Abstract
It has been unclear whether an associations of child ADHD with socio-economic disadvantage (SES) could be accounted for by (a) parental ADHD explaining both low SES and child ADHD, and/or (b) the joint overlap of ODD or CD with low SES and ADHD. Study 1 used a community-recruited case-control sample with detailed evaluation of SES indicators, child ADHD, child externalizing, and parent ADHD symptoms (n = 931 children, 521 ADHD, 577 boys, 354 girls) in a path modeling analysis with latent variables. Study 2 evaluated ADHD and externalizing behavior in a regression model using a poverty index for SES, in 70,927 children (48.2% female) aged 5–17 years from the US 2011–2012 National Survey of Children’s Health (NSCH). In Study 1, lower SES was related to the ADHD latent variable, β = −.18, p < .001; 95%CI [−.25,-.12]. This effect held when parent ADHD and child ODD and CD were in the model, β = −.11, p < .01, 95% CI [−.09,-.03], equivalent to OR = 1.50, 95% CI[1.12–2.04]). In Study 2, these results replicated. Adjusting only for age and sex, children from families who were below 200% of the federal poverty line were more likely to have moderate or severe ADHD than no ADHD, versus children above that line, OR = 2.13, 95% CI[1.79,2.54], p < .001. The effect held after adjusting for disruptive/externalizing problems, OR = 1.61, p < .01, 95%CI [1.32,1.96]. The effect size for comparable models was similar across both studies, lending higher confidence to the results. It is concluded that the SES association with child ADHD is not explained by artifact and requires a mechanistic explanation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The association between socioeconomic disadvantage (SES) and psychological and mental disorders has been established for decades (Dohrenwend and Dohrenwend 1969; Faris and Dunham 1939/1960; Hollingshead and Redlich 1958; McLaughlin et al. 2012; Reiss 2013; Silver et al. 2002). Its association with conduct disorder (CD) and oppositional defiant disorder (ODD) is likewise uncontroversial (Loeber et al. 1995; Merikangas et al. 2010; Miech et al. 1999). On the other hand, while over three dozen studies have also suggested a correlation of variously-defined measures of SES with ADHD, and a recent systematic review and meta-analysis confirmed a small but reliable association (Russell et al. 2016), the reality of this association remains in question.
Overlap of both SES and ADHD with Comorbid Externalizing Behavior as an Artifact
As underscored by Russell et al. (2016), alternative explanations for the SES association with ADHD remain poorly studied. They reported that 23 studies of SES and ADHD have examined covariates; but we note that only six made an attempt to adjust for the conjoint association of low SES with both child ADHD and child externalizing behavior problems or ODD and CD (Bauermeister et al. 2007; Bøe et al. 2012; Counts et al. 2005; Ford et al. 2004; Larsson et al. 2014). Of those, 3 found that the SES-ADHD correlation was not independent of child externalizing problems (Counts et al. 2005; Ford et al. 2004; Szatmari et al. 1989). Citing this point, recent reviewers conclude that a primary or specific association of low SES and ADHD is unclear (Barkley 2014). This is important because if ADHD itself is partially due to low SES, then an array of potential mediating and moderating mechanisms are opened for early prevention ideas for ADHD. But if the association of SES with ADHD is accounted for statistically by the joint overlap of SES with ADHD and comorbid child externalizing problems, no new insights about etiology or possible intervention would have emerged, even though some broad risk factor (like impulsivity) might be shared by ADHD and externalizing, and influenced by low SES. ADHD emerges earlier in development than conduct disorder or delinquency, and earlier than or concurrently with oppositional defiant disorder, so in our path diagrams we do not expect externalizing to have a direct effect on ADHD. Instead, we control for the joint overlap of ADHD and ODD and CD with SES as explained below.
Parent ADHD Association with Both Low SES and Child ADHD as an Explanation
A second reason for uncertainty is that parent ADHD could statistically or mechanistically explain both social disadvantage (due to downward drift) and the child’s ADHD. This potential genotype-environment correlation (Russell et al. 2015) is plausible because in addition to ADHD’s heritability, adults with ADHD tend to earn and save less money and have lower educational and occupational achievement than those without ADHD (Barkley and Fischer 2010; Barkley et al. 2010; Loe and Feldman 2007; Mannuzza et al. 1997; Miech et al. 1999). If this explanation held, then a correlation of low SES with ADHD could be dismissed as relatively unimportant to understanding the specific etiology or phenomenology of ADHD. Two studies (Biederman et al. 2002; Counts et al. 2005) conducted a direct evaluation of parent ADHD; when it was controlled, the SES-child-ADHD association was statistically unreliable in both studies.
In contrast, Larsson et al. (2014) effectively controlled parent ADHD by adjusting for differences in SES indicators in sibling pairs, and supported a causal influence of lower SES on child ADHD. They secondarily checked the result by removing children who had been diagnosed with ODD or CD; without giving details they stated that effects survived. However, this study’s generalizability outside of Sweden is unclear and, like all large population surveys, it is not clear what proportion of ADHD cases were captured or how accurate were the diagnoses.
SES as Mode of Transmission?
The same covariance model that asks whether parent ADHD explains the statistical association of SES and child ADHD, can also ask the reverse: whether low SES statistically mediates the transmission of parent ADHD to child ADHD. If it does, avenues for intervention again are opened up and theories of mechanism could be expanded to account for this. For simplicity, we present only one integrated (correlational) model for the relation of parent ADHD and SES. However, for completeness, we note secondarily the regression model results in which parent ADHD effects operate statistically “through” SES and vice versa. Note that it is also possible that low SES could accentuate parent ADHD (e.g., poor health, stress, and other contributors), so the association of parent ADHD and their SES may be bidirectional.
Methodological Considerations
Before proceeding, two methodological issues bear mention. First, SES is an amalgam of multifaceted constructs including occupational status, income, wealth/poverty, and educational attainment. In Study 1 we attempt to improve the ADHD literature, in which many prior studies of ADHD looked only at one of these features, by creating a latent SES variable that incorporates all of them. In Study 2, we follow other large scale national population studies by using an accepted poverty indicator.
Second, as noted, population surveys have the advantage of generalizability, but often suffer limited measurement depth or validity (e.g., assessing SES by income alone, or ADHD by one or two global questions) due to the costs associated with large samples. Clinically recruited samples tend to be small (Russell et al. 2016) and are vulnerable to referral bias. Community-identified case-finding studies avoid these problems but remain vulnerable to volunteer bias and thus limited generalizability. To address all this, we conducted two studies using complementary sampling approaches, enabling us to comment on generalizability of our conclusions about the role of externalizing comorbidity, while Study 1 provides a strong test of a parent ADHD effect.
