Abstract
Neuroendocrine dysfunction is hypothesized to be an early emerging vulnerability marker for depression. We tested whether the main and interactive effects of maternal psychopathology and early child temperamental vulnerability for depression assessed at age three predicted offspring’s basal cortisol function at age 6 years. 228 (122 males) children participated in the baseline and follow-up assessments. At age three, maternal lifetime psychopathology was assessed with a diagnostic clinical interview, and child temperamental positive affectivity (PA) and negative affectivity (NA) were assessed using laboratory observations. At age six, children’s waking and evening cortisol were assessed on 2 days. Maternal lifetime anxiety predicted offspring’s higher morning cortisol at age six. Child temperamental NA at age three predicted higher evening cortisol at age six. There was a significant interaction between maternal lifetime depression and child temperamental PA at age three in predicting offspring’s morning cortisol at age six. For the offspring of mothers with lifetime depression, higher PA at age 3 predicted lower morning cortisol at age 6. These findings highlight the importance of examining the main and interactive effects of maternal psychopathology and early child temperamental vulnerability in predicting the development of offspring’s stress physiology. Findings hold significance in identifying etiological mechanisms of risk and delineating the complex developmental pathways to psychopathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Depression has been consistently associated with abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis, one of the body’s major stress-response systems (Gold et al. 1988). Two meta-analytic reviews affirm that several indices of the HPA-axis are disrupted in depressed adults (Burke et al. 2005) and depressed youth (Lopez-Duran et al. 2009). Theorists propose that pre-existing differences in HPA-axis functioning may underlie the stress and affective sensitivity observed in depression (Kovacs and Lopez-Duran 2010). In support of this assertion, emerging evidence suggests that elevated levels of the glucocorticoid hormone cortisol, the hormonal end product of the HPA axis, predict the development of depression in youths and adults (Adam et al. 2010; Goodyer et al. 2000; Harris et al. 2000). Understanding the role of HPA-axis functioning in development and psychopathology holds considerable significance in identifying etiological mechanisms and biomarkers of risk for depression.
Parental depression, especially maternal depression, is one of the most robust risk factors for youth and adult depression, as well as other mental and physical health problems (Klein et al. 2005; Weissman et al. 2006). Several studies have reported associations between maternal depression and abnormalities in offspring’s basal cortisol levels, including elevated cortisol levels after waking (Dougherty et al. 2009; Halligan et al. 2004; Mannie et al. 2007; Young et al. 2006) and in the afternoon or evening hours (Brennan et al. 2008; Essex et al. 2002; Young et al. 2006). These associations include offspring in infancy (Brennan et al. 2008), early childhood (Dougherty et al. 2009), pre- and post-pubertal adolescence (Halligan et al. 2004; Mannie et al. 2007; Young et al. 2006), and young adulthood (Mannie et al. 2007). Evidence also suggests that elevated morning cortisol, in particular, mediates the association between maternal postnatal depression and depressive symptoms in adolescents (Halligan et al. 2007). Other studies have examined offspring’s cortisol levels collected in the laboratory and in response to psychosocial stressors; however, assessing cortisol reactivity during childhood poses considerable methodological challenges that have not been resolved (Gunnar et al. 2009). Hence, we chose to focus exclusively on offspring’s basal cortisol diurnal rhythm, which evidences an adult-equivalent rhythm by early childhood and is characterized by cortisol levels peaking in the morning approximately 30 min after waking and decreasing throughout the day to reach a nadir in the late evening (Jessop and Turner-Cobb 2008).
Early temperamental/affective style is another important developmental risk factor for depression, which has been linked to both later depression (Klein et al. 2011) and HPA axis dysregulation (Gunnar and Vazquez 2006). Theorists have long posited that the predisposition for depressive disorders is rooted in early temperament (Akiskal 1989). Temperament refers to early-emerging individual differences in emotional reactivity and regulation (Caspi and Shiner 2006). Notably, Clark et al. (1994) hypothesized that low positive affectivity (PA) and high negative affectivity (NA) are precursors to depressive disorders. PA is characterized by a tendency to experience affects such as joy, interest and excitement, while NA is characterized by a tendency to experience negative emotions such as sadness, anxiety, fear, and anger.
There is evidence that both low PA and high NA are associated with increased basal cortisol levels in adults (e.g., Portella et al. 2005; Nater et al. 2010; Steptoe et al. 2007), and NA-related constructs have been associated with increased morning cortisol in children (Kagan et al. 1987; Schmidt et al. 1997). Very little research has examined associations between PA and HPA-axis indicators in children. In our previous report based on a subset of this sample when the children were 3 years old, we observed a concurrent association between low PA and elevated morning cortisol (Dougherty et al. 2009). Examining associations between PA and cortisol functioning is particularly relevant given the link between anhedonic depression (which is characterized by low PA) and cortisol dysregulation, which has been observed even in young children (Luby et al. 2003).
Emerging research supports a modest but significant association between parental depression and offspring temperament (Kovacs and Lopez-Duran 2010). Several studies have reported that young children at familial risk for depression exhibit low levels of PA and/or high levels of NA (Durbin et al. 2005; Olino et al. 2010; Olino et al. 2011). These findings raise the possibility of differential risk among offspring of depressed parents depending on their temperamental disposition. For example, if parental depression is linked to particular temperament traits in the offspring that predispose to depression, the combination of these two vulnerabilities may impact the development of the neuroendocrine system and increase risk for subsequent depression. Specifically, certain temperamental styles may render children more vulnerable to the familial, possibly genetic, vulnerability of having a depressed parent or to the environmental stressors associated with having a depressed parent, which may in turn impact their neuroendocrine function over time. Alternatively, the genetic factors influencing temperament, depression, and neuroendocrine dysfunction may overlap. To date, no study has examined the unique and joint, interactive effects of maternal depression history and early temperamental vulnerability on offspring HPA-axis function.
