Abstract
Prader-Willi syndrome (PWS) is a neurodevelopmental disorder characterized by hyperphagia and food preoccupations. Although dysfunction of the hypothalamus likely has a critical role in hyperphagia, it is only one of several regions involved in the regulation of eating. The purpose of this research was to examine food-related neural circuitry using functional magnetic resonance imaging in individuals with PWS and matched controls. Individuals with PWS showed increased activation in neural circuitry known to mediate hunger and motivation (hypothalamus, OFC) in response to high- versus low-calorie foods and in comparison to controls. This suggests neural circuitry for PWS is abnormally activated during hunger, particularly for high-calorie foods, and may mediate abnormally strong hunger states, therefore playing a significant role in PWS-induced hyperphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prader-Willi syndrome (PWS) is a neurodevelopmental disorder associated with missing material from the paternal chromosome 15 (q11–q13), derived either by deletion on the paternal copy or by receipt of two copies of the maternal chromosome (Ledbetter et al. 1980; Nicholls et al. 1989). Food preoccupations and hyperphagia are hallmark characteristics of PWS, with an average age of onset of these symptoms at about two years (Dimitropoulos et al. 2001). Associated symptoms often include persistent hunger, food obsessions and compulsions, and food foraging. These symptoms, as well as the presence of hypotonia, result in an increased likelihood of obesity. The cause of the hyperphagia associated with PWS is unknown but thought to be associated, at least partly, with an abnormality in hypothalamic circuitry (Holland 1998; Holland et al. 2003; Thompson et al. 1999). The ventromedial hypothalamus (“satiety” center) and the lateral hypothalamus (“hunger” center) are known to be critically involved in the regulation of energy intake and feeding in the general population (Grossman 1975; Oomura 1973). In addition to hyperphagia, dysfunction of the hypothalamus is postulated to underlie several other essential functions that are often also abnormal in PWS, including temperature, metabolism, sleep-wake cycles, and reproductive function (Holm et al. 1993). Neuroendocrine studies indicate abnormal functioning of the oxytocin-producing neurons of the hypothalamus (Martin et al. 1998; Swaab et al. 1995) and abnormally elevated plasma ghrelin levels among people with PWS (Haqq et al. 2003; Del Parigi et al. 2002).
Although the hypothalamus is likely to have a critical role in hyperphagia, it is only one of several brain centers involved in the regulation of eating behavior. Experimental evidence from neurotracing studies and the animal literature indicates that the hypothalamus receives food-related neural inputs from the amygdala, hippocampal complex, insula, and several polymodal prefrontal association areas (DeFalco et al. 2001; Doyle et al. 1993). Several imaging studies have mapped out neural systems involved in hunger and satiation. For example, Tataranni et al. (1999) found significant activation during an induced hunger state in the hypothalamus, parahippocampal gyrus, thalamus, insular cortex, anterior cingulate, and orbitofrontal cortex (OFC). After satiation with a liquid meal, increased activation was found in several frontal regions including the ventromedial and dorsolateral prefrontal cortex (PFC). LaBar et al. (2001) found that pictures of food presented during functional magnetic resonance imaging (fMRI) elicited greater activation in the amygdala, parahippocampal gyrus, and right fusiform gyrus (FG) when participants were hungry as compared to when they were satiated. Work has not yet characterized the distributed network for food-related processing, with unique roles ascribed to different nodes.
Hunger and satiety neural mechanisms have been examined in a preliminary fashion in PWS by three recent studies. In a study of three adults with PWS, Shapira et al. (2005) suggested that there is significant delay in activation of brain regions associated with satiety (e.g. the hypothalamus). Hinton et al. (2006b) studied 13 individuals with PWS following a 1,200 kcal meal and argued for a deficit in the satiety system as fundamental to the hyperphagia in PWS. However, their results included activation areas mediating hunger, including the hypothalamus, amygdala, basal ganglia, thalamus, and anterior cingulate. Although the authors predicted the same pattern of activation in neural areas associated with hunger as nonobese individuals, since direct comparisons to controls were not made, we cannot be certain that activation of these regions were not abnormally increased among participants with PWS during hunger. In a direct comparison of PWS to healthy-weight controls, Holsen et al. (2006) found different patterns of neural activity in both pre-meal and post-meal states indicating possible abnormality at both the hunger and satiation phase of eating, including the amygdala, OFC, medial PFC, and insula. Together, these investigations provide evidence of satiety-related neural response abnormality in PWS, but abnormalities of the hunger system are, at this point, still quite unclear, especially as this might intersect with the preference for high-calorie foods that is common in PWS.
In an fMRI study using pictures of food, Killgore et al. (2003) examined differences in high- and low-calorie foods during hunger in healthy-weight participants. High-calorie foods activated some regions more than low-calorie foods, including the hypothalamus, thalamus, and the medial and dorsolateral PFC, the latter of which are thought to be involved in inhibition of emotion and regulation of appetitive drives. Despite early perceptions that individuals with PWS eat indiscriminately, collective research with this population has shown that when quantity is equated, individuals with PWS show clear preference for foods based on their taste and macronutrient content, typically favoring tasty high-fat or carbohydrate foods to foods with lower caloric values (Taylor and Caldwell 1985; Fieldstone et al. 1997; Glover et al. 1996; Joseph et al. 2002). Recently, Hinton et al. (2006a) examined the role of food preference and incentive motivation on food-related neural substrates. Findings indicated that although participants with PWS expressed preferences for certain types of food, highly valued foods were not associated with greater activation in neural reward circuitry than less desired foods. However, the authors noted the consistency of participant response on food preferences was correlated with IQ with those with higher IQ making more consistent responses. Thus, this may have influenced analysis of brain activation patterns since conditions (low incentive value versus high incentive value) were tailored to each individual’s self-reported food preferences.