Summary
In all, resolving the question of whether there is a specific association of ADHD and SES is important to conceptualization of ADHD. The present report aims to determine whether a putative association of ADHD with low SES is reliable, or whether it can be explained by two obvious yet unresolved confounds: (a) an artifact in which the unexamined variance shared between SES, ODD/CD, and ADHD accounts for the apparent association of child ADHD with low SES, or (b) the association of parent ADHD with both SES (either causally or bidirectionally) and with child ADHD (e.g., a genotype-environment correlation). In Study 1, we examined these questions in a community sample with very well-characterized ADHD and SES. In Study 2, we evaluated the generalizability of the Study 1 externalizing finding in a representative national sample.
Study 1: Community-Based Case-Control Study
Study 1 Methods
Participant Recruitment and Enrollment
Participants were 931 individual children ages 7–14 years (mean [M] = 9.3 years, standard deviation [SD] = 1.6 years, 62% male) and their biological parents recruited between 2009 and 2015. The 931 included 154 sibling pairs, with four families having three children in the study. Non-independence was handled statistically (below). Families were recruited via community outreach using commercial mailing lists and public advertisements to minimize referral bias. A multi-gate case-finding process was used to ascertain eligible ADHD and typically developing comparison children. All procedures were approved by the local Institutional Review Board and complied with applicable guidelines for protection of human participants.
Participant Selection and Child ADHD and ODD/CD Evaluation
After an initial telephone screening to identify rule outs (listed below), an evaluation visit was scheduled. A semi-structured clinical interview (Kiddie Schedule for Affective Disorders and Schizophrenia, (K-SADS-E)(Puig-Antich and Ryan 1996) was completed with a parent by a trained master’s degree level clinical interviewer. Each interviewer double coded 20 tapes with a criterion interviewer to ensure process fidelity and inter-interviewer reliability (all disorders k > .80 in this report). Video recordings of their interviews were viewed by supervising clinicians on a recurring basis and feedback provided as needed as a fidelity check. At least one parent and one teacher completed the ADHD Rating Scale, Version IV (ADHD-RS-IV) (DuPaul et al. 1998), the Conners ADHD Rating Scale-3rd Edition (CRS-III) (Conners 2008), and the Strengths and Difficulties Questionnaire (SDQ)(Goodman 2001) about the child. All have satisfactory internal and test-retest reliability and validity as documented in the cited manuals and had satisfactory internal reliability in the current sample. Children completed self-report symptom ratings of mood, and an IQ and achievement screen using a reliable and valid short form of the Wechsler Intelligence Scales for Children-4th Edition (WISC-IV) (Wechsler 2003) and the Wechsler Individual Achievement Test-3rd Edition (WIAT) (Wechsler 2009). Interviewers and psychometricians wrote behavioral observations.
Exclusion Criteria
Children were excluded if taking any psychiatric medication other than stimulants. At the time of participation, 34.5% of the ADHD group had a prescription (14% amphetamine salts, 20% methylphenidate or related preparations). However, a handful were enrolled inadvertently with other prescriptions (clonidine .4%, guanfacine .4%). Other exclusions were: history of non-febrile seizure, neurological impairments, a prior diagnosis of mental retardation or autism spectrum disorder, head injury with loss of consciousness, sensorimotor handicap, or other major medical conditions, as reported by the parent. This was done to minimize diagnostic errors involving ADHD. After the diagnostic evaluation, youth were ruled out if they had substance addiction, bipolar disorder, history of psychosis, medical or neurological condition discovered at the clinical screen, or estimated IQ <75, again to reduce diagnostic errors. Control children were also excluded for ADHD, learning disability, or conduct disorder but their psychiatric status was otherwise free to vary, so as to approximate typical population and avoid an artificially healthy comparison group.
Categorical Child Diagnosis
For categorical diagnosis, the preceding information was presented to a “best estimate” clinical team comprising a board-certified child psychiatrist and a licensed child clinical psychologist who independently coded ADHD and other diagnoses, blind to parent ADHD or family SES-related measures. Consistent with DSM-IV ADHD criteria, the clinicians required that another disorder did not better account for symptoms, that there was evidence of impairment, and evidence of cross-situational symptoms as well as duration and age of onset. Their agreement rates for ADHD, ODD, or CD were acceptable (all k > .80) and disagreements were resolved by consensus. A subset of 75 children with subthreshold ADHD (5 symptoms) were included in the study but omitted when checking effects of ADHD vs non-ADHD diagnosis.
Dimensional Analysis of Child ADHD Latent Variable
For the primary latent variable analysis of ADHD as a dimensional measure, teacher ratings were relied upon. This enabled an ADHD measure that was independent of parent report of their own ADHD or SES, thus avoiding source-variance confounding. The teacher scores were the ADHD-RS-IV total score, Conners-III inattentive (cognitive problems) and hyperactive scales, and SDQ hyperactivity total score.
Dimensional Child Externalizing Measure
Child ODD and CD symptoms were counted from parent semi-structured diagnostic interview (K-SADS-E) and treated as manifest variables.
Parent ADHD
Parent ADHD status was evaluated with the Conners Adult ADHD rating scale (CAARS) ADHD index (Conners et al. 1999), and the Barkley Adult ADHD rating Scale (BAARS) (Barkley 2011) current and recalled childhood symptom scales. Each has adequate reliability, validity, and national norms as documented in the cited papers. Spouse ratings of current symptoms were obtained when possible and used when self-ratings were not available. These measures were used as indicators to create dimensional latent variables for mother and father ADHD.
Secondary Analysis: Familial ADHD and Liability for Psychopathology
To check results using an alternative method, we adopted the familial loading score developed by Sham and colleagues for studies of schizophrenia (Andreasen et al. 1978; Van Os et al. 1997; Verdoux et al. 1996) in order to sum familial psychiatric history into a total dimensional liability score, while taking into account family size. In our adaptation, one parent completed a family psychiatric history grid in which they reported how many first- and second-degree relatives had probable or definite case of ADHD, conduct problems, antisocial behavior problems, schizophrenia, major depressive disorder, and bipolar disorder. These were converted into a weighted loading score in which first degree relatives “proportion affected” (to normalize on family size) was weighted at .5 and 2nd degree relatives by .25; these were summed. A total familial loading score was computed for (a) ADHD and for (b) all psychiatric conditions combined. These scores have had adequate reliability in past studies (Verdoux et al. 1996) but because its validity is based only on prediction of proband status, it was used as a secondary, not a primary method.