In the current report, we aim to extend our initial findings of associations between elevated morning cortisol and maternal depression and child low PA based on a subset of the current sample when the offspring were three years old (Dougherty et al. 2009). Given the small sample size (N = 94) and the limited number of mothers with a lifetime history of depression in the previous report, we did not have sufficient power to examine the interactive effects of maternal depression and child temperament on offspring’s basal cortisol levels. In addition, our previous report was based on a cross-sectional design, with all measures obtained at approximately the same time and was limited to 1 day of cortisol assessment. The aim of the present study was to examine both the main and moderated effects of maternal lifetime history of depression and early child temperament on offspring’s basal morning and evening cortisol levels approximately 3 years later. We also assessed a number of confounds known to be associated with cortisol, including age, gender, time of waking, child’s health status, hours of sleep, children’s current anxious and depressive symptoms and current diagnosis of major depressive disorder (MDD) (Gunnar and Vazquez 2006). In addition, to test the specificity of maternal lifetime history of depression, we examined the effects of maternal lifetime anxiety disorder on offspring’s cortisol function, as associations between maternal anxiety symptoms and offspring’s cortisol profiles have also been observed (O’Connor et al. 2005; Van den Bergh et al. 2008).
The current sample was drawn from the larger community sample of young children who were participating in a longitudinal study examining early temperament and risk for psychopathology (Olino et al. 2010). We focused on examining these associations during early childhood for two reasons: (1) early childhood is prior to the typical risk period for mood disorders and affords the opportunity to investigate vulnerability for depression in the absence of the disorder (Egger and Angold 2006); and (2) evidence suggests that differences in temperamental affectivity and neuroendocrine functioning in the offspring of depressed parents emerge during the first few years of life (Kovacs and Lopez-Duran 2010).
In light of research showing that early temperamental affectivity is altered in the offspring of mothers with lifetime depression, we hypothesized that the combination of maternal lifetime history of depression and child temperamental vulnerability at age 3 years would predict offspring’s cortisol function at age 6 years. We hypothesized that children with mothers with a history of depression and who exhibited low PA and/or high NA at age 3 would evidence elevated basal cortisol levels at age 6 years. In addition, based on our previous findings, we expected that the combination of maternal lifetime depression and child low PA would be specifically linked to elevated morning cortisol.
Lastly, we had two exploratory aims. First, we examined whether children’s basal cortisol levels at age 6 were associated with early exposure to maternal depression during the child’s first 3 years of life, as prior studies have suggested that an association may exist (e.g., Ashman et al. 2002; Essex et al. 2002). We compared cortisol levels among children of mothers with no history of depression, children of mothers with a history of depression prior to the child’s birth but not during the child’s first 3 years of life, and children of mothers with a history of depression during the child’s first 3 years of life. Second, given that melancholia is the subtype of depression most frequently associated with hypercortisolemia (Thase 2009), and in our previous report (Dougherty et al. 2009) maternal depression with melancholic features was specifically associated with offspring’s elevated morning cortisol at age 3 years, we examined whether children of mothers with a history of melancholic depression are particularly likely to exhibit elevated cortisol at age six.
Method
Participants
The initial sample was 559 children (54 % males) and their parents participating in a longitudinal study examining early temperament and risk for psychopathology (Dougherty et al. 2011; Olino et al. 2010). The mean age of the children at baseline was 3.5 years (SD = 0.26). Potential participants were identified via a commercial mailing list, and eligible families had a 3 year-old child with no significant medical conditions or developmental disabilities, and at least one English-speaking biological parent. Most of the participants came from middle-class families (M = 45.1; SD = 10.9), as measured by Hollingshead’s Four Factor Index of Social Status (Hollingshead 1975), and the vast majority (95 %) of children came from two-parent homes. Of the children, 86.9 % were White, 8.6 % were Hispanic, and the remainder of the sample was comprised of a variety of other racial/ethnic groups.
Four hundred and seventy children and their parents (84.1 %) participated in the follow-up assessment when the children were approximately 6 years old (M = 6.08 SD = 0.41). Families were asked to provide morning and evening salivary cortisol samples across 2 days (i.e., 4 samples per child). Of the 470 children, 255 (120 females, 135 males) participated in the cortisol assessment from whom we received a total of 1,005 cortisol samples. We compared the 255 participants who provided cortisol samples to the 215 who did not on the parent, child, and demographic variables included in the study. We found that non-participating mothers had higher rates of lifetime depression than participating mothers (31.4 % vs. 21.3 %), χ 2 (1, N = 541) = 7.04, p < 0.01; however, there were no significant differences between nonparticipating mothers and participating mothers on maternal self-reported depression severity at the age six assessment, age of onset of MDD, number of MDD episodes, and rates of comorbid disorders.
Of the 1,005 cortisol samples collected, we excluded 6 samples (0.6 %) that had extremely high cortisol values (> 4 standard deviation above the mean) (Gunnar and Talge 2007), and 218 cortisol samples (21.7 %) that were not compliant with the protocol (see below for details). In addition, 13 children were excluded for taking corticosteroid (N = 2), stimulant (N = 2), analgesic (N = 1), or antibiotic (N = 6) medications, and/or were sick with a fever (N = 3), as these factors have been found to potentially affect cortisol levels (Granger et al. 2009; Gunnar and Talge 2007). Children were included in analyses if they had at least one valid cortisol sample, leaving a total of 228 participants.
Written informed consent was obtained by parents after the study procedures had been fully explained. Characteristics of the sample are presented in Table 1. The study was approved by the human subjects review committee at Stony Brook University, and informed consent was obtained from parents.
Measures
Baseline Assessment (Age 3)
Parental Psychopathology
Biological mothers were interviewed by telephone using the Structured Clinical Interview for DSM-IV, non-patient version (SCID-NP; First et al. 1996). The SCID-NP is a widely used semi-structured diagnostic interview that has been documented to have good reliability and validity (Williams et al. 1992). SCID-NPs were obtained from 226 mothers. Two mothers could not be interviewed, but we were able to obtain information on one of the mothers by interviewing the co-parent using the family history method (Andreasen et al. 1977). Thus, of the 228 children who provided valid cortisol data at the follow-up assessment, we had diagnostic information on 227 mothers. Two Masters-level clinicians conducted the diagnostic interviews. Based on audiotapes of 30 assessments, interrater reliability (indexed by kappa) for lifetime depression and anxiety was 0.93 and 0.91, respectively. Mothers with lifetime depression were significantly more likely to have comorbid lifetime history of anxiety disorder (68.0 %) than mothers without a lifetime history of depression (24.3 %), χ 2 (1, N = 227) = 33.23, p < 0.01. See Table 1 for the clinical characteristics of the mothers.