The purpose of the present investigation was to examine neural mechanisms of hunger using pictures of food stimuli with different reward value in comparison to nonfood stimuli. We hypothesized increased activation to food stimuli in PWS compared to matched controls in several regions, including the hypothalamus, lateral OFC, thalamus, amygdala, right lateral FG, and the mid-insular region. Furthermore, based on past research indicating a preference for high calorie foods among the PWS population, we hypothesized increased activation to high- versus low-calorie foods in these regions. We used fMRI to examine neural circuitry in participants with PWS and comparison participants matched on IQ and body mass index (BMI) while they made perceptual judgments on pairs of objects and high- or low-calorie foods during a hunger state. This is the first neuroimaging study of PWS to include a BMI and cognitively matched comparison sample; such matching is an important control for these potential confounds, as cognitive delay or obesity could cause unique activation patterns independent of PWS. BMI-matching, which has been used repeatedly in PWS-related research, is important for controlling for effects due to general obesity. As a first step to understanding PWS-related hyperphagia, the current study focused on hunger circuitry. Although recent studies have shown preliminary evidence of a satiation deficit, the hunger circuitry has not been systematically examined and cannot be excluded from contributing to the hyperphagia. We hypothesize that abnormally strong hunger states by themselves can contribute to hyperphagia, and as a first step we test for differences in hunger state neural activation between those with PWS and matched controls.
Materials and Methods
Participants
Nine individuals with PWS (three male, two left-handed; age: 8–38 years old) underwent fMRI scanning.Footnote 1 These individuals were recruited from national and local PWS parent conferences and newsletters, from queries to our research web page, and through our genetic disabilities specialty clinic at the Child Study Center. Parents provided genetic records confirming PWS diagnosis by chromosomal and DNA molecular analysis for participation. Seven participants had chromosome 15 deletions, one had uniparental disomy, and one had a translocation. Given the predominance of deletion cases, subtype analyses were not performed. Comparison participants included 10 individuals with developmental delay and similar BMI (MR/DD; four male, three left-handed; age: 18–29 years old) recruited from local vocational programs and from queries to our research web page. This MR/DD sample consisted of eight individuals with nonspecific cognitive delay and two with a known genetic disorder (Williams syndrome and 48XXYY karyotype) with no history of eating disorder. Because this is among the first fMRI investigations of individuals with PWS, we selected individuals for the comparison group based on similar level of BMI as well as cognitive delay to control for the potential influence of cognitive impairment on the ability to complete an fMRI scan and task accuracy.Footnote 2
Demographic characteristics did not differ significantly between groups (see Table 1).
Three of the PWS participants and eight comparison participants were taking psychiatric medication at the time of the study.Footnote 3 Seven individuals with PWS were taking injections of growth hormone. Effect of medication status on our regions of interest (ROI) was evaluated by performing direct comparisons between those on medication and participants without medication. No differences were observed in our ROIs with respect to psychiatric medication or growth hormone status. Although mean age did not differ between groups, because the PWS group included children in the sample, a separate group contrast dividing the PWS group into two groups by median age (16.03 yrs) was conducted. None of the ROI activations were significantly different (at p < .05) for older versus younger participants in the PWS group. We also performed cursory analyses on BMI and no differences were found; however, it is important to note these analyses were underpowered given the sample size. All participants fasted for a minimum of 3 h before the scanning session. Mean fasting time differed between groups (PWS M = 3.4 h, SD = .96; MR/DD M = 5.6, SD = 2.1; t(17) = −2.96, p < .01). Because of the difference in fasting time, food intake prior to the scanning session was recorded for caloric intake estimation. Mean calories consumed did not differ between groups (PWS M = 424.3 kcal, SD = 234 kcal; MR/DD M = 399 kcal, SD = 276.7 kcal; t(17) = .206, p = .83).
Experimental Tasks
Changes in blood oxygen level-dependent (BOLD) contrast were measured in a block design while participants performed a perceptual discrimination task, indicating by a button press whether side-by-side full-color photographs of high-calorie food, low-calorie food, or nonfoods (furniture) were of the “same” or “different” object type (Fig. 1).
Low-calorie photographs included images depicting fresh vegetables and fruits. High-calorie photographs included images such as cookies, French fries, chocolate cake, and pizza. Average calorie estimate per food condition was approximately 50 and 200 cal (per serving size) for the low-calorie and high-calorie conditions respectively. The same/different task parameters were selected not only to ensure participants were attending to the stimuli but also to drive the perceptual systems engaged during food and nonfood discrimination during a hunger state. Each image consisted of only one type of food or object (e.g. no mixed vegetables) and were presented only once during the scan. Each of the two runs was composed of eight blocks (21 s each, with a 14 s rest between blocks) with six image pairs per block. Each run compared blocks of furniture, high-calorie, and low-calorie foods in a counterbalanced order. Stimulus duration was set at 2,250 ms and the interstimulus interval (ISI) at 1,250 ms.Footnote 4
Food Preference Assessment
To evaluate individual preferences of high- and low-calorie foods, participants completed a food preference test following the completion of the imaging protocol. This test required participants to rate photographic flash cards of 74 foods on a 7-point Likert scale from ‘dislike extremely’ to ‘like extremely.’ Mean preference ratings were calculated for high- and low-calorie foods separately for each participant. These ratings were used to confirm that participants did not prefer low-calorie to high-calorie foods; they were not used to tailor the experimental tasks as in previous experiments. If mean preference ratings were significantly higher for low-calorie foods in comparison to high-calorie foods for a given participant, that participant’s data would not be entered into the group analyses.