Social-Economic Disadvantage (SES)
SES was operationalized with measures of parental education, family household income, and parental occupational status as follows. (a) For parental education, we used the highest parent education as self-reported by parents. Parent education reported with 1 = Grade School, 2 = Some High School, 3 = High School Equivalent, 4 = Regular High school degree, 5 = Some College but no degree, 6 = Associates degree, 7 = Bachelor’s degree, 8 = Masters or Law degree, 9 = Doctorate, PhD, or MD. (b) Total household income was reported by the primary caregiver in bins to maximize response rate, with 1 = < $25,000, 2 = <$35,000, 3 = <$50,000, 4 = <$75,000, 5 = <$100,000, 6 = <$130,000, 7 = <$150,000, 8 = more than $150,000 (16.6% of families chose <$35,000 or less, suggesting they were at or below the poverty line). These were converted to dollars for descriptive purposes but analyzed as an ordinal 1–7 scale. (c) Occupational status score. Parental occupation was obtained by self-report during the parent interview. Then, occupation was scored by the Nam-Powers-Boyd system which provides a final occupation score of 0–100 (Nam and Boyd 2004). To establish inter-rater reliability, occupational scoring was confirmed by two independent raters who each rated a subset of the sample (n = 50) with acceptable agreement (Intra-class correlation >.90).Footnote 1 The highest parent occupation score was used as the analysis variable.
Data Reduction
Three latent variables were created. A latent SES variable was created using income, highest parental education, and highest parental occupational score as indicators. A child ADHD latent variable dimensional score was created using teacher ratings (to avoid source-variance confound between dependent and independent variables) listed earlier. A latent ADHD variable was created separately for mothers and for fathers, comprising all available data from among self-report ratings on CAARS, BAARS current, BAARS child, and spouse ratings.
Data Analysis
Models were developed using established path modeling logic (Kenny 1979). In path diagram logic, models are designed to be consistent with causal theory (even though, in this case, observations are cross-sectional). A hypothesized causal path gets a one-directional arrow (and computes a regression coefficient). An unexamined association that exists between two constructs gets a curved dual-headed arrow (and computes a correlation).Footnote 2 We developed the model based on the conceptual logic of how the variables relate as described in the introduction.
The model described in the introduction was then tested using a structural equation model (SEMs; (Shumacker and Lomax 1996) using Mplus 7.4 (Muthen and Muthen 1998–2012). Model fit was examined using multiple fit indices: the comparative fit index (CFI)(Bentler 1990), the Tucker-Lewis index (TLI) (Tucker and Lewis 1973), and the root mean squared error of approximation (RMSEA) (Browne et al. 1993). CFI and TLI values above .90 and RMSEA values below .06 indicate adequate model fit (Hu and Bentler 1999). Full information maximum likelihood (FIML) was used to handle missing data (Arbuckle 1996).
SEM models for the ADHD dimensional latent variable used the robust maximum likelihood estimator; to compare effect sizes to the literature on odds ratios, we converted beta weights to odds ratios using published formulas (Borenstein et al. 2009). When ADHD was treated as a category, the model used the robust weighted least squares estimator to produce fit statistics and robust maximum likelihood (with essentially the same results) to enable us to calculate odds ratios for comparison to other studies.Footnote 3 Both estimators can identify and handle non-normally distributed data. Non-independent observations (i.e., siblings) were handled using the Mplus cluster command. Statistical mediation, used in secondary data checks below, was tested using the model indirect command.
The primary model simultaneously modeled SES, mother and father ADHD, child ODD and CD, child ADHD, and age and sex. This was repeated with ADHD as a categorical diagnosis. (In preliminary tests, non-linear (quadratic and cubic) effects were trivial, so only linear effects are reported). In secondary analysis, we computed alternative models and placed all of these in the supplemental materials (available online in Fig. S-3, and summarized in Table S3 online) including separate path models for alternative claims about the causal relation among parent ADHD and SES, for alternative ways of controlling child ODD and CD, and other secondary checks noted later. In particular, we computed effect sizes for a model without parent ADHD for direct comparison of effect size found in Study 1 with that found in Study 2. Robustness analysis was conducted on alternative definitions of child and parent ADHD, including child diagnostic group analyses, family history defined as the familial history loading score, average of parent symptom scores, and highest of parent symptoms scores.
Study 1 Results
Preliminary Data Review
Demographic and descriptive statistics are presented in Table 1. Preliminary measurement models for parent ADHD, child ADHD, and SES fit well and are provided Fig. S-1 and S-2 (available online).
Primary Hypothesis Test (Omnibus model)
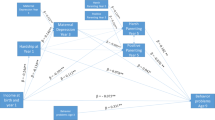
Figure 1 displays the primary model and its results. This model had satisfactory fit, χ 2 (24, N = 931) = 87.29, p < .001, CFI = .99, TLI = .98, RMSEA = .05. This model adjusts for the association of low SES with parent ADHD and the association of SES with child ODD and CD (i.e., it removes the overlap of SES, ODD or CD, and ADHD, which is accomplished by the singled-headed arrows from SES to child ODD and CD, and accounts for residual covariance of ODD and CD with child ADHD). It revealed an enduring small but reliable non-zero association of low SES with child ADHD in the latent variable model, β = −.11, SE = .04, p < .01; 95%CI[−.19,-.03]. The equivalent odds ratio is OR = 1.50, 95% CI[1.12–2.04]. The figure also shows that SES was related to ODD and CD symptoms independently of the SES effect on child ADHD. The complete list of all parameters (including residuals, not shown in figure to ease readability) is available in Table S-1 (online supplement).
Study 1: Omnibus Latent Variable Model Relating SES, Parent ADHD Symptomatology, Child ODD and CD, and Teacher-rated Child ADHD Symptomatology. Note: χ 2 (137, N = 931) = 276.56, p = .00, CFI = .98, TLI = .97, RMSEA = .03. *p < .05, **p < .01. Correlations among the focal variables were estimated as despicted but parameters are not displayed to ease readability. Those correlations among the focal variables (all p < .001) were: SES and Maternal ADHD = −.30; SES and Paternal ADHD = −.34; Maternal ADHD and Paternal ADHD = .23; Child ADHD and ODD = .26. The correlations among the residuals of the endogenous variables (all p < .001) were: Child ADHD and CD = .13; ODD and CD = .44. With parent ADHD removed from the model, the main effect of SES on Child ADHD symptoms after controlling for child ODD and CD symptoms (and age and sex) was β = −.18, p < .001 (95% CI = −.25, −.11). When child ODD and CD were removed from the model, the SES-ADHD association controlling for parent ADHD was β = −.09, p = .03 (95% CI = −.17, −.01). See text and supplemental materials for details
Secondary Analysis Question One (Residual Effect After Control of Externalizing)
Here, the elements of Fig. 1 relating to parent ADHD were removed to create a model that could be comparable to Study 2. The association of low SES with child ADHD in this model was moderate in size, with adjusted SES effect, β = −.18, p < .001; 95% CI [−.25,-.11]. This model again fit well, χ 2 (34, N = 931) = 97.03, p = .00, CFI = .99, TLI = .98, RMSEA = .05. This adjusted effect size corresponds to an odds ratio = 1.97; 95% CI[1.52, 2.56] or a Cohen’s d = .37, a small-to-medium effect by convention. Further details are in Fig. S-4 (online).