Child Temperament
Each child and a parent visited the laboratory for a 2-h observational assessment of temperament that included a standardized set of 12 episodes selected from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith et al. 1995). Episodes were designed to elicit a range of affect and temperament-relevant behaviors. Using an independent sample, we previously reported moderate stability of laboratory ratings of temperament from ages 3 to 7, and moderate concurrent and longitudinal associations between Lab-TAB ratings and home observations (Durbin et al. 2007). Each task was videotaped through a one-way mirror and later coded. To prevent carryover effects, no episodes presumed to evoke similar affective responses occurred consecutively and each episode was followed by a brief play break to allow the child to return to a baseline affective state. The parent remained in the room with the child for all episodes except Stranger Approach and Box Empty (see below).
The episodes, in order of presentation, were: (1) Risk Room–child played freely with novel and ambiguous stimuli (e.g., cloth tunnel, Halloween mask), followed by the experimenter asking the child to approach each object; (2) Tower of Patience–child and experimenter took turns building a tower of cardboard blocks according to a schedule of increasing delays, forcing the child to wait longer between turns; (3) Arc of Toys–child played freely in a room full of toys and then was asked to clean up; (4) Stranger Approach–a male research assistant entered the room where the child had been left alone, speaking to the child while gradually walking closer; (5) Make That Car Go–experimenter and child raced two remote-controlled racecars; (6) Transparent Box–child was given inoperable keys to open a transparent box that contained an appealing toy; (7) Pop-Up Snakes–experimenter and child played a practical joke on the mother, asking her to open what appeared to be a can of potato chips, but actually contained coiled spring snakes; (8) Impossibly Perfect Green Circles–experimenter asked the child to draw several circles, mildly criticizing each one, and asking the child to draw another; (9) Popping Bubbles–experimenter and child played together with a bubble-shooting toy; (10) Exploring New Objects–child was given an opportunity to explore new objects (e.g., tent, pet carrier, “gooey” toys); (11) Snack Delay–child was instructed to wait for the experimenter to ring a bell before eating a snack; (12) Box Empty–child was left alone with a wrapped empty box to open, under the pretense that an appealing toy was inside.
Coding of Temperament Data
The coding system has been described in greater detail elsewhere (Dyson et al. 2012; Olino et al. 2011). Briefly, facial, bodily and vocal indicators of positive affect (PA), fear, sadness, and anger were coded during each episode, regardless of the emotion an episode was designed to elicit. This coding scheme captured atypical emotional behaviors, while also increasing scale reliability by generating affect ratings for all tasks. Each relevant display of facial, vocal, and bodily affect was coded on a three-point intensity scale. Ratings were summed within each episode, the totals for the 12 episodes were then totaled, and the three channels were aggregated to create scores for PA, sadness, fear, and anger reflecting facial, vocal, and bodily indicators. Analyses used aggregated PA and NA scores. PA consisted of the average of z-scores for facial, bodily, and vocal PA across all episodes. NA consisted of the average of z-scores for facial, bodily, and vocal anger, sadness, and fear. PA and NA had adequate internal consistencies (α = 0.89 and 0.85, respectively) and interrater reliabilities (intraclass correlation [ICC] = 0.93 and 0.85, respectively; N = 28). PA correlated 0.04 (p = 0.60) with NA. As PA and NA values were positively skewed, transformations (square root and logarithmic, respectively) were applied, and transformed values were used in all analyses.
Follow-Up Assessment (Age 6)
Salivary Cortisol Collection
Salivary cortisol was collected two times per day over 2 consecutive days: 30 min after waking and 30 min before bedtime. These sampling times were chosen in order to capture the morning peak or rise in cortisol following waking and the nadir levels before bed. Cortisol was gathered by passive drool: participants expressed their saliva through a small straw into a polypropylene tube, and parents labeled tubes with the time and date. Parents were instructed not to allow their child to eat or drink or brush their teeth in the 1/2-h before sampling. Samples were returned by mail and frozen at −20° C, then sent to Trier, Germany to be assayed for cortisol. Assays were conducted in duplicate using a time-resolved immunoassay with fluorometric detection (DELFIA). Intra-assay coefficients of variation (CVs) were between 4.0 % and 6.7 %, and inter-assay CVs ranged from 7.1 to 9.0 %. Consistent with previous research, morning and evening cortisol values showed a positively skewed distribution. We applied a log10 transformation to the data to yield unskewed values.
Samples were excluded from analyses if they were not taken in compliance with the collection schedule based on parent report of collection times. Following Broderick et al. (2004), noncompliance was defined as taking the morning sample more than 15 min before or after the intended collection time, or taking the bedtime sample more than 1 h before or after the intended collection time. Applying these criteria, 433 (86.1 %) morning samples were compliant, and 353 (70.5 %) evening samples were compliant. Of the compliant samples, 527 were collected on a weekday, and 251 were collected on a weekend. Some participants did not report whether the sample was collected on a weekday or weekend. Both the main effect of type of day (weekend vs. weekday) F(1, 772) = 0.28, p = 0.60, and the interaction between time of day (morning vs. evening) and type of day, F(1, 772) = 2.22, p = 0.14 were not significant. The correlation between day 1 and day 2 compliant morning samples was r = 0.58, p < 0.001, and the correlation between day 1 and day 2 compliant evening samples was r = 0.50, p < 0.001. Compliant morning and evening cortisol values were averaged, respectively, across days.
Covariates
A number of health variables and psychosocial factors known to be associated with HPA-axis functioning were included as covariates in analyses: age, gender, hours of sleep, and children’s current anxious and depressive symptoms (Gunnar and Vazquez 2006). Parents completed the Child Behavior Checklist/1½–5 (CBCL/6–18; Achenbach and Rescorla 2001) anxious/depressed scale (α = 0.77) to assess children’s current anxious and depressive symptoms. Time of waking was significantly associated with morning cortisol (r = −0.27, p < 0.001), but time between waking and the morning cortisol assessment was not significantly associated with morning cortisol (r = −0.05, p = 0.47); thus, only time of waking was included as a covariate in analyses involving morning cortisol. Given our focus on risk for depression, children were assessed for MDD at baseline and follow-up using the Preschool Age Psychiatric Assessment (PAPA; Egger et al. 1999). Adequate test-retest reliability for PAPA diagnoses has been reported using independent interviews (kappas: 0.49–0.74; Egger et al. 2006). In addition, PAPA diagnoses have been associated with impairment (Bufferd et al. 2011) and observations of children’s behavior (Dougherty et al. 2011). In the subsample included in this report, no children met criteria for MDD at age 3 years and seven children met criteria for MDD at age 6 years. Interrater reliability estimates, which were based on the full sample, for MDD at age 3 and 6 years were acceptable (kappas = 1.00 (N = 21) and 0.64 (N = 35), respectively).