fMRI Data Acquisition
Imaging data was acquired on a Siemens 3.0T Trio scanner with a quadrature head coil equipped for echo-planar imaging at the Yale Magnetic Resonance Research Center. Functional images were acquired using a gradient-echo single-shot echo-planar sequence over 34 axial sequence slices aligned parallel to AC–PC plane with an inplane resolution of 4 × 4 × 4 mm with no gap (TR = 1,950, TE = 25 ms, flip angle = 60). BOLD activation data was acquired during two runs (5:01 min each, 157 echo-planar images per run). Images were back-projected onto a translucent screen placed near the end of the MRI scanner and were viewed through a periscopic prism system on the head coil. T1-weighted structural images (TR = 300, TE = 2.47 ms, FOV = 256, matrix = 256 × 256, flip angle = 60, NEX = 2), 4 mm thick, in the same location as the echo-planar data for in-plane registration and high resolution 3D structural images (3D MPRAGE, contiguous, sagittal acquisition, 176 images with 1 mm isotropic voxels, TR = 2,530, TE = 3.52 ms, TI = 1100, FOV = 256, matrix = 256 × 256, flip angle = 7, NEX = 1) were collected during the same session.
Data Analysis
Image processing, analyses, and tests of statistical significance were performed using Brainvoyager 2000 commercial software (Brain Innovation). Motion-corrected images were spatially smoothed using a Gaussian filter with a full-width half-maximum value of 7 mm. Data for each individual was aligned with high-resolution 2D and 3D anatomical images for display and localization. For group analysis, individual data sets underwent piecewise linear transformation into a proportional three-dimensional grid defined by Talairach and Tournoux (1988) and coregistered with the high-resolution 3D data set that was resampled to give 1 mm3 voxels. In the averaged group data sets, functional activation was examined using a random effects general linear model (GLM) analysis contrasting the three experimental conditions: high-calorie foods, low-calorie foods, and furniture. Within-group analyses of response to high-calorie and low-calorie versus nonfood images were conducted separately prior to direct comparison between-group interaction analyses of group (PWS versus MR/DD) by condition contrast (high-calorie versus nonfood; low-calorie versus nonfood; high-calorie versus low-calorie).
Analyses were divided into two types, each with different alpha thresholds: (1) those for which we had a small number of a priori ROIs, and (2) “whole brain” examination for other areas of activation. Based on hypotheses regarding hyperphagia in PWS and the literature regarding food-related neural circuitry in healthy-weight adults, our a priori ROIs included the (1) hypothalamus, (2) lateral OFC, (3) thalamus, (4) amygdala, (5) mid-insular cortex, and (6) right lateral FG (the center of which has been termed the fusiform face area). ROIs were anatomically defined by consensus among the authors and by consulting Duvernoy’s (1991) atlas of human brain anatomy. For these a priori ROIs we used an alpha threshold of p < 0.05, uncorrected, with a minimum cluster size of six contiguous voxels. To protect against Type 1 error, a more conservative alpha level of p < .001, uncorrected, and a cluster size of six contiguous voxels was set for all other brain regions during the “whole brain” examination.
Results
Behavioral Performance
There were no significant differences between groups on task accuracy (see Table 2).
Similarly, within-group comparisons of accuracy during the three task comparisons (high versus low, high versus nonfood, low versus nonfood) did not differ significantly for the PWS group. Within-group accuracy differences for the MR/DD comparison group were found for high versus nonfood and for low versus nonfood, with the nonfood condition having higher accuracy in both comparisons (t (8) = −3.5, p < .01; t (8) = −2.89, p < .05 respectively).
Food preference ratings were divided into high- and low-calorie foods and preference did not differ within or between groups (Table 2). Although our results do not indicate significant preference to high-calorie foods compared to low-calorie foods for participants with PWS, earlier research has shown that individuals with PWS have a preference for sweet foods relative to other foods (Taylor and Caldwell 1985). Our participants with PWS rated high- and low-calorie foods equally high with mean ratings falling under the ‘like very much’ category. These ratings were done while fasting which may explain the high preference for most foods of both calorie types.
fMRI Results
High-calorie Food Versus Nonfood Control
For the PWS group, at the ROI significance level (p < .05), there was bilateral activation of the hypothalamus, thalamus, and mid-insula region, and right activation in the FG and amygdala to high-calorie foods in contrast to the control condition (Table 3). The MR/DD comparison group showed activity in the FG, and regions of the inferior frontal, superior frontal and cingulate gyri (Table 3).
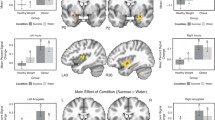
Direct comparison of the groups revealed interesting findings with respect to our hypothalamic ROI. For high-calorie foods, the PWS group showed significantly stronger bilateral hypothalamus and right amygdala activation than the comparison group. The comparison group showed significantly more activity for high-calorie foods in the right putamen at the whole-brain threshold (Table 3; Fig. 2).