Secondary Analysis Question Two (Residual Effect Controlling Only Parent ADHD)
Here, the ODD/CD elements in Fig. 1 were omitted to enable further scrutiny of simple effects for dissemination. Fit was excellent, χ 2 (111, N = 931) = 211.42, p < .001, CFI = .98, TLI = .98, RMSEA = .03, and the adjusted SES to child-ADHD effect was, as expected, still reliable (adjusted SES main effect on child ADHD: β = −.09, p = .03; 95% CI[−.17,-.01]. This adjusted effect size corresponds to OR = 1.42 (95% CI[1.22,1.82] or a Cohen’s d = .19. This is a small effect by Cohen’s convention. The total indirect path (obtained using the delta method, i.e., by multiplying the parent ADHD to SES path estimate by the SES to child ADHD path estimate, while also estimating the covariance between these two estimates) was reliable both for paternal ADHD, βindirect effect of paternal symptoms = −.054, p < .001, 95% CI[−.08,-.02] and maternal ADHD, βindirect effect of maternal symptoms = .-.047, p < .001 95% CI[−07,-.02]. Further details are in Fig. S-5 (online).
Secondary Question Three (Does Social Disadvantage Statistically Mediate ADHD Intergenerational Transmission)
The third analysis is essentially a reconfiguration of the same model as just described, but with a different conceptual meaning. It asks whether low SES accounts for (mediates) ADHD transmission from parent to child. Thus, child ADHD was regressed on SES and on maternal and paternal symptoms, and SES was regressed on maternal and paternal ADHD. The indirect (mediational) path was reliable for paternal symptoms, βindirect effect of paternal symptoms = .024, p = .04, 95% CI[.001,.05]; while the indirect effect of maternal symptoms was marginal, βindirect effect of maternal symptoms = .019, p = .055, 95% CI[=.00,.04]. This model fit the data well, χ 2 (110, N = 931) = 215.50, p = .00, CFI = .98, TLI = .98, RMSEA = .03. These results are consistent with the possibility that that low SES participates in inter-generational transmission of ADHD. This model is depicted in online Fig. S-6.
Robustness Tests
To evaluate whether results were attributable to our particular way of measuring family ADHD loading, we re-tested the parent model for both dimensional and categorical outcomes using several other ways of operationalizing familial risk loading: with the family loading score for (a) all psychiatric disorders and (b) only for ADHD, and for each of these using composite parental symptom scores (average of z scores) that (a) gave equal weight to current symptoms and childhood symptoms, and (b) gave equal weight to all symptom measures, regardless of reference period, all with no change in the story—in fact all effects sizes for SES were larger than in our primary model. These secondary data are provided in Table S-2, online. Thus, how we operationalized parent ADHD or family psychiatric liability did not account for the results.
With child ADHD as a categorical diagnosis (subthreshold excluded, n = 856, recall Table 1 for group sizes) the results of our omnibus model (as in Fig. 1) fell shy of significance: the SES-child diagnosis main effect was β = −.09, p = .13, 95% CI[−.21,.03]; OR = 1.39, 95% CI[.90,2.16]. This model fit the data well, χ 2 (91, N = 856) = 191.80 p = .00, CFI = .95, TLI = .93, RMSEA = .04. When parent ADHD symptoms were omitted, the results held (main effect of SES on child ADHD, β = −.28, p < .001; 95% CI[−.37,-.19]; OR =2.90, 95% CI = 1.99, 4.24, p < .00001). This model fit the data well, χ 2 (12, N = 856) =36.80, p = .03, CFI = .97, TLI = .93, RMSEA = .05. When parent ADHD was included but child ODD and CD were omitted, the main effect of SES on child ADHD was again marginal: β = −.12, p = .06; 95% CI = −.21, .00; OR =1.29, 95% CI[1.00,1.67]. This model fit the data well, χ 2 (71, N = 856) =117.45, p = .03, CFI = .96, TLI = .95, RMSEA = .03.
Study 1: Summary
Study 1 used a detailed, well supported set of measures of child ADHD, child externalizing, SES, and parent ADHD in local community sample. The results supported the conclusion that low SES is associated with ADHD and that this link is not explained by parent ADHD effects on both SES and child ADHD, by the artifact of ADHD’s overlap with both SES and ODD or CD, or the joint consideration of these effects. These results were robust in the model with dimensional measure of ADHD but fell short of significance if the lower-power categorical diagnosis of ADHD was relied upon as the outcome.
Study 2: Generalizability of ADHD-SES Effect in a National Sample
Study 2 Methods
Sample: NSCH Survey Description
This study was conducted using the 2011/2012 iteration of the National Survey of Children’s Health (NSCH) (USA National Survey of Children's Health 2011-2012: Child and adolescent health measurement initiative 2014). The NSCH is a representative, stratified, nationwide telephone survey that was conducted across the US in 2003/04, 2007/08, and 2011/12. Telephone numbers were called at random to identify households with one or more children under 18 years old. In each household, one child was randomly selected to be the subject of the parent interview. The National Center for Health Statistics of the Centers for Disease Control and Prevention conducts the survey and produces a public-use data set. Two previous studies used the 2003–2004 or 2007–2008 iterations of the NSCH survey to examine the association between poverty level and ADHD status (Kim et al. 2011; Waring and Lapane 2008). One prior study examined that association in the 2011–2012 NSCH survey (Visser et al. 2014). However, ADHD definition was not scrutinized, nor were externalizing problems covaried in those reports.
Analytic Sample
A total of 95,677 children of age 0–17 years were included in 2011/2012, but only ages 5–17 were examined here. While ADHD is identified in children under age 5, including them in this analysis was judged to be problematic for three reasons. First, diagnosis of ADHD in this age range has raised concerns due to the difficult overlap of ADHD symptoms with normal behavior in very young children (Connor 2002; Ford-Jones 2015). Second, only a third of children with ADHD are identified during that period (Visser et al. 2015), so we would have many false negatives in that non-ADHD group. Third, prior to 2011 no professional diagnostic guidelines existed for this age group for ADHD, and guidelines then promulgated applied only to ages 4 and 5 (Wolraich et al. 2011), raising further questions about the meaning and accuracy of the reports in the younger age ranges in the 2011/2012 survey. We therefore followed prior convention and limited analyses to age 5–17 (Lingineni et al. 2012; Waring and Lapane 2008), yielding unweighted N = 70,927 (48.17% female).
Measures: A. Definition of Socioeconomic Disadvantage Using Poverty Level
In study 2, we also followed prior convention by using poverty level as an index of SES disadvantage. Poverty level was determined based on Department of Health and Human Services Federal Poverty guidelines. The original variable had four categories (below 100% federal poverty level [FPL], 100%–199% FPL, 200%–299% FPL, 300%–399% FPL, 400% FPL or greater). To maximize statistical power, we used dichotomized poverty level (POVERTY; <200% vs. ≥ 200%). In 2012, 200% of the federal poverty guideline for a family of 4 was an annual income of <= $56,000.