Data Analysis Strategy
We examined whether maternal lifetime depression and child temperament at age 3 predicted children’s morning and/or evening cortisol at age 6 using analysis of covariance (ANCOVA) and multiple linear regression analyses. Interactions between maternal lifetime depression and child temperament were examined using multiple linear regression. Maternal depression was dummy coded for absence or presence of lifetime depression. All temperament variables were standardized. Interaction terms were created by multiplying the two independent variables. Covariates included in all analyses were time of waking (for analyses involving morning cortisol), age, gender, hours of sleep, child current anxious and depressive symptoms, and maternal lifetime anxiety disorder. As outliers are particularly problematic for hypothesized interactions, we carefully screened our data for univariate and multivariate outliers (Tabachnick and Fidell 2007). Examination of z scores (|z| ≥ 3.30) revealed one univariate outlier for child NA. This same outlier was also identified as a multivariate outlier using Mahalanobis distance with p < 0.001 and was removed from analyses.
Results
Descriptive Data
Table 1 presents descriptive statistics for morning and evening cortisol levels, maternal psychopathology, child temperament, and demographic, health, and other covariates. Cortisol values in Table 1 reflect raw values (in nmol/L), but a logarithmic transformation of these cortisol values was used in all analyses. A diurnal rhythm in cortisol was observed in the sample, t(194) = 31.46, p < 0.001, indicating that morning cortisol was significantly higher than evening cortisol. Morning and evening cortisol were significantly correlated (r = 0.14, p < 0.05). Maternal lifetime depression was not significantly associated with child PA, t(225) = −0.08, p = 0.94, or NA, t(224) = −1.63, p = 0.11. Children of mothers with and without a lifetime history of depression did not differ on age, gender, ethnicity, or parental education. Child PA was positively associated with child age (r = 0.22, p = 0.001); Child PA and NA were not significantly associated with any other demographic variable.
Maternal Psychopathology and Child Cortisol
We examined whether maternal lifetime depression predicted children’s morning and evening cortisol at age 6. No significant differences were observed for morning cortisol at age 6 between offspring of mothers with a history of depression (M = 7.59, SD = 4.51, N = 49) and without a history of depression (M = 8.26, SD = 4.66, N = 171), F(1, 211) = 1.76, p = 0.19. In addition, no significant differences were observed for evening cortisol at age 6 between offspring of mothers with a history of depression (M = 0.95, SD = 1.55, N = 45) and without a history of depression (M = 1.16, SD = 1.88, N = 150), F(1, 187) = 1.14, p = 0.29.
The effect of early exposure to maternal depression on offspring’s cortisol levels at age six was examined. A one-way ANOVA comparing children of mothers with no history of depression (N = 177), a history of depression prior to the child’s birth but not during the child’s first 3 years of life (N = 37), and a history of depression during the child’s first 3 years of life (N = 13) yielded no significant effects for either morning cortisol, F(2, 221) = 2.45, p = 0.08, or evening cortisol, F(2, 194) = 1.03, p = 0.36. In addition, we compared children of mothers with a history of melancholic depression (N = 18), mothers with lifetime non-melancholic depression (N = 32), and mothers with no depression history (N = 177) on offspring’s cortisol levels at age 6 years. No main effect of group was observed for morning cortisol, F(2,220) = 0.37, p = 0.70 or evening cortisol, F(2,193) = 1.69, p = 0.19.
Next, we examined whether maternal history of anxiety disorder predicted offspring cortisol at age 6 after controlling for maternal depression history. Offspring of mothers with a history of anxiety had significantly higher morning cortisol at age 6 (M = 8.96, SD = 5.05, N = 75) than offspring of mothers without a history of anxiety (M = 7.68, SD = 4.34, N = 145), F(1, 211) = 6.01, p = 0.02, Cohen’s d = 0.27. No significant difference were observed for evening cortisol at age 6 between offspring of mothers with a history of anxiety (M = 1.05, SD = 1.50, N = 69) and without a history of anxiety, (M = 1.14, SD = 1.96, N = 126), F(1, 187) = 0.44, p = 0.51. No interaction between maternal lifetime depressive and anxiety disorder diagnoses was observed for morning, F(1, 210) = 0.34, p = 0.56, or evening cortisol levels, F(1,186) = 0.32, p = 0.57.
Child Temperament and Cortisol
We examined whether child PA or NA at age 3 predicted morning or evening cortisol levels at age 6. Neither child PA (b = 0.01, SE = 0.09, pr = 0.01, p = 0.88), nor NA (b = 0.02, SE = 0.07, pr = 0.02, p = 0.79) at age 3 significantly predicted morning cortisol at age 6. In addition, child PA was not significantly associated with evening cortisol levels at age 6 (b = − 0.19, SE = 0.14, pr = −0.10, p = 0.19). However, high child NA significantly predicted higher evening cortisol at age 6 (b = 0.27, SE = 0.12, pr = 0.16, p = 0.03). Finally, the interaction between PA and NA did not predict morning (b = 0.11, SE = 0.35, pr = 0.02, p = 0.76) or evening (b = −0.59, SE = 0.59, pr = −0.07, p = 0.31) cortisol levels.
Interactions Between Maternal Psychopathology and Child Temperament
We examined whether the interactions between maternal lifetime depression and child temperament at age 3 predicted children’s morning and evening cortisol levels at age 6. As seen in Table 2, there was a significant interaction between maternal depression and child PA on morning cortisol but no significant interaction between child NA and maternal depression. No significant interactions between child temperament and maternal depression were observed for evening cortisol.
