9.
Levine, M. (1986). Leiter international performance scale: A handbook. Los Angeles: Western Psychological Services.
Lewis, R., Kapur, S., Jones, C., DaSilva, J., Brown, G., Wilson, A., et al. (1999). Serotonin 5-HT2 Receptors in Schizophrenia: A PET study using [18 F] setoperone in neuroleptic-naive patients and normal subjects. The American Journal of Psychiatry, 156, 72–78.
Lopez-Figueroa, A. L., Norton, C. S., Lopez-Figueroa, M. O., Armellini-Dodel, D., Burke, S., Akil, H., et al. (2004). Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biological Psychiatry, 55, 225–233. doi:10.1016/j.biopsych.2003.09.017.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. doi:10.1007/BF02172145.
Losh, M., & Piven, J. (2007). Social-cognition and the broad autism phenotype: Identifying genetically meaningful phenotypes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48, 105–112. doi:10.1111/j.1469-7610.2006.01594.x.
MacLean, J. E., Szatmari, P., Jones, M. B., Bryson, S. E., Mahoney, W. J., Bartolucci, G., et al. (1999). Familial factors influence level of functioning in pervasive developmental disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 38, 746–753. doi:10.1097/00004583-199906000-00023.
Mann, J. J., Brent, D., & Arango, V. (2001). The neurobiology and genetics of suicide and attempted suicide: A focus on the serotonergic system. Neuropsychopharmacology, 24, 467–477. doi:10.1016/S0893-133X(00)00228-1.
McBride, P. A., Anderson, G. M., Herzig, M., Sweeney, J., Kream, J., Cohen, D. J., et al. (1989). Serotonergic responsivity in male young adults with autistic disorder: Results of a pilot study. Archives of General Psychiatry, 46, 213–221.
McBride, P. A., Anderson, G. M., & Shapiro, T. (1996). Autism research: Bringing together approaches to pull apart the disorder. Archives of General Psychiatry, 53, 980–983.
Meltzer, C. C., Price, J. C., Mathis, C. A., Greer, P. J., Cantwell, M. N., Houck, P. R., et al. (1999). PET imaging of serotonin type 2A receptors in late life neuropsychiatric disorders. The American Journal of Psychiatry, 156, 1871–1878.
Meyer, J., Kapur, S., Houle, S., DaSilva, J., Owczarek, B., Brown, G., et al. (1999). Prefrontal cortex 5-HT2 receptors in depression: An [18F] setoperone PET imaging study. The American Journal of Psychiatry, 156, 1029–1034.
Minton, M. A., Sheline, Y. I., Moerlein, S. M., Vlassenk, A. G., Huang, Y., & Snyder, A. Z. (2003). Decreased hippocampal 5-HT2a receptor binding in major depression: In vivo measurement with [18F]altanserin positron emission tomography. Biological Psychiatry, 55, 217–224. doi:10.1016/j.biopsych.2003.08.015.
Mulder, E. J., Anderson, G. M., Kema, I. P., de Bildt, A., van Lang, N. D. J., den Boer, J. A., et al. (2004). Platelet serotonin in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution and behavioral correlates. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 491–499. doi:10.1097/00004583-200404000-00016.
Murphy, D. G., Schmitz, N., Toal, F., Murphy, K., Daly, E., Erlandsson, K., et al. (2006). Cortical 5-HT2A receptor binding and social communication in adults with Asperger’s syndrome: An in vivo SPECT study. The American Journal of Psychiatry, 163, 934–936. doi:10.1176/appi.ajp. 163.5.934.
Murphy, M., Bolton, P. F., Pickles, A., Fombonne, E., Piven, J., & Rutter, M. (2000). Personality traits of the relatives of autistic probands. Psychological Medicine, 30, 1411–1424. doi:10.1017/S0033291799002949.
Palmen, S. J., Hulshoff Pol, H. E., Kemner, C., Schnack, H. G., Sitskoorn, M. M., Appels, M. C., et al. (2005). Brain anatomy in non-affected parents of autistic probands: A MRI study. Psychological Medicine, 35, 1411–1420. doi:10.1017/S0033291705005015.
Pazos, A., Probst, A., & Palacio, J. M. (1987). Serotonin receptors in the human brain—iv. autoradiographic mapping of serotonin-2 receptors. NeuroImage, 21, 123–139.
Peterson, E., Schmidt, G. L., Tregellas, J. R., Winterrowd, E., Kopelioff, L., Hepburn, S., et al. (2006). A voxel-based morphometry study of gray matter in parents of children with autism. Neuroreport, 17, 1289–1292. doi:10.1097/01.wnr.0000233087.15710.87.