Measures: B. ADHD
The survey provided three options for describing the child’s ADHD, which likely provide varying degrees of validity: (a) Ever ADHD: “For each condition, please tell me if a doctor or other health care provider ever told you that [the selected child] has the condition, even if [he/she] does not have the condition now.” Attention deficit disorder (ADD) or ADHD was listed as one condition. Here, with prevalence of nearly 12%, serious questions can be raised as to the validity of all of these as true ADHD cases. Therefore, we note, (b) Current ADHD: “Does [S.C.] currently have [condition]?”. Those who were ever told they had ADHD but no longer have it would include cases in which the clinician found an alternative explanation for the problem or the parent simply did not see serious enough problems to be persuaded by the health care provider’s opinion, as well as true “recovered” cases. A third check is also available: (c) Severity of ADHD: “Would you describe [his/her] [condition] as mild, moderate, or severe?” A “mild” case would seem more likely to fail to meet DSM-IV diagnostic criteria in a formal evaluation than a moderate or severe case.
To maximize validity, therefore, the analysis was conducted with a three-level ADHD variable (mutually exclusive categories): (a) “not ADHD” (88.4%, n = 62,458); (b) “Possible ADHD” (ADHD diagnosed in the past but not current; or currently has mild ADHD; 6.3%, n = 4469); or (c) “probable ADHD” (currently, moderate or severe ADHD; 5.2%, n = 3696). The prevalence of the probable category (~5%) is congruent with estimates from epidemiological studies that used structured clinical interviews or combined parent and teacher standardized ratings to estimate prevalence of ADHD (Polanczyk et al. 2007), and thus was expected to have the best validity for defining ADHD as defined in our Study 1.
Measures: C. Externalizing Comorbidity
We created a 3-level variable for externalizing: (a) “no externalizing” (never diagnosed), (b) “possible externalizing disorder” (ever diagnosed with a conduct/disruptive disorder but does not currently have behavior problems), (c) “probable externalizing disorder” (past and current conduct/disruptive disorder).
Data Analysis and Covariates
The association between ADHD and poverty level was modeled using multinomial logistic regression with the appropriate use of the weighting, ID, and state variables as required for the NSCH data set. Missing data was handled with full information maximum likelihood estimation, in Mplus, as in Study 1. Montecarlo integration was necessary to estimate the model given the multinomial outcome and presence of missing data. The resulting parameter estimates were based on the full sample of 70,927 except for the completely unadjusted model which was based on a sample of 70,862 (65 cases were missing on the poverty variable). We estimated an unadjusted model, an adjusted Model 1 for demographic variables (sex, age, race/ethnicity), and an adjusted Model 2 for demographic variables plus conduct/disruptive behavior disorder. We report odds ratios and 95% confidence intervals. Statistical significance was set at p < .05.
Study 2 Results
Table 2 provides the results of the multinomial logistic regression models for the base model and the adjusted models. It shows that in the model adjusted only for sex and age, when children were in families with income at <200% FPL, they were more likely to have “moderate/severe” or “probable” ADHD than no ADHD, OR = 2.13, 95% CI[1.79, 2.54], p < .001. Adjusting also for conduct/disruptive behavior disorder, the effect remained reliable, OR = 1.61, 95% CI [1.32, 1.96], p < .001. This corresponds to a Cohen’s d = .26. The effect of poverty level on “possible ADHD” was smaller and not independent of disruptive behavior problems.
Study 2: Summary
Study 2 replicated the Study 1 externalizing finding using a nationally representative data set. The result is consistent with the conclusion that the association of poverty status with ADHD exists independently of the overlap of SES with both ADHD and disruptive behavior problem diagnoses. It replicates that subset of the Study 1 finding and shows the result is generalizable.
General Discussion
The association of socioeconomic disadvantage with psychopathology and neurodevelopmental disorders has gained renewed importance in light of growing understanding of the broad impacts of social class on child development (American Psychological Association 2007), interest in the social determinants of health and mental health, insights into neural development and its dependence on early enrichment both socially and biologically (Bowles et al. 2009; Van Praag et al. 2000), and by fresh appreciation that environmentally-responsive epigenetic mechanisms mediate life stress effects on behavior (Champagne and Curley 2005; McEwen et al. 2012; Roth 2012; Thayer and Kuzawa 2011). Low SES (or its proxies such as parental education or income) is associated with life stress and neighborhood disadvantage as well as developmental health risks such as lower cognitive ability and educational outcomes (American Psychological Association 2007; Bradley and Corwyn 2002; Evans 2004).
What has been unclear is whether ADHD is somehow an exception to all of this. The present findings are important because they move a good distance toward resolving that prior ambiguity. Study 1 confirmed using state-of-the-art research evaluation methods that ADHD is associated with carefully defined lower SES, in a community recruited sample after adjusting for the overlap of SES and ADHD with comorbid ODD or CD. Study 2 provided replication and generalizability study in a national sample, despite different methods, ascertainment, and measures. The effect size replication was notable. In study 1, the effect size for the model adjusting for the overlap of SES, child ADHD, and ODD and CD (but ignoring parent ADHD) (β = −.18) is equivalent to OR = 1.97 (or Cohen’s d = .37). In study 2, the analogous model had OR = 1.61 (d = .26). The meta-analysis (Russell et al. 2016) suggested a population effect size of OR = 1.8–2.2 across a range of indices uncorrected for any other variables. The present results are broadly in line with that figure when considering our adjustment for confounds that Russel et al. did not adjust for.
This convergence in results and effect sizes with these additional statistical controls provides compelling evidence that in the United States, low SES is associated with ADHD specifically, not merely due to the overlap of SES with both ADHD and disruptive behavior disorders, or due to the overlap of SES with a shared liability factor underlying externalizing. This overall picture supports prior conclusions and underscores the value of further study of mediators and moderators relevant to ADHD itself and not just for associated externalizing problems. The statistical association of SES and ADHD reported here is small by Cohen’s conventions. However, it is comparable to other important risk factors for ADHD and other important effects on public health. Such a statistical effect size may be of substantial public health significance due to the large number of children who are affected by social disadvantage.
The second key issue we studied was whether parent ADHD could explain the SES-ADHD link (Russell et al. 2015). We could only test this in Study 1, but there we provided the best direct test to date of whether a putative low SES -ADHD association is explained by parent ADHD (a partial proxy for a genotype-environment correlation). The parent ADHD latent variables only partially explained the SES effect on child ADHD. The adjusted association of low SES and child ADHD was again small by Cohen’s convention. We can conclude that parent ADHD contributes to both child ADHD and lower SES, but that low SES has a small additional statistical association with child ADHD.