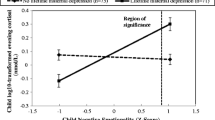
To further understand the nature of the interaction, we plotted the relations between estimated levels of offspring’s morning cortisol levels and child PA for offspring of mothers with and without a lifetime depression. As seen in Fig. 1, for the offspring of mothers with a history of depression, there was a significant negative association between child PA at age 3 and morning cortisol at age 6 (b = −0.42, SE = 0.20, pr = −0.14, p = 0.04). In contrast, for children of mothers with no history of depression, there was no significant association between child PA at age 3 and morning cortisol at age 6 (b = 0.09, SE = 0.09, pr = 0.07, p = 0.33). To determine the degree of child PA at which differences in morning cortisol emerged for offspring of mothers with and without a history of depression, Hayes and Matthes (2009)’s guidelines were used for testing regions of significance according to the Johnson-Neyman technique (Johnson and Fay 1950). This approach uses the asymptotic variances, covariances, and other regression parameters to determine the upper and lower boundaries of the focal predictor variable at which groups representing a multilevel moderator are significantly different (p < 0.05) in terms of the outcome of interest. In this case, child PA was the focal predictor variable and the moderator was maternal depression history. The degree of child PA at which group differences in waking cortisol emerged is shown in Fig. 1. At levels of child PA greater than 0.54 (standardized z-score), offspring of mothers with a history of depression demonstrated significantly lower morning cortisol than offspring of mothers without a history of depression. In contrast, at levels of child PA below 0.54, offspring of mothers with or without a history of depression did not differ in morning cortisol.
Children’s morning cortisol levels at age 6 as a function of maternal lifetime depression history and child positive affectivity at age 3. The line on the X axis at 0.54, indicates the value of positive affectivity (standardized z-score) at and above which offspring of mothers with and without lifetime depression differ significantly in terms of age 6 morning cortisol levels. Bars reflect standard errors of measurement
No significant interactions between maternal anxiety and child temperament were observed, and no significant three-way interactions for maternal depression or anxiety disorders and child PA and NA were observed for morning cortisol or evening cortisol.
Diurnal Slope
Given that disturbances in the typical pattern of diurnal cortisol may provide valuable information on HPA axis functioning, we examined main and interactive effects of maternal psychopathology and child temperament on the diurnal cortisol slope. The diurnal cortisol slope was calculated by subtracting evening cortisol levels from morning cortisol levels, and dividing by the number of hours between the morning and evening samples. No significant associations were found.
Child Depression
In order to determine whether the results were driven by a current depressive disorder in the offspring, we excluded the seven children who met criteria for current depression at the follow-up assessment, and results were similar.
Discussion
We examined the main and interactive effects of maternal history of psychopathology and early child temperamental affectivity in predicting children’s basal cortisol functioning 3 years later. We found that the offspring of mothers with a history of anxiety evidenced elevated morning cortisol at age six. Children who exhibited higher NA at age three evidenced elevated evening cortisol at age six. Lastly, we found that the offspring of mothers with a history of depression who also exhibited higher levels of PA at age three evidenced lower morning cortisol levels at age six. These results persisted after controlling for child health and psychosocial variables known to be associated with HPA-axis functioning and after excluding children with current depression. Our results suggest that early risk for psychopathology may include a complex interplay between familial risk, affective vulnerability, and neuroendocrine dysfunction. The relations between these risk factors appear to reflect distinct etiological pathways and underlying processes, given that different temperamental and familial characteristics predicted morning and evening cortisol levels and that children’s morning and evening cortisol levels were only minimally correlated. These unique relations may be related to the differential proportion of occupied mineralocorticoid and glucocorticoid receptors, which mediate the effects of cortisol, at the peak and nadir of the diurnal cycle (Gunnar and Vazquez 2006).
Controlling for maternal depression history, we found that the offspring of mothers with a history of anxiety evidenced elevated morning cortisol levels at age 6. Little research has focused on the association between maternal anxiety and offspring basal cortisol, and maternal anxiety and depression are rarely examined simultaneously as done in the current study, which is important given the high comorbidity between the disorders. Nevertheless, emerging evidence supports such an association. Van den Bergh et al. (2008) reported that maternal postnatal anxiety predicted a high, flattened diurnal cortisol profile in the adolescent offspring, as evidenced by a smaller decrease from awakening to evening cortisol. For females only, this diurnal profile was associated with depressive symptoms, which suggests the involvement of the HPA axis in the association between maternal anxiety and female adolescent depression. In addition, O’ Connor et al. (2005) found that maternal prenatal anxiety predicted prepubertal offspring’s elevated morning and afternoon cortisol levels, but not evening cortisol levels. The inconsistencies in the literature regarding whether offspring’s cortisol is linked to maternal anxiety, depression or their comorbidity may be explained by overlapping genetic factors between anxiety and depression (Kendler et al. 2007). Some studies suggest that the underlying vulnerability linking anxiety and depression may lie in personality traits, such as neuroticism (Hettema et al. 2006), and indeed, greater neuroticism has been linked to elevated waking cortisol levels in adults (Portella et al. 2005). Moreover, elevated cortisol in the offspring of anxious or depressed mothers may be due to associated disruptions in parenting and exposure to stressful early rearing environments. These findings highlight the need for future research to examine the effects of both maternal anxiety and depression on offspring’s cortisol, as well as potential mediating variables, such as genetic factors, maternal personality, and early experience.
We found that high levels of child NA at age three predicted subsequent elevations in cortisol prior to bedtime, a time when cortisol levels are typically most quiescent. Elevated evening cortisol has been associated with current depression in youths (Goodyer et al. 1996) and has been found to predict a more chronic course of depression in adolescents (Goodyer et al. 2001). A twin study examining the heritability of cortisol levels in children found that evening cortisol levels appear to be more impacted by state-dependent factors and current levels of distress and social-environmental contributions than morning cortisol levels (Bartels et al. 2003). In contrast, using a latent state trait modeling on children’s cortisol, Kertes and van Dulmen (2012) observed that trait factors accounted for approximately half of evening cortisol levels. It is possible that associations between environmental demands and evening cortisol may be closely tied to one’s temperamental, trait-like, disposition towards negative emotions. Children high in NA may have greater difficulty in modulating their biological and behavioral stress responses, making them more susceptible to the cumulative challenges of the day.
