Abstract
Increased intrasubject variability (ISV), or short-term, within-person fluctuations in behavioral performance is consistently found in Attention-Deficit/Hyperactivity Disorder (ADHD). ADHD is also associated with impairments in motor control, particularly in boys. The results of the few studies that have examined variability in self-generated motor output in children with ADHD have been inconsistent. The current study examined variability in motor control during a finger sequencing task among boys with and without ADHD as well as the relationship between intrasubject variability during motor and cognitive control tasks. Changes in performance over the course of the task and associations with ADHD symptom domains were also examined to elucidate the nature of impaired motor control in children with ADHD. Fifty-one boys (ages 8 to 12 years) participated in the study, including 28 boys with ADHD and 23 typically developing (TD) boys. Participants completed a finger sequencing task and a Go/No-Go task providing multiple measures of response speed and variability. Boys with ADHD were slower and more variable in both intertap interval on the finger sequencing task and reaction time on the Go/No-Go task, with measures of speed and variability correlated across the two tasks. For the entire cohort, the only unique predictor of parent ratings of hyperactive-impulsive symptoms was variability in intertap interval during finger sequencing, whereas inattentive symptoms were only predicted by reaction time variability on the Go/No-Go task. These findings suggest that inefficient motor control is implicated in the pathophysiology of ADHD, particularly in regards to developmentally inappropriate levels of hyperactivity and impulsivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Developmentally inappropriate levels of inattention, hyperactivity, and impulsivity are the defining features of Attention-Deficit/Hyperactivity Disorder (ADHD) (American Psychiatric Association 2000). The causal pathways contributing to the expression of these persistent and impairing symptoms remain unclear although multi-process models of ADHD prevail (e.g., Sonuga-Barke 2005; Willcutt et al. 2008). One of the most consistent findings in the ADHD literature is increased intrasubject variability (ISV), or short-term, within-person fluctuations in behavioral performance with a particular focus on reaction times (Kuntsi and Klein 2012). Increased ISV in reaction times among individuals with ADHD has been reported across a range of cognitive tasks and across various methods used to quantify ISV (e.g., Castellanos et al. 2005; Epstein et al. 2011). The neural mechanisms underlying ADHD-associated increases in variability remain unclear, although there is evidence of a common factor to variability across tasks (Frazier-Wood et al. 2012; Russell et al. 2006; Vaurio et al. 2009).
Explanatory models of increased intrasubject variability in ADHD implicate higher-order self-regulatory processes (Douglas 1999; Stuss et al. 2003), subcortically mediated problems in state regulation (Sergeant et al. 2003), insufficient suppression of the default mode network (Sonuga-Barke and Castellanos 2007), and deficient attentional processes (Leth-Steensen et al. 2000). The extent to which an individual displays trial-to-trial variability in their reaction time is also influenced by efficiency of response preparation and selection (Mostofsky and Simmonds 2008). Given the growing literature reporting anomalous motor development in children with ADHD (D’Agati et al. 2010; Denckla and Rudel 1978; Gilbert et al. 2011; Klotz et al. 2011; MacNeil et al. 2011; Rommelse et al. 2008; Zelaznik et al. 2012), further investigation of whether parallel deficits in cognitive and motor control systems are present in children with ADHD is warranted. Improving our understanding of variability in self-generated motor control in children with ADHD in relation to reaction time variability during a traditional cognitive task is important for elucidating the processes contributing to variability in behavior more generally. In addition, examination of whether variability in motor and cognitive control is differentially related to ADHD symptom domains of inattention and hyperactivity/impulsivity may inform our understanding of the heterogeneity of ADHD.
Studies have begun to explore the neural basis of ISV using both structural and functional imaging methods (see review by MacDonald et al. 2009). Structural alterations associated with increased ISV include lesions of the left or right dorsolateral PFC or the superior medial frontal cortex (Stuss et al. 2003) and reduced corpus callosum surface area (Anstey et al. 2007). Consistent with these structural MRI findings, functional brain correlates of increased response variability include increased activation in a region of the superior medial frontal cortex consistent with the rostral supplementary motor area (or “pre-SMA”) (Simmonds et al. 2007) and in left and right middle frontal regions (Bellgrove et al. 2004). The combined findings from structural and functional MRI studies suggest that regions and circuitry important to motor response preparation and selection, in particular the pre-SMA, appear crucial in controlling variable responding (see review by Mostofsky and Simmonds 2008), such that alterations in both motor and cognitive control systems contribute to increased ISV.