The parent ADHD result, when taken together with the sibling-based, causally-informative findings of Larsson et al. (2014) in their study in Sweden, is consistent with a causal link between low SES and ADHD. However, our cross-sectional study cannot directly inform causality. Because we could not assess parent ADHD in the national survey, we do not know the generalizability of that finding.
Despite the strength of the present findings, other limitations must be considered. Although we had multiple measures of parent ADHD, all were self-report or parental recalled childhood ADHD symptoms. A complete evaluation of parent ADHD would have included other informants. Perhaps for this reason as well, source-variance confound may explain why the secondary models using child ADHD as a categorical measure failed to detect an SES-ADHD effect. Further, potential variation across race, culture, or national context was not evaluated here (although statistical covarying of race/ethnicity did not change results).
What if this association reflects a causal influence, as implied by Larsson et al. (2014)? What might an influence of social context on ADHD mean in terms of an integrated formulation about ADHD risk and etiology? Here we venture a speculative comment to guide future work. Despite ADHD’s substantial heritability (Chang et al. 2013; Faraone and Mick 2010), effects of socio-economic context could be embedded in epigenetic (or gene by environment interaction) effects (Foulon et al. 2015), potentially even moderating heritability. To this last point, at least in some countries socioeconomic status appears to moderate the heritability of IQ (Hanscombe et al. 2012; Turkheimer et al. 2003) and of brain development (Hackman et al. 2010). This is untested for ADHD. It is possible that a negative recursive cycle occurs in which adults with ADHD experience some degree of socio-economic set back (Barkley et al. 2010) which then amplifies the expression of ADHD in their offspring.
Integrated models of genetic and social contexts are emerging in relation to health and illness (Thayer and Kuzawa 2011). Epigenetic mediation of SES-associated stressor effects on child ADHD is an interesting but as yet speculative possibility (Nigg 2012, 2016). Social disadvantage may entail a context in which physical and social stressors contribute to physical and mental health conditions, particularly for developing children (Santiago et al. 2011). To date, the study of mediators has emphasized breakdowns in parenting and other social mediators (Russell et al. 2015). However, a widened study of mediators is warranted. Several risk factors are correlated with lower SES, such as poor diet and higher exposure to pollutants, that are associated with ADHD or altered child attentional development (Liston et al. 2009; Liston et al. 2006; Nigg 2016).
Particularly promising could be further consideration of allostatic load (Blair et al. 2011; Ganzel et al. 2010; McEwen 2015; Rogosch et al. 2011). That hypothesis suggests that the cumulative effect of exposure to stressful events creates a context in which both physical and social stressors build on one another and contribute to further stress (McEwen 2007), and to neuroendocrine and cardiovascular dysregulation (Brosschot et al. 2005). According to this theory, the elasticity and resiliency of one’s biological systems are reduced and respond to challenges less robustly and recover less efficiently (McEwen and Gianaros 2010). In particular, this idea has been extensively developed in relation to gene by environment studies that consider sensitivity to experiences “for good or for ill” as one feature of genetic variation that accounts for differential response to environments (Pluess 2017; Pluess and Belsky 2013).
Conclusion
As the association of socio-economic context with ADHD becomes established, understanding of mechanisms becomes paramount (Froehlich et al. 2007; Russell et al. 2015). Socioeconomic disadvantage embeds a constellation of risk factors, many of which may influence ADHD. This is not to overlook the importance of patterns of resilience, as well as the historical, political, and cultural context embedded in the manifestation of poverty. Explanations and interventions ultimately will have to consider not only individual characteristics but structural landscapes that produce and sustain socioeconomic disadvantage (Mills 2015).
Notes
A potential confound in the Nam-Powers-Boyd system is that it scores an occupation score = “0” for a range of reasons. In our sample, the breakdown was as follows: Unemployed (n = 23), plus n = 54 others that might be debatable: active military (n = 1), retired (n = 7), disabled (n = 5), students (n = 22), homemaker (n = 19). In a robustness analysis, we recoded these n = 54 scores that as missing. We repeated this for all the models (with slight change in N as reported below). Results were essentially unchanged and conclusions remained the same. We therefore report the results with the code as recommended (Nam and Boyd 2004). The robustness results are available on request.
In a path model, a hypothesized bidirectional effect gets two straight arrows, one pointing in each direction. However, models containing bidirectional effects can only be estimated given strong assumptions and other variables (or instruments) that cause each of the two variables involved in the bidirectional effect; therefore, we did not consider them viable here.
To obtain fit statistics for an SEM with a dichotomous outcome variable requires the robust weighted least squares estimator. We ran all of our models using both the robust weighted least squares and robust maximum likelihood estimators and received nearly identical results. We present the results using the robust weighted least squares estimator so that the reader has sufficient information to assess model fit.
References
American Psychological Association. (2007). Task Force on Socioeconomic Status. Report of the APA Task Force on Socioeconomic Status. Retrieved from https://www.apa.org/pi/ses/resources/publications/task-force-2006.pdf
Andreasen, N. C., Endicott, J., Spitzer, R. L., & Winokur, G. (1978). The family history method using diagnostic criteria. Nursing Research, 27(2), 140.
Arbuckle, J. L. (1996). Full information estimation in the presence of incomplete data. In G. A. Marcoulides & R. E. Schumacker (Eds.), Advanced structural equation modeling (pp. 243–277). Mahwah: Erlbaum.
Barkley, R. A. (2011). Barkley Adult ADHD Rating Scale-IV (BAARS-IV). New York: Guilford Publications.
Barkley, R. A. (Ed.). (2014). Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th ed.). New York: Guilford Publications.
Barkley, R. A., & Fischer, M. (2010). The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry, 49(5), 503–513.
Barkley, R. A., Murphy, K. R., & Fischer, M. (2010). ADHD in adults: What the science says. New York: Guilford Publications.
Bauermeister, J. J., Shrout, P. E., Chávez, L., Rubio-Stipec, M., Ramírez, R., Padilla, L., et al. (2007). ADHD and gender: are risks and sequela of ADHD the same for boys and girls? Journal of Child Psychology and Psychiatry, 48(8), 831–839.
Bentler, P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246.
Biederman, J., Faraone, S. V., & Monuteaux, M. C. (2002). Differential effect of environmental adversity by gender: Rutter’s index of adversity in a group of boys and girls with and without ADHD. American Journal of Psychiatry, 159(9), 1556–1562.
Blair, C., Raver, C. C., Granger, D., Mills-Koonce, R., & Hibel, L. (2011). Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology, 23(03), 845–857.
Bøe, T., Øverland, S., Lundervold, A. J., & Hysing, M. (2012). Socioeconomic status and children’s mental health: results from the Bergen Child Study. Social Psychiatry and Psychiatric Epidemiology, 47(10), 1557–1566.