To our knowledge, this is the first study to demonstrate that the interaction between familial risk for depression and temperamental high PA in children prior to any depressive illness predicted lower levels of morning cortisol during early childhood. Even though the effect for child PA was in the predicted negative direction, groups only differed in the high PA range, rather than in the low PA range as hypothesized. In addition, we did not replicate our previous findings demonstrating main effects of maternal lifetime depression and child PA on children’s morning cortisol (Dougherty et al. 2009); nevertheless, both sets of findings link maternal depression and child PA specifically to morning cortisol. Furthermore, the current study examined the longitudinal, rather than concurrent, associations of familial and temperamental vulnerability with basal cortisol levels, which was not the case in our previous report. In addition, our sample in this study was considerably larger and we sampled cortisol over 2 days, increasing the reliability of the findings.
On the one hand, given evidence that elevated morning cortisol may be a predictive marker for subsequent depression (Adam et al. 2010; Goodyer et al. 2000; Harris et al. 2000), it is possible that lower morning cortisol may be a marker for resiliency. High levels of PA have been associated with adaptive outcomes, such as high sociability (Fox et al. 2001), and has been shown to buffer the effects of stress (Tugade and Fredrickson 2004; Wichers et al. 2007). Thus, high levels of child temperamental PA may protect against the effects of a familial vulnerability for depression on children’s neuroendocrine function.
On the other hand, lower levels of morning cortisol have also been associated with risk, including depression in adults (Brockting et al. 2012; Stetler and Miller 2005) and externalizing problems in children (for a review see Alink et al. 2008). Likewise, high PA has also been linked to maladaptive outcomes. Children with high PA tend to have a strong motivation to approach and seek rewards, but when their goals are blocked, these children can also be more reactive and prone to experiencing anger and frustration. High PA has been linked to bipolar disorder and antisocial personality disorder in adults (Zuckerman 1994), and externalizing and internalizing problems in children (e.g., Degnan et al. 2011; Stifter et al. 2008). Furthermore, Olino et al. (2010) reported that at high and moderate levels of positive emotionality, greater negative emotionality was associated with higher rates of parental depression. If children high in PA are more prone to behavior problems, parenting high PA children may be challenging, which may pose particular difficulties to mothers with a history of depression. Thus, it is possible that the interactions between a child high in PA and a mother with a history of depression may contribute to a chronically stressed environment, the impact of which may lead to blunted cortisol levels, particularly as prolonged exposure to stress is linked with HPA-axis down regulation and blunted cortisol levels (Miller et al. 2007). Both interpretations of the observed interaction are speculative at this point as it is unknown at what levels of morning cortisol signify adaptive or maladaptive responses. Moreover, further examination of the characteristics of children high in PA is warranted to understand this interactive effect, including how high PA in children contributes to parenting, family stress, and child behavior problems.
Strengths and Limitations
This study had several strengths. First, the sample is large, particularly for a neuroendocrine study in young children. This allowed us to exclude participants who were not compliant with the protocol and to examine interactions between familial and temperamental vulnerability, which has not been done in previous research. Second, the longitudinal design tested whether maternal psychopathology, including both maternal depression and anxiety, and child temperament predicted offspring cortisol 3 years later. Third, temperament was assessed using a comprehensive laboratory observation measure. This ensured that temperament assessments were not influenced by maternal psychopathology or other respondent biases. Fourth, maternal psychiatric history was determined using a well-validated semi-structured diagnostic interview. Fifth, we collected multiple cortisol samplings across 2 days to increase reliability of measurement.
The present study also had several limitations. First, laboratory observations of children’s temperament rely on a single occasion and setting, which precludes an assessment of traits across contexts and over time. However, findings have supported the validity of similar observational approaches in predicting behavior in naturalistic settings and at later points in development (Durbin et al. 2007). Second, compliance with the cortisol assessment was low (54.3 %) and non-participating mothers were more likely to have a history of depression compared to non-participating mothers, which reduced power and may restrict the generalizability of our results. Moreover, it is possible that findings could be biased if non-participating families and/or non-participating depressed mothers systematically differed on a variable that influences offspring cortisol. Future research needs to examine this possibility and examine various ways of retaining participants in cortisol assessments. Third, although our prospective design ensured that maternal psychopathology and child temperamental affectivity temporally preceded our measures of HPA axis functioning, we are unable to infer causality or directionality.
Fourth, given that we did not assess multiple cortisol samples during the first hour after waking, it is unknown whether we captured the peak morning cortisol level in our assessment, and we were also not able to specifically assess the cortisol awakening response (CAR), which captures the change in cortisol from waking to peak morning levels and may be a more sensitive predictor of depression in youth (Adam et al. 2010). Fifth, there is evidence to support the context-dependent nature of stress system activity; however we did not examine the role of contextual factors, such as the early rearing environment and life stress, on children’s stress system or how contextual factors interact with familial and/or temperamental vulnerabilities. Lastly, the point at which elevated or blunted cortisol levels increase risk is unknown; therefore, future research should address this issue, which will also help establish the clinical utility of this vulnerability marker.
In closing, further long-term follow-up is necessary to establish whether these familial, temperament, and neuroendocrine factors contribute to the development of depression and other forms of psychopathology and what other processes mediate and moderate their roles in risk across development. Knowledge gained from these findings should contribute to identifying mechanisms of risk and delineating the complex developmental pathways to psychopathology.
Abbreviations
- HPA-axis:
-
hypothalamic-pituitary-adrenal axis
- PA:
-
positive affectivity
- NA:
-
negative affectivity
References
Achenbach, T. M., & Rescorla, L. A. (2001). Manual for the ASEBA school-age forms and profiles. Burlington: University of Vermont, Department of Psychiatry.
Adam, E. K., Doane, L. D., Zinbarg, R. E., Mineka, S., Craske, M. G., & Griffith, J. W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35, 921–931.
Akiskal, H. S. (1989). Validating affective personality types. In L. Robins & J. Barrett (Eds.), The validity of psychiatric diagnosis (pp. 217–227). New York: Raven.
Alink, L. R., Ijzendoorn, M. H., Bakermans-Kranenburg, M. J., Mesman, J., Juffer, F., & Koot, H. M. (2008). Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology, 50, 427–450.