PWS versus MR/DD group comparison: High-calorie food versus nonfood. Greater PWS activation to high-calorie foods is shown in shades of red, while greater MR/DD activation to high-calorie foods is shown in shades of blue (threshold at p < .05, uncorrected and cluster-filtered at a minimum of 40 contiguous voxels for ease of visualization). Significantly activated ROIs include: the hypothalamus (8, −2, −2, peak t = 3.40, p = .003; −9, 2, −2; peak t = 3.60, p = .002) and amygdala (20, −1, −15, peak t = 2.15, p = .04). Right and left are reversed by radiologic convention. Note: Although group comparisons of contrasts can be difficult to interpret without seeing the individual group data (see Table 3 for individual group data), individual maps were inspected to confirm all red areas indicate PWS greater activation to food versus furniture and all blue areas indicate MR/DD group greater activation to food versus furniture. However, for the MR/DD group, only the putamen is significant at the whole brain examination level (p < .001)
Low-calorie Food Versus Nonfood Control
Next, regions responding to low-calorie foods were examined by contrasting low-calorie food and nonfood control stimuli (see Table 4).
There were striking differences between groups. The PWS group showed no cerebral activity to low-calorie foods in contrast to furniture at the p < .001 threshold. At the ROI threshold of p < .05, only the right FG was significantly active compared to nonfood. In contrast, the comparison group showed significant activity in the thalamus, cingulate, FG, and medial and superior frontal gyri. Direct group contrast also indicated that the comparison group showed significantly more activity than the PWS group at the whole-brain threshold in the thalamus, cingulate gyrus, and putamen.
High-calorie Versus Low-calorie Food
To identify regions specific to caloric value, high-calorie foods were contrasted to low-calorie foods. Several of our ROIs, including the hypothalamus, amygdala, and OFC, were found to be more activated to high-calorie than low-calorie foods in the PWS group (Table 5).
In contrast, the comparison group did not show any activation to high-calorie foods in contrast to low-calorie foods in our ROIs or at the whole-brain threshold. Surprisingly, the comparison group showed greater activity to low-calorie foods at the whole-brain threshold in the following areas: bilateral FG, left inferior frontal gyrus, putamen, medial frontal gyrus, and right insula.
Direct comparison of the two groups on the high- versus low-calorie food contrast showed that participants with PWS had more activity to high-calorie food compared to low-calorie food as compared to the MR/DD controls. Significant differences were found in several a priori ROIs, including the hypothalamus, insula, and OFC (Fig. 3). Although the direct comparison map shows activation spreading from the hypothalamus into the ventral striatal areas, peak activation originates in the hypothalamus and no separate peaks are indicated in either the putamen or lentiform nuclei (see Fig. 3).
PWS versus MR/DD group comparison: High-calorie food versus low-calorie food. Greater PWS activation is shown in shades of red (there were no areas of activation greater for the MR/DD group; threshold at p < .001, uncorrected and cluster-filtered at a minimum of 40 contiguous voxels for ease of visualization). Significantly activated ROIs include: the hypothalamus (6, −3, −3, peak t = 5.05, p = .0001; −6, −4, −3; peak t = 4.35, p = .0004), insula (38, −1, 19, peak t = 3.85, p = .001; −36, 0, 19; peak t = 3.70, p = .002), and the orbitofrontal cortex (21, 38, −8; peak t = 3.95, p = .001). Right and left are reversed by radiologic convention
Thus, it is not clear whether these reward system areas are uniquely involved or not. The PWS group also showed greater activation than MR/DD controls at the whole-brain threshold in the superior temporal gyrus and cerebellum (Table 5).
Discussion
Individuals with PWS showed hyperactivation to high-calorie foods in neural circuitry known to mediate food-related behavior and showed significantly more activation of these circuits compared to the MR/DD controls. Although these findings should be replicated by future studies with larger sample sizes, the hyperactivation of the hypothalamus, insula, amygdala, and OFC in PWS to high-calorie foods suggests that these areas may be involved in the hyperphagia and eating disorder that is central to PWS. Furthermore, this suggests that caloric value plays an important role in differentiating food-related neural circuitry in individuals with PWS. The implications of the activation in these regions and other ROI are described below.
Hypothalamus, Thalamus, Mid-insular Cortex, and Amygdala
Although the limitations in spatial resolution with fMRI make localization of activity to specific hypothalamic nuclei quite difficult, our data seemed to be most concentrated in the lateral hypothalamus, the region that animal research has consistently shown to play a regulatory role in feeding behaviors (Anand and Brobeck 1951; Rolls 1999). Damage to lateral hypothalamic cells in rats has produced a lasting decrease in food intake indicating the role of the lateral hypothalamus in regulating both food-seeking and food-intake behavior (Clark et al. 1991). In nonhuman primates, a population of neurons has been described in the lateral hypothalamus which responds not only to the taste and smell of food but also to the sight of food (Mora et al. 1976; Burton et al. 1976). Moreover, these responses are modulated by hunger state. Using reward devaluation paradigms, whereby foods are eaten to the point of satiety and are no longer found rewarding even when the animal remains hungry, Rolls et al. (1989) showed reduced activity in the lateral hypothalamus to the devalued stimulus but sustained activity to novel foods. Thus, these neurons fail to respond when a food is no longer rewarding, implicating the involvement of the lateral hypothalamus in the reward circuitry of food intake. Given the role of the hypothalamus in directing food-seeking behaviors (Coons et al. 1965), our data suggests that overactivity of the hypothalamus to food cues may have a role in driving unrestrained eating among those with PWS. Additionally, abnormalities of this circuit in PWS may underlie the difficulties of achieving the equivalent of reward devaluation. This would suggest that the hypothalamic circuitry in PWS may be related to a heightened response to high-calorie foods and may perhaps sustain that response past satiety to drive unrestrained eating in this population.