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to Meta-Analysis. Chichester: John Wiley & Sons, Ltd..
Bowles, S., Gintis, H., & Groves, M. O. (Eds.). (2009). Unequal chances: Family background and economic success. Princeton: Princeton University Press.
Bradley, R. H., & Corwyn, R. F. (2002). Socioeconomic status and child development. Annual Review of Psychology, 53(1), 371–399.
Brosschot, J. F., Pieper, S., & Thayer, J. F. (2005). Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology, 30(10), 1043–1049.
Browne, M. W., Cudeck, R., Bollen, K. A., & Long, J. S. (1993). Alternative ways of assessing model fit. Sage Focus Editions, 154, 136–136.
Champagne, F. A., & Curley, J. P. (2005). How social experiences influence the brain. Current Opinion in Neurobiology, 15(6), 704–709.
Chang, Z., Lichtenstein, P., Asherson, P. J., & Larsson, H. (2013). Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry, 70(3), 311–318. https://doi.org/10.1001/jamapsychiatry.2013.287.
Conners, C. K. (2008). Conners 3rd Edition Manual. North Tonawanda: Multi-Health Systems, Inc..
Conners, C. K., Erhardt, D., & Sparrow, E. (1999). Conners Adult ADHD Rating Scales (CAARS) Technical Manual. North Tonawanda: Multi-Health Systems, Inc..
Connor, D. F. (2002). Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. Journal of Developmental and Behavioral Pediatrics, 23(1 Suppl), S1–S9.
Counts, C. A., Nigg, J. T., Stawicki, J. A., Rappley, M. D., & Von Eye, A. (2005). Family adversity in DSM-IV ADHD combined and inattentive subtypes and associated disruptive behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry, 44(7), 690–698.
Dohrenwend, B. P., & Dohrenwend, B. S. (1969). Social status and psychological disorder: A causal inquiry. New York: John Wiley & Sons, Ltd..
DuPaul, G. J., Power, T. J., Anastopoulos, A. D., & Reid, R. (1998). ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. New York: Guilford Publications.
Evans, G. W. (2004). The environment of childhood poverty. American Psychologist, 59(2), 77–92. https://doi.org/10.1037/0003-066X.59.2.77.
Faraone, S. V., & Mick, E. (2010). Molecular genetics of attention deficit hyperactivity disorder. Psychiatric Clinics of North America, 33(1), 159–180.
Faris, R. E. L., & Dunham, H. W. (1939/1960). Mental disorders in urban areas: An ecological study of schizophrenia and other psychoses. New York: Hafner Publishing Company.
Ford, T., Goodman, R., & Meltzer, H. (2004). The relative importance of child, family, school and neighbourhood correlates of childhood psychiatric disorder. Social Psychiatry and Psychiatric Epidemiology, 39(6), 487–496.
Ford-Jones, P. C. (2015). Misdiagnosis of attention deficit hyperactivity disorder: 'Normal behaviour' and relative maturity. Paediatrics & Child Health, 20(4), 200–202.
Foulon, S., Pingault, J. B., Larroque, B., Melchior, M., Falissard, B., & Cote, S. M. (2015). Developmental predictors of inattention-hyperactivity from pregnancy to early childhood. PLoS One, 10(5), e0125996. https://doi.org/10.1371/journal.pone.0125996.
Froehlich, T. E., Lanphear, B. P., Epstein, J. N., Barbaresi, W. J., Katusic, S. K., & Kahn, R. S. (2007). Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Archives of Pediatrics and Adolescent Medicine, 161(9), 857–864.
Ganzel, B. L., Morris, P. A., & Wethington, E. (2010). Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological Review, 117(1), 134–174. https://doi.org/10.1037/a0017773.
Goodman, R. (2001). Psychometric properties of the Strengths and Difficulties Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 40(11), 1337–1345. https://doi.org/10.1097/00004583-200111000-00015.
Hackman, D. A., Farah, M. J., & Meaney, M. J. (2010). Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651–659.
Hanscombe, K. B., Trzaskowski, M., Haworth, C. M., Davis, O. S., Dale, P. S., & Plomin, R. (2012). Socioeconomic status (SES) and children's intelligence (IQ): in a UK-representative sample SES moderates the environmental, not genetic, effect on IQ. PLoS One, 7(2), e30320. https://doi.org/10.1371/journal.pone.0030320.
Hollingshead, A. B., & Redlich, F. C. (1958). Social class and mental illness: Community study. New York: John Wiley & Sons, Ltd..
Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55.
Kenny, D. A. (1979). Correlation and Causality. New York: John Wiley & Sons.
Kim, J., Mutyala, B., Agiovlasitis, S., & Fernhall, B. (2011). Health behaviors and obesity among US children with attention deficit hyperactivity disorder by gender and medication use. Preventive Medicine, 52(3–4), 218–222.
Larsson, H., Sariaslan, A., Långström, N., D'Onofrio, B. M., & Lichtenstein, P. (2014). Family income in early childhood and subsequent attention deficit/hyperactivity disorder: a quasi-experimental study. Journal of Child Psychology and Psychiatry, 55(5), 428–435.
Lingineni, R. K., Biswas, S., Ahmad, N., Jackson, B. E., Bae, S., & Singh, K. P. (2012). Factors associated with attention deficit/hyperactivity disorder among US children: results from a national survey. BMC Pediatrics, 12, 50. https://doi.org/10.1186/1471-2431-12-50.
Liston, C., Miller, M. M., Goldwater, D. S., Radley, J. J., Rocher, A. B., Hof, P. R., et al. (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience, 26(30), 7870–7874.
Liston, C., McEwen, B. S., & Casey, B. J. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences, 106(3), 912–917.
Loe, I. M., & Feldman, H. M. (2007). Academic and educational outcomes of children with ADHD. Journal of Pediatric Psychology, 32(6), 643–654.
Loeber, R., Green, S. M., Keenan, K., & Lahey, B. B. (1995). Which boys will fare worse? Early predictors of the onset of conduct disorder in a six-year longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry, 34(4), 499–509.
Mannuzza, S., Klein, R. G., Bessler, A., Malloy, P., & Hynes, M. E. (1997). Educational and occupational outcome of hyperactive boys grown up. Journal of the American Academy of Child and Adolescent Psychiatry, 36(9), 1222–1227.
McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews, 87(3), 873–904.
McEwen, B. S. (2015). Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism, Clinical and Experimental, 64(3 Suppl 1), S2–S10. https://doi.org/10.1016/j.metabol.2014.10.029.
McEwen, B. S., & Gianaros, P. J. (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186(1), 190–222.
McEwen, B. S., Eiland, L., Hunter, R. G., & Miller, M. M. (2012). Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology, 62(1), 3–12.