Andreasen, N. C., Endicott, J., Spitzer, R. L., & Winokur, G. (1977). The family history method using diagnostic criteria: reliability and validity. Archives of General Psychiatry, 34, 1229–1235.
Ashman, S. B., Dawson, G., Panagiotides, H., Yamada, E., & Wilkinson, C. W. (2002). Stress hormone levels of children of depressed mothers. Development and Psychopathology, 14, 333–349.
Bartels, M., de Geus, E. J. C., Kirschbaum, C., Sluyter, F., & Boomsma, D. I. (2003). Heritability of daytime cortisol levels in children. Behavior Genetics, 33, 421–433.
Brennan, P. A., Pargas, R., Walker, E. F., Green, P., Newport, D. F., & Stowe, Z. (2008). Maternal depression and infant cortisol: Influences of timing, comorbidity and treatment. Journal of Child Psychology and Psychiatry, 49, 1099–1107.
Brockting, C., Lok, A., Visser, I., Assies, J., Koeter, M., & Schene, A. (2012). Lower cortisol levels predict recurrence in remitted patients with recurrent depression: A 5.5 year prospective study. Psychiatry Research, pii, S0165–S1781.
Broderick, J. E., Arnold, D., Kudielka, B. M., & Kirschbaum, C. (2004). Salivary cortisol sampling compliance of patients and healthy volunteers. Psychoneuroendocrinology, 29, 636–650.
Bufferd, S. J., Dougherty, L. R., Carlson, G. A., & Klein, D. N. (2011). Parent-reported mental health in preschoolers: findings using a diagnostic interview. Comprehensive Psychiatry, 52, 359–369.
Burke, H. M., Davis, M. C., Otte, C., & Mohr, D. C. (2005). Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology, 30, 846–856.
Caspi, A., & Shiner, R. (2006). Personality development. Handbook of child psychology: Vol. 3: Social, emotional and personality development (6th ed., pp. 300–365). Hoboken: John Wiley & Sons.
Clark, L. A., Watson, D., & Mineka, S. (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103, 103–116.
Degnan, K. A., Hane, A. A., Henderson, H. A., Moas, O., Reeb-Sutherland, B. C., & Fox, N. A. (2011). Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Developmental Psychology, 47, 765–780.
Dougherty, L. R., Klein, D. N., Olino, T. M., Dyson, M. W., & Rose, S. (2009). Increased waking salivary cortisol and depression risk in preschoolers: the role of maternal history of melancholic depression and early child temperament. Journal of Child Psychology and Psychiatry, 50, 1495–1503.
Dougherty, L. R., Bufferd, S. J., Carlson, G. A., Dyson, M. W., Olino, T. M., & Klein, D. N. (2011). Preschoolers’ observed temperament and psychiatric disorders assessed with a parent diagnostic interview. Journal of Clinical Child and Adolescent Psychology, 40, 295–306.
Durbin, C. E., Klein, D. N., Hayden, E. P., Buckley, M. E., & Moerk, K. C. (2005). Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology, 114, 28–37.
Durbin, E. C., Hayden, E. P., Klein, D. N., & Olino, T. M. (2007). Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7. Emotion, 7, 388–399.
Dyson, M. W., Olino, T. M., Durbin, C. E., Goldsmith, H. H., & Klein, D. N. (2012). The structure of temperament in preschoolers: A two-stage factor analytic approach. Emotion, 12, 44–57.
Egger, H. L., & Angold, A. (2006). Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry, 47, 313–337.
Egger, H. L., Ascher, B. H., & Angold, A. (1999). The preschool age psychiatric assessment (PAPA). Durham: Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center.
Essex, M. J., Klein, M. H., Cho, E., & Kalin, N. H. (2002). Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry, 52, 776–784.
First, M. B., Gibbon, M., Spitzer, R. L., & Williams, J. B. W. (1996). Structured clinical interview for DSM-IV axis I disorders: Non-patient edition (SCID-1, Version 2.0). New York: Biometrics Research, New York State Psychiatric Institute.
Fox, N. A., Henderson, H. A., Rubin, K. H., Calkins, S. D., & Schmidt, L. A. (2001). Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development, 72, 1–21.
Gold, P. W., Goodwin, F. K., & Chrousos, G. P. (1988). Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. The New England Journal of Medicine, 319, 348–353.
Goldsmith, H. H., Reilly, J., Lemery, K. S., Longley, S., & Prescott, A. (1995). The Laboratory Temperament Assessment Battery: Preschool version. Unpublished manuscript.
Goodyer, I. M., Herbert, J., Altham, P. M. E., Pearson, J., Secher, S. M., & Shiers, H. M. (1996). Adrenal secretion during major depression in 8-to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine, 26, 245–256.
Goodyer, I. M., Herbert, J., Tamplin, A., & Altham, P. M. (2000). First-episode major depression in adolescents: affective, cognitive and endocrine characteristics of risk status and predictors of onset. The British Journal of Psychiatry, 176, 142–149.
Goodyer, I. M., Park, R. J., & Herbert, J. (2001). Psychosocial and endocrine features of chronic first-episode major depression in 8–16 year olds. Biological Psychiatry, 50, 351–357.
Granger, D. A., Hibel, L. C., Fortunato, C. K., & Kapelewski, C. H. (2009). Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34, 1437–1448.
Gunnar, M. R., & Talge, N. M. (2007). Neuroendocrine measures in developmental research. In L. A. Schmidt & S. J. Segalowitz (Eds.), Developmental psychophysiology: Theory, systems, and methods (pp. 343–366). Cambridge: University Press.
Gunnar, M. R., & Vazquez, D. M. (2006). Stress neurobiology and developmental psychopathology. In D. Cicchetti & D. J. Cohen (Eds.), Developmental psychopathology. Vol. Developmental neuroscience (2nd ed., pp. 533–577). Hoboken: John Wiley.
Gunnar, M. R., Talge, N. M., & Herrera, A. (2009). Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34, 953–967.
Halligan, S. L., Herbert, J., Goodyer, I. M., & Murray, L. (2004). Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry, 55, 376–381.
Halligan, S. L., Herbert, J., Goodyer, I., & Murray, L. (2007). Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry, 62, 40–46.