Petit-Tabou, M. C., Landeau, B., Osmont, A., Tillet, I., Barre, L., & Baron, J. C. (1996). Estimation of neo-cortical serotonin-2 receptor binding potential by single dose fluorine-18-Setoperone kinetic PET data analysis. Journal of Nuclear Medicine, 37, 95–104.
Piven, J., Palmer, P., Jacobi, D., Childress, D., & Arndt, S. (1997). Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. The American Journal of Psychiatry, 154, 185–190.
Polleux, F., & Lauder, J. M. (2004). Toward a developmental neurobiology of autism. Mental Retardation and Developmental Disabilities Research Reviews, 10, 303–317. doi:10.1002/mrdd.20044.
Rapin, I. (1997). Autism. The New England Journal of Medicine, 337, 97–104. doi:10.1056/NEJM199707103370206.
Rojas, D. C., Smith, J. A., Benkers, T. L., Camou, S. L., Reite, M. L., & Rogers, S. J. (2004). Hippocampus and amygdala volumes in parents of children with autistic disorder. The American Journal of Psychiatry, 161, 2038–2044. doi:10.1176/appi.ajp. 161.11.2038.
Ruser, T. F., Arin, D., Dowd, M., Putnam, S., Winklosky, B., Rosen-Sheidley, B., et al. (2008). Communicative competence in parents of children with autism and parents of children with specific language impairment. Journal of Autism and Developmental Disorders, 37, 1323–1336. doi:10.1007/s10803-006-0274-z.
Rutter, M. (1999). Autism: Two-way interplay between research and clinical work. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 40, 169–188. doi:10.1017/S0021963098003461.
Schain, R. J., & Freedman, D. X. (1961). Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. The Journal of Pediatrics, 58, 315–320. doi:10.1016/S0022-3476(61)80261-8.
Skuse, D. H. (2001). Endophenotypes and child psychiatry. The British Journal of Psychiatry, 178, 395–396. doi:10.1192/bjp. 178.5.395.
Sparrow, S., Bala, D., & Cichetti, D. (1984). Vineland Adaptive Behaviour Scales (Survey Form). Circle Pines, MN: American Guidance Service.
Stone, W. L., McMahon, C. R., Yoder, P. J., & Walden, T. A. (2008). Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatrics and Adolescent Medicine, 161, 384–390. doi:10.1001/archpedi.161.4.384.
Szatmari, P., Maziade, M., Zwaigenbaum, L., Merette, C., Roy, M. A., Joober, R., et al. (2007). Informative phenotypes for genetic studies of psychiatric disorders. American Journal of Medical Genetics. Part B, 144, 581–588.
Veenstra-Vander-Weele, J., Kim, S., Lord, C., Courchesne, R., Akshoomoff, N., Levinthal, B. L., et al. (2002). Transmission disequillibrium studies of the serotonin 5ht2a receptor gene (htr2a) in autism. American Journal of Medical Genetics, 114, 277–283. doi:10.1002/ajmg.10192.
Whitaker-Azmitia, P. (2001). Serotonin and brain development: Role in human developmental diseases. Brain Research Bulletin, 56, 479–485. doi:10.1016/S0361-9230(01)00615-3.
Yatham, L. N., Liddle, P. F., Dennie, J., Shiah, I. S., Adam, M. J., Lane, C. J., et al. (1999). Decrease in brain serotonin 2 receptor binding in patients with major depression following desipramine treatment: A positron emission tomography study with F18-labeled setoperone. Archives of General Psychiatry, 56, 705–711. doi:10.1001/archpsyc.56.8.705.
Young, S. N., Leyton, M., & Benkelfat, C. (1999). PET studies of serotonin synthesis in the human brain. Advances in Experimental Medicine and Biology, 467, 11–18.
Acknowledgements
This project has been supported by the National Alliance for Autism Research (NAAR; grant #98-179) and the Hamilton Health Sciences Research Foundation. Drs. Goldberg, Szatmari, and Zwaigenbaum have been supported by fellowship awards from the Ontario Mental Health Research Foundation (OMHF). Dr. Anderson acknowledges support of the Korczak Foundation for Autism Research and the Gettner Research Fund. Dr. Raman Chirakal and Dr. J. J. Chen are gratefully acknowledged for the production of setoperone. Dr. Karen Gulenchyn, Dr. Troy Farncombe, Margo Thompson and the PET technologists are thanked for their assistance in acquiring the data. We would like to thank Heather Allin, Eric Duku and Ann Thompson for their advice and efforts in assisting with this study. A special thanks to the parents for participating and for their ongoing support of this and related studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldberg, J., Anderson, G.M., Zwaigenbaum, L. et al. Cortical Serotonin Type-2 Receptor Density in Parents of Children with Autism Spectrum Disorders. J Autism Dev Disord 39, 97–104 (2009). https://doi.org/10.1007/s10803-008-0604-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-008-0604-4