McLaughlin, K. A., Costello, E. J., Leblanc, W., Sampson, N. A., & Kessler, R. C. (2012). Socioeconomic status and adolescent mental disorders. American Journal of Public Health, 102(9), 1742–1750. https://doi.org/10.2105/ajph.2011.300477.
Merikangas, K. R., He, J.-P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., et al. (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989.
Miech, R. A., Caspi, A., Moffitt, T. E., Wright, B. R. E., & Silva, P. A. (1999). Low socioeconomic status and mental disorders: a longitudinal study of selection and causation during young adulthood 1. American Journal of Sociology, 104(4), 1096–1131.
Mills, C. (2015). The psychiatrization of poverty: Rethinking the mental health-poverty nexus. Social and Personality Psychology Compass, 9(5), 213–222.
Muthen, L. K., & Muthen, B. O. (1998-2012). Mplus User's Guide. Seventh Edition. Los Angeles, CA: Muthen and Muthen.
Nam, C. B., & Boyd, M. (2004). Occupational status in 2000; over a century of census-based measurement. Population Research and Policy Review, 23(4), 327–358.
Nigg, J. T. (2012). Future directions in ADHD etiology research. Journal of Clinical Child and Adolescent Psychology, 41(4), 524–533. https://doi.org/10.1080/15374416.2012.686870.
Nigg, J. T. (2016). Where do epigenetics and developmental origins take the field of developmental psychopathology? Journal of Abnormal Child Psychology, 44(3), 405–419. https://doi.org/10.1007/s10802-015-0121-9.
Pluess, M. (2017). Vantage sensitivity: Environmental sensitivity to positive experiences as a function of genetic differences. Journal of Personality, 85(1), 38–50.
Pluess, M., & Belsky, J. (2013). Vantage sensitivity: individual differences in response to positive experiences. Psychological Bulletin, 139(4), 901–916. https://doi.org/10.1037/a0030196.
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J., & Rohde, L. A. (2007). The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry, 164(6), 942–948.
Puig-Antich, J., & Ryan, N. (1996). Schedule for affective disorders and schizophrenia. Pittsburgh: Western Psychiatric Institute and Clinic.
Reiss, F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Social Science and Medicine, 90, 24–31.
Rogosch, F. A., Dackis, M. N., & Cicchetti, D. (2011). Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology, 23(04), 1107–1124.
Roth, T. L. (2012). Epigenetics of neurobiology and behavior during development and adulthood. Developmental Psychobiology, 54(6), 590–597.
Russell, A. E., Ford, T., & Russell, G. (2015). Socioeconomic associations with ADHD: findings from a mediation analysis. PLoS One, 10(6), e0128248.
Russell, A. E., Ford, T., Williams, R., & Russell, G. (2016). The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry and Human Development, 47(3), 440–458.
Santiago, C. D., Wadsworth, M. E., & Stump, J. (2011). Socioeconomic status, neighborhood disadvantage, and poverty-related stress: Prospective effects on psychological syndromes among diverse low-income families. Journal of Economic Psychology, 32(2), 218–230.
Shumacker, R. E., & Lomax, R. G. (1996). A beginner’s guide to structural equation modeling. NJ Erlbaum: Mahwah.
Silver, E., Mulvey, E. P., & Swanson, J. W. (2002). Neighborhood structural characteristics and mental disorder: Faris and Dunham revisited. Social Science and Medicine, 55(8), 1457–1470.
Szatmari, P., Boyle, M., & Offord, D. R. (1989). ADDH and conduct disorder: degree of diagnostic overlap and differences among correlates. Journal of the American Academy of Child and Adolescent Psychiatry, 28(6), 865–872.
Thayer, Z. M., & Kuzawa, C. W. (2011). Biological memories of past environments: epigenetic pathways to health disparities. Epigenetics, 6(7), 798–803.
Tucker, L. R., & Lewis, C. (1973). A reliability coefficient for maximum likelihood factor analysis. Psychometrika, 38(1), 1–10.
Turkheimer, E., Haley, A., Waldron, M., D'Onofrio, B., & Gottesman, I. I. (2003). Socioeconomic status modifies heritability of IQ in young children. Psychological Science, 14(6), 623–628. https://doi.org/10.1046/j.0956-7976.2003.psci_1475.x.
USA National Survey of Children's Health 2011-2012: Child and adolescent health measurement initiative. (2014). Retrieved from Centers for Disease Control Center for Health Statistics: www.childhealthdata.org
Van Os, J., Marcelis, M., Sham, P., Jones, P., Gilvarry, K., & Murray, R. (1997). Psychopathological syndromes and familial morbid risk of psychosis. The British Journal of Psychiatry, 170(3), 241–246.
Van Praag, H., Kempermann, G., & Gage, F. H. (2000). Neural consequences of enviromental enrichment. Nature Reviews Neuroscience, 1(3), 191–198.
Verdoux, H., Van Os, J., Sham, P., Jones, P., Gilvarry, K., & Murray, R. (1996). Does familiality predispose to both emergence and persistence of psychosis? A follow-up study. The British Journal of Psychiatry, 168(5), 620–626.
Visser, S. N., Danielson, M. L., Bitsko, R. H., Holbrook, J. R., Kogan, M. D., Ghandour, R. M., et al. (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. Journal of the American Academy of Child and Adolescent Psychiatry, 53(1), 34–46.e32.
Visser, S. N., Zablotsky, B., Holbrook, J. R., Danielson, M. L., & Bitsko, R. H. (2015). Diagnostic experiences of children with Attention-Deficit/Hyperactivity Disorder. National Health Statistics Reports, 81, 1–7.
Waring, M. E., & Lapane, K. L. (2008). Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics, 122(1), e1–e6.
Wechsler, D. (2003). Wechsler intelligence scale for children–Fourth Edition (WISC-IV). San Antonio: The Psychological Corporation.
Wechsler, D. (2009). Wechsler Individual Achievement Test, 3rd Edition (WIAT-III). New York: Pearson, Inc..
Wolraich, M., Brown, L., Brown, R. T., DuPaul, G., Earls, M., Feldman, H. M., et al. (2011). ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics, 128(5), 1007–1022. https://doi.org/10.1542/peds.2011-2654.
Funding
This work was funded by NIMH grant R37–59105 (Nigg, PI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this work.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all parents participating in the study, one parent provided written informed consent for all children participating in the study, and written informed assent was obtained from all participating children.
Electronic Supplementary Material
ESM 1
(DOCX 987 kb)
Rights and permissions
About this article
Cite this article
Miller, L.L., Gustafsson, H.C., Tipsord, J. et al. Is the Association of ADHD with Socio-Economic Disadvantage Explained by Child Comorbid Externalizing Problems or Parent ADHD?. J Abnorm Child Psychol 46, 951–963 (2018). https://doi.org/10.1007/s10802-017-0356-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-017-0356-8