Accumulating evidence suggests impairments in motor control are common in ADHD (Brossard-Racine et al. 2012; D'Agati et al. 2010; Denckla and Rudel 1978; Fliers et al. 2008; Gilbert et al. 2011; Klotz et al. 2011; Rommelse et al. 2008; Zelaznik et al. 2012). Specifically, development of premotor and supplementary motor areas of the brain is delayed in ADHD (Shaw et al. 2007). Inhibition of the motor cortex, measured with transcranial magnetic stimulation, is diminished in children with ADHD compared to typically developing (TD) peers (Gilbert et al. 2011; Wu et al. 2012). Functional MRI during finger sequencing reveals children with ADHD show decreased activation on contralateral primary motor cortex (M1) as compared with TD peers (Mostofsky et al. 2006). During Go/No-go performance, children with ADHD show decreased pre-SMA activation as compared with TD controls (Suskauer, Simmonds, Fotedar, et al. 2008), and fail to show the same pattern of association between pre-SMA activation and lower ISV seen in TD controls, instead showing greater activation in PFC with lower ISV (Suskauer, Simmonds, Caffo, et al. 2008). Anomalous motor development in ADHD has also been shown in behavioral measures of motor control, including excessive mirror overflow (MacNeil et al. 2011), defined as unintentional and unnecessary movements that accompany voluntary action. Children with ADHD also fail to meet age norms on timed repetitive and sequential movements and develop accurate rhythm more slowly (Denckla and Rudel 1978). Furthermore, studies have shown that ADHD is associated with impaired motor timing during a timed tapping task (Zelaznik et al. 2012) and time estimation/production tasks (Rommelse et al. 2008; van Meel et al. 2005), and increased variability in arm trajectories during a motor adaptation task (Izawa et al. 2012). Finally, motor impairments observed in ADHD are associated with poorer functioning in the adaptive domains of home living behavior, socialization, and home direction (Wang et al. 2011).
Very few studies have examined whether ADHD is associated with increased variability in self-generated motor output, as opposed to motor output guided by some form of external timing or stimulus (c.f., Egeland et al. 2012; Frazier-Wood et al. 2012; Klotz et al. 2011; Rommelse et al. 2008). The results of the few studies that have examined this have been somewhat inconsistent. In two of these studies (Frazier-Wood et al. 2012; Rommelse et al. 2008), participants were instructed to tap their index finger as many times as they could during an 18-s interval. The performance of children with ADHD did not differ from that of controls in terms of variability in intertap interval. In contrast, Egeland et al. (2012) found that children with ADHD performed more poorly than healthy controls on a similar finger tapping test (number of key presses in 10 s) and on a grooved pegboard test, which requires greater motor control. Most recently, Klotz et al. (2011) found that finger tapping rates during a finger sequencing task were slower in children with ADHD, although variability in finger tapping did not differ between groups. In addition, speed and variability in finger tapping rates were related to speed and variability in reaction time during a simple reaction time task and to standardized measures of motor development. The inconsistent findings regarding variability in motor control in ADHD suggest that further examination is needed, along with consideration of age and gender due to the different trajectories of motor development for boys and girls (Larson et al. 2007).
In addition, motor symptoms may be differentially related to ADHD symptom domains, although the findings from previous studies are inconsistent. Four previous studies examined motor control in relation to ADHD subtype or symptom domains, one of which did not find evidence of subtype differences (Brossard-Racine et al. 2012), whereas the remaining studies had contradictory findings. Egeland et al. (2012) did not report differences in simple finger tapping between children with the combined and inattentive subtypes of ADHD, although they identified different mechanisms mediating motor control programs in each subtype. MacNeil et al. (2011) reported increased mirror overflow was related to greater hyperactive/impulsive symptoms, but not inattention symptom, whereas Fliers et al. (2008) reported stronger associations between inattentive symptoms and motor control problems than with hyperactive-impulsive symptoms. The mixed findings regarding the relationship between motor control problems and ADHD symptom domains suggest that further investigation is warranted. Furthermore, examination of whether ISV during a self-generated finger sequencing tasks is differentially related to ADHD symptom domains after accounting for ISV during a traditional cognitive task is important for understanding the underlying mechanisms of increased ISV in ADHD.
The current study builds on the existing literature by examining intrasubject variability across tests of motor and inhibitory control in boys with ADHD in comparison to typically developing boys. Due to previous studies reporting motor deficits may be limited to boys with ADHD (e.g., MacNeil et al. 2011), and because motor development differs for boys and girls (Larson et al. 2007) this initial study focused exclusively on boys. The specific hypotheses were: (1) Boys with ADHD will show slower and more variable finger tapping during a finger sequencing task, (2) Boys with ADHD will show slower and more variable reaction times on a Go/No-Go task, and (3) Speed and variability will be correlated across tasks. The effects of age and changes in speed and variability over the course of the finger sequencing task were also examined. In addition, exploratory analyses examined which measures of speed and variability were most strongly related to ADHD symptom domains as reported by parents.