Two areas known to be involved in the sensation of taste, the thalamus and mid-insular cortex (Reilly 1998; Pritchard et al. 1999), also showed greater activation to high-calorie foods than to low-calorie foods for participants with PWS. Thalamic activation was lateralized to the left hemisphere with peak activation consistent with a localization in the ventral posteromedial nucleus, known also as the gustatory thalamus (Carpenter 1985). The gustatory thalamus plays an important role in taste-guided behavior with connections to the lateral hypothalamus, insular cortex, amygdala, and OFC (Reilly 1998; Ongur and Price 2000). Lesions to the gustatory thalamus have long been associated with impairment in taste perception, although more recent research has questioned the extent of this impairment and suggests the importance of this region for more preparatory behaviors such as food foraging (Reilly 1998). This latter argument would be more consistent with the food-seeking behaviors found in PWS.
Neurons in the mid-insular region, within the primary taste cortex, are narrowly tuned for taste and are particularly sensitive to sweet stimuli (Yaxley et al. 1988). Our results indicate the peaks of activated mid-insular regions are slightly more superior than previous findings, however, are still likely to include part of the primary taste cortex. This region is not modulated by hunger state but functions to evaluate taste of a stimulus (Rolls 1999; Tataranni et al. 1999; Araujo and Rolls 2004). Recent imaging research has indicated that mere pictures of food shown during fMRI can stimulate regions of the cortex involved in taste even when taste was not elicited by a physical gustatory stimulus (Simmons et al. 2005). Similarly, our research showed bilateral activation of the mid-insular cortex in response to viewing high-calorie food contrasted with both nonfood and low-calorie food among individuals with PWS. The increased reward value of high-calorie foods for individuals with PWS may be related to the activation in this region.
Individuals with PWS also showed greater activation in the right amygdala, compared to the matched sample, to high-calorie foods in contrast to nonfoods. The amygdala’s role in arousal and the evaluation of emotionally valenced stimuli is consistent with these findings (Zald 2003; Cahill et al. 1995; Schneider et al. 1997). The extent to which the food stimuli are highly arousing and motivating may be dependent on both hunger and pleasantness of the foods. For individuals with PWS, the pictures of high-calorie foods may not only be more desirable, but participants are also likely to be hungrier than comparison participants due to the satiety deficit. The participant’s internal state may dramatically influence the motivational value of the food pictures and we would expect internal states to differ between PWS and non-PWS participants with food being more highly motivational to participants with PWS.
Right Lateral Fusiform Gyrus
For both participant groups, the lateral posterior region of the FG showed greater activation during calorie subtype versus nonfood contrasts. This area of the FG has been shown to respond preferentially to visual objects for which the viewer has a special interest or motivation, such as faces or objects for which they are perceptually expert (Gauthier et al. 2000; Grelotti et al. 2005; Kanwisher et al. 1997). Since individuals must fast prior to our fMRI scan, the food pictures serve as visual hunger cues that more fully engage this visuospatial-attention circuit. The difference in activation between food and nonfood stimuli may reflect enhanced perceptual processes within the fusiform, modulated by “limbic up” motivational inputs (Morris et al. 1998; Vuilleumier 2000). Given the heightened interest in food, participants with PWS may be more motivated to look at food than nonfood, and activity in the FG may represent this increased attention to food. This may also be true for our fasting comparison sample. Our findings are consistent with that of LaBar et al. (2001) who reported increased right FG activation during fasting compared to satiated states.
Lateral Orbitofrontal Cortex
In our results, high-calorie foods contrasted to low-calorie foods activated a small region of the lateral OFC among individuals with PWS in comparison to the matched sample (see Fig. 3). Primate research has demonstrated OFC connections to the hypothalamus and the insula’s primary taste cortex and has led to the identification of the secondary taste cortex in the OFC region (Baylis et al. 1995; Rolls 1999). Unlike the primary taste cortex, the secondary taste cortex has been shown to be modulated by hunger, with decreased neuron response after satiation of a particular taste. These neurons are subject to food-specific satiety in that satiation to one particular food does not reduce firing in response to another food type. In addition, the OFC includes neurons that respond to smell and sight of food and these too are modulated by satiety. Together, these OFC neurons code the reward value of food in that the neuronal responses to sight, smell, and taste of food decrease as satiety increases (Critchley and Rolls 1996). Activation of this particular region of the lateral OFC has been shown in recent imaging research with healthy adults to be hunger-dependent and positively correlated with an individual’s subjective ratings of pleasantness of a liquid food stimulus, further illuminating the importance of reward value to this area (Kringelbach et al. 2003). Our findings suggest that the activation of the lateral OFC in response to high-calorie foods is related to the heightened reward value of these foods in PWS as compared to matched controls.