Harris, T. O., Borsanyi, S., Messari, S., Stanford, K., Brown, G. W., Cleary, S. E., et al. (2000). Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. The British Journal of Psychiatry, 177, 505–510.
Hayes, A. F., & Matthes, J. (2009). Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavioral Research Methods, 41, 924–936.
Hettema, J. M., Neale, M. C., Myers, J. M., Prescott, C. A., & Kendler, K. S. (2006). A population based twin study of the relationship between neuroticism and internalizing disorders. The American Journal of Psychiatry, 163, 857–864.
Hollingshead, A. B. (1975). Four factor index of social status. Unpublished manuscript.
Jessop, D. S., & Turner-Cobb, J. M. (2008). Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress: The International Journal on the Biology of Stress, 11, 114.
Johnson, P. O., & Fay, L. C. (1950). The Johnson–Neyman technique, its theory and application. Psychometrika, 15, 349–367.
Kagan, J., Reznick, J. S., & Snidman, N. (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–1473.
Kendler, K. S., Gardner, C. O., Gatz, M., & Pederson, N. L. (2007). The sources of comorbidity between major depression and generalizing anxiety disorder in a Swedish national twin sample. Psychological Medicine, 37, 453–462.
Kertes, D. A., & van Dulmen, M. (2012). Latent state trait modeling of children’s cortisol at two points of the diurnal cycle. Psychoneuroendocrinology, 37, 249–255.
Klein, D. N., Lewinsohn, P. M., Rohde, P., Seeley, J. R., & Olino, T. M. (2005). Psychopathology in the adolescent and young adult offspring of a community sample of mothers and fathers with major depression. Psychological Medicine, 35, 353–365.
Klein, D. N., Kotov, R., & Bufferd, S. J. (2011). Personality and depression: explanatory models and review of the evidence. Annual Review of Clinical Psychology, 7, 269–295.
Kovacs, M., & Lopez-Duran, N. (2010). Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: implications for prevention. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51, 472–496.
Lopez-Duran, N. L., Kovacs, M., & George, C. J. (2009). Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology, 34, 1272–1283.
Luby, J. L., Heffelfinger, A. K., Mrakotsky, C., Brown, K. M., Hessler, M. J., Wallis, J. M., et al. (2003). The clinical picture of depression in preschool children. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 340–348.
Mannie, Z. N., Harmer, C. J., & Cowen, P. J. (2007). Increased waking salivary cortisol levels in young people at familial risk of depression. The American Journal of Psychiatry, 164, 617–621.
Miller, G. E., Chen, E., & Zhou, E. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenal axis in humans. Psychological Bulletin, 133, 25–45.
Nater, U. M., Hoppmann, C., & Klumb, P. L. (2010). Neuroticism and conscientiousness are associated with cortisol diurnal profiles in adults-role of positive and negative affect. Psychoneuroendocrinology, 35, 1573–1577.
O’Connor, T. G., Ben-Shlomo, Y., Heron, J., Golding, J., Adams, D., & Glover, V. (2005). Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry, 58, 211–217.
Olino, T. M., Klein, D. N., Dyson, M. W., Rose, S. A., & Durbin, C. E. (2010). Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology, 119, 468–478.
Olino, T. M., Lopez-Duran, N. L., Kovacs, M., George, C. J., Gentzler, A. L., & Shaw, D. S. (2011). Developmental trajectories of positive and negative affect in children at high and low familial risk for depressive disorder. Journal of Child Psychology and Psychiatry, 52, 792–799.
Portella, M. J., Harmer, C. J., Flint, J., Cowen, P., & Goodwin, G. M. (2005). Enhanced early morning salivary cortisol in neuroticism. The American Journal of Psychiatry, 162, 807–809.
Schmidt, L. A., Fox, N. A., Rubin, K. H., & Sternberg, E. M. (1997). Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology, 30, 127–140.
Steptoe, A., Gibson, E. L., Hamer, M., & Wardle, J. (2007). Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology, 32, 56–64.
Stetler, C. A., & Miller, G. E. (2005). Blunted cortisol responses to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology, 114, 697–705.
Stifter, C. A., Putnam, S., & Jahromi, L. (2008). Exuberant and inhibited toddlers: stability of temperament and prediction to behavior problems. Development and Psychopathology, 20, 401–421.
Tabachnick, B. G., & Fidell, L. S. (2007). Using multivariate statistics (5th ed.). Boston: Allyn and Bacon.
Thase, M. E. (2009). Neurobiological aspects of depression. In I. H. Gotlib & C. L. Hammen (Eds.), Handbook of depression (pp. 187–217). New York: Guilford Press.
Tugade, M. M., & Fredrickson, B. L. (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology, 86, 320–333.
Van den Bergh, B. R. H., Van Calster, B., Smits, T., Van Huffel, S., & Lagae, L. (2008). Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology, 33, 536–545.
Weissman, M. M., Wickramaratne, P., Nomura, Y., Warner, V., Pilowsky, D., & Verdeli, H. (2006). Offspring of depressed parents: 20 years later. The American Journal of Psychiatry, 163, 1001–1008.
Wichers, M. C., Myin-Germeys, I., Jacobs, N., Peeters, F., Kenis, G., Derom, C., Vlietinck, R., et al. (2007). Evidence that moment-to-moment variation in positive emotions buffer genetic risk for depression: a momentary assessment twin study. Acta Psychiatrica Scandinavica, 115, 451–457.
Williams, J. B., Gibbon, M., First, M. B., Spitzer, R. L., Davies, M., Borus, J., et al. (1992). The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Archives of General Psychiatry, 49, 630–636.
Young, E. A., Vazquez, D., Jiang, H., & Pfeffer, C. R. (2006). Saliva cortisol and response to dexamethasone in children of depressed parents. Biological Psychiatry, 60, 831–836.
Zuckerman, M. (1994). Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press.
Conflicts of Interest
Authors report no conflicts of interest. This work was supported by the following grants: NIMH RO1 MH069942 (DNK) and a GCRC Grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dougherty, L.R., Smith, V.C., Olino, T.M. et al. Maternal Psychopathology and Early Child Temperament Predict Young Children’s Salivary Cortisol 3 Years Later. J Abnorm Child Psychol 41, 531–542 (2013). https://doi.org/10.1007/s10802-012-9703-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-012-9703-y