Method
Participants
Participants between the ages of 8 to 12 years old were recruited through community-wide advertisement, volunteer organizations, local schools, medical institutions, and word of mouth. A brief telephone interview was conducted with a parent to determine whether their child met initial inclusion criteria. Next, a structured diagnostic interview using the Diagnostic Interview for Children and Adolescents-IV (DICA-IV; Reich et al. 1997) was conducted over the phone with the child’s parent. Participants were then scheduled for a study visit and were mailed additional parent ratings used to confirm diagnostic status, including the Conners’ Parent and Teacher Rating Scales-Revised Long Version (CPRS-R:L and CTRS-R:L; Conners 2002), and the ADHD Rating Scale-IV, home and school versions (ADHD-RS; DuPaul et al. 1998). During the initial study visit, participants were administered the Wechsler Intelligence Scale for Children-IV (Wechsler 2003) and the Word Reading subtest from the WIAT-II (Wechsler 2002) in order to rule out intellectual and reading disabilities.
Participants included in the ADHD group must have been previously diagnosed with ADHD as reported by parents during initial phone screen. An ADHD diagnosis was confirmed based on the following criteria: (1) an ADHD diagnosis on the DICA-IV psychiatric interview and (2) a T-score of 60 or higher on scale L (DSM-IV: inattentive) or M (DSM-IV: hyperactive-impulsive) on the CPRS-R:L or CTRS-R:L (when available), or a score of 2 or 3 on at least 6 out of 9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ADHD-RS. This information was then reviewed by a child neurologist (S.H.M.) for a final confirmation of an ADHD diagnosis. Participants in the control group could not meet diagnostic criteria for any psychiatric disorder based on DICA-IV and their scores had to be below clinical cutoff scores on the parent-report measures (CPRS-R:L and the ADHD-RS) and on the teacher-report measures (CTRS-R:L and the ADHD-RS), when available.
Exclusion criteria for the all participants consisted of the following: (1) Full Scale IQ (FSIQ) score below 80 based on the WISC-IV, (2) history of intellectual disability, seizures, traumatic brain injury or other neurological illnesses, (3) diagnosis of conduct, mood, generalized anxiety, separation anxiety or obsessive–compulsive disorders based on the DICA-IV, (4) psychotropic medications other than stimulant medication, and (5) significant discrepancy between FSIQ and WIAT-II. Children with ADHD and comorbid oppositional defiant disorder (ODD) were not excluded from participation to increase the generalizability of our sample given the high rates of comorbidity between ADHD and ODD (Lahey et al. 1999) and because we did not have specific predictions about the impact of comorbid ODD on motor and cognitive control in children with ADHD.
A total of 51 boys between the ages of 8–12 years old participated in the study (see Table 1). The ADHD group was composed of 28 boys (age Mean [SD] = 10.2 [1.4] years), representing all three subtypes (20 combined type, 7 inattentive type, and 1 hyperactive/impulsive type). The remaining 23 boys composed the typically developing (TD) group (Age Mean [SD] = 10.8 [1.3] years). Participants with ADHD taking stimulant medication were asked to withhold medication on the day prior and day of testing. Groups were balanced on age and perceptual reasoning abilities based on the Perceptual Reasoning Index (PRI) from the WISC-IV. All subjects met right handedness criteria according to the Edinburgh Handedness Inventory (Oldfield 1971) or to the Physical and Neurological Exam for Subtle Signs (Denckla 1985). This study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and assent was obtained from the participating child.
Procedures
Finger-Sequencing Paradigm
In preparation for the finger-sequencing paradigm, four Biopac goniometers (Biopac Systems Inc., Goleta, CA) were taped onto the dorsal side of the subject’s hand and to the proximal phalangeal surface of the index and ring finger on both hands. Goniometers were taped to ensure consistent flexion recordings from the metacarpophalangeal joint. After all four goniometers were inspected for proper placement; the goniometers were calibrated to 0 and 45° finger flexions using Acqknowledge recording software (MacNeil et al. 2011). Subjects were directed to place their non-tapping hand in front of them while their tapping hand was bent and placed on the arm rest. Subjects were given finger-sequencing directions, asked to practice the finger tapping sequencing once per hand, and asked to repeat the directions to ensure understanding. Lastly, a video camera was placed in front of the subject to record the finger tapping sequence for later viewing.