Conclusions
Prior research using animal models has indicated that the regions identified in this study as overactive in PWS are interconnected, each having a specific role in processing information about feeding and the reward value of food. Although the hypothalamus is thought to be centrally involved in the PWS phenotype, these other regions may play equally important roles in the pathophysiology of the observed hyperphagia in PWS. This is one of the first reported fMRI investigations of the eating disorder in individuals with PWS using visual presentation of foods during a hunger state. Although these findings should be replicated by future studies with larger sample sizes, these results suggest that the food neural circuitry for PWS differs from that of non-PWS groups.
Our findings regarding brain regions that activate during food-related tasks are fairly consistent with what we know about food neural circuitry from previous neuroimaging research. There are some differences, however, that may be accounted for by variations in methodology across studies. For example, differences in the type of control stimuli used (e.g., furniture, trees and flowers, tools, etc.) may account for variations in activations. In addition, the statistical methodology employed has not been consistent across studies. We used a random effects design, which takes into account both within- and between-subjects variance, in order to be able to generalize to the broader PWS and normal control populations, while other studies of food neural circuitry in healthy individuals and PWS used fixed effect analyses (i.e., Killgore et al. 2003; LaBar et al. 2001; Holsen et al. 2006). In addition, because using random effects modeling makes the analyses inherently robust, we feel the use of uncorrected P-values is acceptable and is consistent with previous work in this area (Kilgore et al. 2003; Simmons et al. 2005).
The current study has several limitations. First, the relatively small sample limits the generalization of our findings. The prevalence of this population (1 in 15,000) makes recruitment difficult, particularly when recruiting for a study which requires specialized inclusion criteria for participation as an fMRI study does. Results from this study should primarily serve to support future studies with larger samples. However, our sample size is not smaller than other recent neuroimaging studies in PWS (Holsen et al. 2006; Shapira et al. 2005) and we were able to detect significant effects using random effects modeling. A larger sample size would have provided greater statistical power, thereby possibly revealing additional ROI and would also be beneficial for examining sub-characteristics of the population (i.e., genetic subtype, medication effects, BMI, etc.). For example, although medication status did not result in significant differences between groups for our ROI analyses, a larger sample is still warranted to adequately test the effect of both psychiatric medication and growth hormone on food-related neural circuitry. We note, however, that our findings with regard to growth hormone are consistent with structural findings that indicate no differences in the anterior pituitary gland, the site of growth hormone synthesis, among individuals with PWS who are growth hormone deficient (Miller et al. 1996). Additionally, we acknowledge that the age range among the PWS sample is wide. Due to the small sample size, we were unable to systematically examine the effect of age on our ROIs but note that future studies should investigate potential developmental effects. However, all participant data was examined by a clinical neuroradiologist and by a neuoranatomy expert and no gross pathology that would affect coregistration was detected. In addition, we confirmed that data from our youngest participants examined prior to group averaging did not qualitatively differ from the rest of the sample, thus we believe including them in the anatomical averaging and group comparison analysis did not result in incorrect anatomical landmarking. Previous neuroimaging studies in PWS have included participants as young as 9 years of age in group comparison analyses (Holsen et al. 2006). Another limitation concerned the difference in fasting time between groups. This difference was due to dietary constraints for some participants with PWS requiring them to have a very light afternoon snack prior to the scan. It seems unlikely that less fasting would lead to greater activation, and if anything, we would expect more hunger in PWS to lead to more substantial group differences.
In summary, despite the aforementioned limitations, the present study identifies several neural systems likely involved in the neurobiological mechanisms of hyperphagia in PWS. Based on these preliminary findings, in addition to recent evidence of a satiety response deficit, the hyperphagia in PWS may also involve abnormal motivation to eat. It will be important for future research to continue to test the effects of abnormal hunger and satiation on these brain structures to further develop the model of hyperphagia in PWS. A better understanding of the neurobiological mechanisms of this eating disorder should allow for future development of more effective treatment options for individuals with PWS and provide insight into the mechanisms of food-related obesity in the general population.
Notes
This research was approved by the Human Investigations Committee of the Yale University School of Medicine. In order to prepare our participants for the MRI procedure, we conducted a mock scanning session using a replica of the actual MRI scanner, equipped with audio equipment to simulate the MRI noise level.
Prior to scanning this sample, a pilot study of six healthy-weight adults was conducted to solidify the study design and replicate previous findings with healthy-weight participants. No attempt was made to match the pilot sample to the PWS sample. Pilot data indicated food-related activation in areas consistent with previous research in normal-weight individuals, including the insula, thalamus, FG, and OFC (Dimitropoulos and Schultz 2004).
In PWS: (1) paroxetine, (2) paroxetine, risperidone, (3) fluoxetine. In MR/DD: (1) escitalopram, topiramate, (2) methylphenidate hydrochloride, (3) risperidone, (4) atomoxetine, zonisamide, sertraline, (5) methylphenidate hydrochloride , (6) divalproex sodium, olanzapine, (7) divalproex sodium, risperidone, (8) bupropion.
Our first three PWS participants received these stimuli presented over three runs instead of two (Stimulus duration = 3,000 ms, ISI = 1,500 ms) but short response times and high accuracy on the task led us to shorten the protocol to two runs. A direct comparison between data from those who received three runs versus two runs did not reveal any differences in activation level in our primary ROIs (p > .20).
References
Anand, B. K., & Brobeck, J. R. (1951). Localization of a feeding center in he hypothalamus of the rat. Proceedings of the Society for Experimental Biology and Medicine, 77, 323–324.