Subjects were instructed to tap their fingers to their thumb, sequentially, starting with their index finger and ending at their pinky (Index-Middle-Ring-Pinky). The sequence was repeated until the administrator told the subject to stop (at least 10 sequences, 40 finger taps). Subjects completed 5 blocks per hand alternating hands after every block. Between each block the subjects reset their hands and prepared for the next block. The starting finger sequencing hand was counterbalanced to control for possible effects. Goniometer tracings are sampled at 100 Hz and recorded using AcqKnowledge software 3.9.1v (Biopac Systems Inc.).
Goniometer tracings were analyzed using AcqKnowledge software 3.9.1v (Biopac Systems Inc.). Index finger flexion peaks were used to identify a complete finger tapping sequence. Peak index finger flexions were identified using peak identification tools in Acqknowledge. If necessary, index finger taps were confirmed using video playback. Index finger tapping errors (tapping the index finger twice in succession) were removed through visual inspection to reduce incomplete finger sequences. During video playback, raters recorded the amount of time that each subject took to complete 40 finger taps. This time window was used to control for sequencing errors and number of finger taps. The first index finger tap was excluded from analysis due to increased intertap variability (time from the first index finger tap to the second index finger tap). Each remaining index finger flexion peak time was recorded for further analysis. The first 40 taps completed by the participant were visually identified and only those finger taps were used to examine tap speed and variability. Tap speed was determined by calculating the length of the interval in seconds between index finger taps (i.e., longer intervals indicating slower finger sequencing speed) and computing the mean tap interval for each block, referred to as intertap interval. The standard deviation of the intertap interval for each block served as the measure of variability in finger sequencing.
Go/No-Go paradigm
A subset of participants (n = 18 per group) also completed a Go/No-Go (GNG) task described in previous studies (Mostofsky et al. 2003; Wodka et al. 2007). The Go/No-Go task was programmed using Presentation software (Neurobehavioral Systems, Albany, CA, USA) for Windows XP. Participants were seated in front of a computer monitor with a keyboard. The task stimuli consisted of green spaceships for “Go” trials and red spaceships for “No-Go” trials, presented one at a time. Participants were instructed to push the spacebar with their index finger as quickly as possible in response to green spaceships only. The use of familiar stimulus–response associations (green for “go”; red for “no-go”) minimized the perceptual and cognitive demands of the tests. Cues were weighted towards green spaceships at a ratio of 3:1. Go and no-go trials appeared in pseudorandom order, derived using the randomization function in Microsoft Excel, with the restriction that there were never fewer than 3 go trials before a no-go cue and never more than 2 no-go trials in a row. There were 11 practice trials (8 go cues; 3 no-go cues) followed by 240 experimental trials (192 go cues; 48 no-go cues). Stimuli were present on-screen for 300 ms with an interstimulus interval of 2000 ms during which a fixation cross was present on-screen. Response accuracy and reaction time were recorded for the first 217 trials of the task. Response speed and variability measures on the Go/No-Go task were quantified using mean and standard deviation of reaction time (MRT and SDRT) and ex-Gaussian indicators, allowing for a more defined characterization of RT variability (Leth-Steensen et al. 2000). Mu and sigma represent the mean and standard deviation of the normal component of the distribution, respectively, and tau represents the exponential component of the RT distribution, or positive skew.
Statistical Analysis
Diagnostic differences in speed and variability during the finger sequencing task were examined with separate repeated measures ANOVAs with a between-subjects factor of Group (ADHD, TD) and two within-subjects factors of Hand (Left, Right) and Block (1–5). Polynomial linear contrasts were used to examine the linear effect of block. For the subsample of participants whom also completed the Go/No-Go task (n = 18 per group), univariate ANOVAs were employed to examine group differences in response speed and variability for the traditional reaction time measures (MRT and SDRT) and the ex-Gaussian estimates (mu, sigma, and tau). However, ex-Gaussian parameters could not be estimated for the finger sequencing task because there are not enough trials for adequate model fit (Heathcote et al. 1991). Therefore, traditional RT measures were used to examine correlations among finger sequencing speed and MRT on the Go/No-Go task and among finger sequencing variability and SDRT on the Go/No-Go task. In addition, linear regressions were employed to examine the associations among measures of cognitive (Go/No-Go MRT and SDRT) and motor (finger sequencing speed and variability) control with ADHD symptom domains on the Conners Parent Rating Scale (Hyperactive/Impulsive Symptoms T-score and Inattentive Symptoms T-score). All analyses were conducted controlling for the participant’s age and notable differences are mentioned when relevant. Additional analyses were also conducted using the coefficient of variation, computed as SDRT divided by MRT, as a measure of finger sequencing and Go/No-Go variability and tau as predictors in the regression models. The results were very similar and are therefore not reported.