Araujo, I. E., & Rolls, E. T. (2004). Representation in the human brain of food texture and oral fat. The Journal of Neuroscience, 24(12), 3086–3093.
Baylis, L. L., Rolls, E. T., & Baylis, G. C. (1995). Afferent connections of the caudolateral orbitofrontal cortex taste area of the primate. Neuroscience, 64, 801–812.
Burton, M. J., Rolls, E. T., & Mora, F. (1976). Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Experimental Neurology, 51, 668–677.
Cahill, L., Babinsky, R., Markowitsch, H. J., & McCaugh, J. L. (1995). The amygdala and emotional memory. Nature, 377, 295–296.
Carpenter, M. B. (1985). Core text of neuroanatomy (3rd ed.). Baltimore: Williams & Wilkins.
Clark, J. M., Clark, A. J. M., Bartle, A., & Winn, P. (1991). The regulation of feeding and drinking in rats with lesions of the lateral hypothalamus made by N-methyl-D-aspartate. Neuroscience, 4, 631–640.
Coons, E. E., Levak, M., & Miller, N. E. (1965). Lateral hypothalamus: Learning of food-seeking response motivated by electrical stimulation. Science, 150, 1320–1321.
Critchley, H. D., & Rolls, E. T. (1996). Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. Journal of Neurophysiology, 75, 1673–1686.
DeFalco, J., Tomishima, M., Liu, H., Zhao, C., Cai, X., Marth, J. D., et al. (2001). Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science, 291, 2608–2613.
Del Parigi, A., Tschop, M., Heiman, M., Salbe, A., Vozarova, B., Sell, S., et al. (2002). High circulating ghrelin: A potential cause for hyperphagia and obesity in PWS. Journal of Clinical Endocrinology & Metabolism, 87(12), 5461–5464.
Dimitropoulos, A., Feurer, I., Butler, M., & Thompson, T. (2001). Emergence of compulsive behavior and tantrums in children with Prader-Willi syndrome. American Journal on Mental Retardation, 106(1), 39–51.
Dimitropoulos, A., & Schultz, R. (2004, March). Hyperphagia in Prader-Willi syndrome: Using fMRI to explore brain mechanisms in response to food stimuli. Paper presented at the 37th Annual Gatlinburg Conference on Research and Theory in Mental Retardation and Developmental Disabilities, San Diego, California.
Doyle, P., Rohner-Jeanrenaud, F., & Jeanrenaud, B. (1993). Local cerebral glucose utilization in brains of lean and genetically obese (fa/fa) rats. American Journal of Physiology, 264(1), E29–E36.
Duvernoy, H. M. (1991). The human brain: Structure, three-dimensional sectional anatomy and MRI. New York: Springer-Verlag.
Fieldstone, A., Zipf, W. B., Schwartz, H. C., & Berntson, G. G. (1997). Food preferences in Prader-Willi syndrome, normal weight and obese controls. International Journal of Obesity, 21, 1046–1052.
Gauthier, I., Skudlarski, P., Gore, J. C., & Anderson, A. W. (2000). Expertise for cars and birds recruits areas involved in face recognition. Nature Neuroscience, 3(2), 191–197.
Glover, D., Maltzman, I., & Williams, C. (1996). Food preferences among individuals with and without Prader-Willi syndrome. American Journal on Mental Retardation, 101(2), 195–205.
Grelotti, D. J., Klin, A. J., Gauthier, I., Skudlarski, P., Cohen, D. J., Gore, J. C., et al. (2005). fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia, 43(3), 373–385.
Grossman, S. P. (1975). Role of the hypothalamus in the regulation of food and water intake. Psychological Review, 82(3), 200–224.
Haqq, A., Farooqi, I., O’Rahilly, S., Stadler, D., Rosenfeld, R., Pratt, K., et al. (2003). Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in PWS. Journal of Clinical Endocrinology and Metabolism, 88(1), 174–178.
Hinton E. C., Holland A. J., Gellatly M. S., Soni S., & Owen A. M. (2006a). An investigation into food preferences and the neural basis of food-related incentive motivation in Prader-Willi syndrome. Journal of Intellectual Disability Research, 50(Pt 9), 633–642.
Hinton E. C., Holland A. J., Gellatly M. S., Soni S., Patterson M., Ghatei M. A., & Owen A. M. (2006b). Neural representations of hunger and satiety in Prader-Willi syndrome. International Journal of Obesity, 30(2), 313–321.
Holland, A. J. (1998). Understanding the eating disorder affecting people with Prader-Willi syndrome. Journal of Applied Research in Intellectual Disabilities, 11(3), 192–206.
Holland, A., Whittington, J., & Hinton, E. (2003). The paradox of Prader-Willi syndrome: a genetic model of starvation. The Lancet, 362, 989–991.
Holm, V. A., Cassidy, S. B., Butler, M. G., Hanchett, J. M., Greenswag, L. R., Whitman, B. Y., et al. (1993). Prader-Willi syndrome: Consensus Diagnostic Criteria. Pediatrics, 91, 398–402.
Holsen L. M., Zarcone J. R., Brooks W. M., Butler M. G., Thompson T. I., Ahluwalia J. S., Nollen N. L., & Savage C. R. (2006). Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity, 14(6), 1028–1037.
Joseph, B., Egli, M., Koppekin, A., & Thompson, T. (2002). Food choice in people with Prader-Willi syndrome: Quantity and relative preference. American Journal on Mental Retardation, 107(2), 128–135.
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience, 17(11), 4302–4311.
Killgore, W. D. S., Young, A. D., Femia, L. A., Bogorodzki, P., Rogowska, J., & Yurgelun-Todd, D. A. (2003). Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage, 1381–1394.
Kringelbach, M. L., Doherty, J. O., Rolls, E. T., & Andrews, C. (2003). Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex, 13, 1064–1071.
LaBar, K. S., Gitelman, D. R., Parrish, T. B., Kim, Y., Nobre, A. C., Mesulam, M. (2001). Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience, 115(2), 493–500.
Ledbetter, D. H., Riccardi, V. M., Youngbloom, S. A., Strobel, R. J., Keenan, B. S., Crawford, J. D., & Louro, J. M. (1980). Deletion (15q) as a cause of the Prader-Willi syndrome (PWS). American Journal of Human Genetics, 32, 77A.
Martin, A., State, M., Anderson, G. M., Kaye, W. M., Hanchett, J. M., McConahay, C. W., et al. (1998). Cerebrospinal fluid levels of oxytocin in Prader-Willi syndrome: A preliminary report. Biological Psychiatry, 44, 1349–1352.
Miller, L., Angula, M., Price, D., & Taneja, S. (1996). MR of the pituitary in patients with Prader-Willi syndrome: size determination and imaging findings. Pediatric Radiology, 26, 43–47.
Mora, F., Rolls, E. T., & Burton, M. J. (1976). Modulation during learning of the responses of neurons in the hypothalamus to the sight of food. Experimental Neurology, 53, 508–519.
Morris, J. S., Friston, K. J., Buchel, C., Frith, C. D., Young, A. W., Calder, A. J., Dolan, R.J. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121, 47–57.
Nicholls, R. D., Knoll, J. H. M., Butler, M. G., Karam, S., & Lalande, M. (1989). Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader-Willi syndrome. Nature, 342, 281–285.
Ongur, D., & Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex, 10, 206–219.
Oomura, Y. (1973). Central mechanism of feeding. Advances in Biophysics, 5, 65–142.
Pritchard, T. C., Macaluso, D. A., & Eslinger, P. J. (1999). Taste perception in patients with insular cortex lesions. Behavior Neuroscience, 113(4), 663–71.
Reilly, S. (1998). The role of the gustatory thalamus in taste-guided behavior. Neuroscience and Biobehavioral Reviews, 22(6), 883–901.
Rolls, E. T. (1999). The brain and emotion. New York: Oxford University Press.
Rolls, E. T., Sienkiewicz, Z. J., & Yaxley, S. (1989). Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience, 1, 53–60.
Schneider, F., Grodd, W., Weiss, U., Klose, U., Mayer, K. R., Nagele, T., et al. (1997). Functional MRI reveals left amygdala activation during emotion. Psychiatry Research: Neuroimaging, 76, 75–82.
Shapira, N. A., Lessig, M. C., He, A. G., James, G. A., Driscoll, D. J., Liu, Y. (2005). Satiety dysfunction in Prader-Willi syndrome demonstrated by fMRI. Journal of Neurology, Neurosurgery, and Psychiatry, 76, 260–262.
Simmons, W. K., Martin, A., & Barsalou, L. W. (2005). Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex, 15(10), 1602–1608.
Swaab, D. F., Purba, J. S., & Hofman, M. A. (1995). Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: A study of five cases. Journal of Clinical Endocrinology and Metabolism, 80, 573–579.
Talairach, J., & Tournoux, P. (1988). Co-planar steriotaxic atlas of the human brain. New York: Thieme.
Tataranni, P. A., Gautier, J., Chen, K., Uecker, A., Bandy, D., Salbe, A. D., et al. (1999). Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences, 96, 4569–4574.
Taylor, R. L., & Caldwell, M. L. (1985). Type and strength of food preference of individuals with Prader-Willi syndrome. Journal of Mental Deficiency Research, 29, 109–112.
Thompson, T., Butler, M. G., MacLean, Jr., W. E., Joseph, B., & Delaney, D. (1999). Cognition, behavior, neurochemistry, and genetics in Prader-Willi syndrome. In H. Tager-Flusberg (Ed.), Neurodevelopmental disorders (pp. 155–178). Cambridge: The MIT Press.
Vuilleumier, P. (2000). Faces call for attention: Evidence from patients with visual extinction. Neuropsychologia, 38, 693–700.
Yaxley, S., Rolls, E. T., & Sienkiewicz. (1988). The responsiveness of neurons in the insular gustatory cortex of the macaque monkey is independent of hunger. Physiology & Behavior, 42, 223–229.
Zald, D. H. (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41, 88–123.
Acknowledgments
This work was supported by grant PO1 HD 03008 from the National Institute of Child Health and Human Development and T32 MH 18268 from the National Institute of Mental Health. We thank the individuals and their families for their participation in this study. We would also like to thank Carley Piatt, Elinora Hunyadi, Jennifer Hetzke, Cheryl Klaiman, and the technicians of the Yale MR Imaging Center for their assistance with this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimitropoulos, A., Schultz, R.T. Food-related Neural Circuitry in Prader-Willi Syndrome: Response to High- Versus Low-calorie Foods. J Autism Dev Disord 38, 1642–1653 (2008). https://doi.org/10.1007/s10803-008-0546-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-008-0546-x