Results
Finger Sequencing Speed
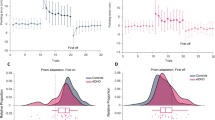
The mean interval in seconds between each completed finger sequence as reflected in the time of the index finger tap was computed as a measure of finger sequencing speed (i.e., shorter interval indicates faster finger sequencing). Participants were faster with their dominant hand, F (1, 49) = 15.7, p < 0.001, and as the task progressed, block linear, F (1, 49) = 61.4, p < 0.001. Children with ADHD tended to complete the finger sequence more slowly than their typically developing peers, F (1, 49) = 3.4, p = 0.07. In addition, although the difference in the average finger sequence speed collapsed across the five blocks for each hand did not vary across groups, Group X Hand F < 1, a significant Group x Hand x Block linear interaction emerged, F (1, 49) = 5.2, p < 0.05, (see Fig. 1). Post-hoc comparisons revealed that the TD group showed changes in finger sequencing speed over the course of the task that differed for each hand whereas the ADHD group showed equivalent changes in finger sequencing speed over the course of the task with both hands.
Specifically, for the TD group during non-dominant hand finger sequencing, blocks two to five were all significantly faster than block one, ps < 0.005. During dominant hand finger sequencing for the TD group, only blocks three to five were significantly faster than the first block, ps < 0.01, whereas blocks one and two did not differ, p = 0.25. In contrast, children with ADHD showed a similar pattern in changes in tap speed during finger tapping with their non-dominant and dominant hands. For both hands, tap speed did not change from block one to block two, p > 0.66, whereas they tapped faster with both hands in block five compared to the first two blocks, ps < 0.05 . When age was included as a covariate, the Group x Hand x Block interaction remained significant, F (1, 48) = 4.9, p < 0.05, but the hand effects were no longer significant.
Finger Sequencing Variability
The standard deviation (SD) of the interval in between index finger taps was computed as a measure of intrasubject variability. Finger sequencing variability was greater during left hand finger sequencing compared to right hand finger sequencing, F (1, 49) = 4.5, p < 0.05. However, this effect did not vary by group, Group X Hand and Group X Hand X Block, Fs <1, and the main effect of hand was not significant after controlling for age, F (1, 48) = 1.0, p = 0.32.
Greater intrasubject variability during finger sequencing was observed in children with ADHD, F (1, 49) = 6.0, p < 0.05, and this effect varied as a function of block, with a significant Group x Block linear interaction, F (1, 49) = 6.6, p < 0.05, (see Fig. 2). Post-hoc comparisons revealed that typically developing children became slightly less variable across blocks whereas children with ADHD became slightly more variable across blocks. Accordingly, significant group differences in tap variability were only observed during the final two blocks, block 4 Mdiff = 0.10 s, p = 0.001, and block 5 Mdiff = 0.09 s, p = 0.001. When age was included as a covariate, the Group x Block linear effect remained significant, F (1, 48) = 6.6, p < 0.05, although the main effect of group collapsed across blocks was no longer significant, F (1, 48) = 3.3, p = 0.075.
Group differences in tap variability during sequential motor task. The standard deviation (SD) of the interval in between index finger taps was computed as a measure of intrasubject variability. Greater intrasubject variability during finger sequencing was observed in children with ADHD, F (1, 49) = 6.0, p < 0.05, and this effect varied as a function of block, with a significant Group x Block linear interaction, F (1, 49) = 6.6, p < 0.05
Go/No-Go Reaction Time Measures
Speed and variability in reaction time (RT) on the Go/No-Go Task was examined with both traditional reaction time measures (MRT, SDRT) and ex-Gaussian measures (mu, sigma, and tau). Children with ADHD showed slower and more variable reaction times on the Go/No-Go task, MRT: F (1, 35) = 9.5, p < 0.005; SDRT: F (1, 35) = 13.3, p = 0.001. Ex-Gaussian measures indicated that speed and variability for the normal part of the RT distribution did not differ between groups (mu and sigma, Fs < 1), whereas groups significantly differed in tau, F (1,35) = 11.7, p < 0.005. These results did not change when age was included as a covariate.
Associations among Speed and Variability Measures
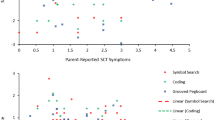
Bivariate correlations were used to examine associations among speed and variability in sequential finger movements and both traditional reaction time measures (MRT and SDRT) and ex-Gaussian measures (mu, sigma and tau) on the Go/No-Go task for the subsample of participants that completed both tasks (n = 18 per group). Finger sequencing speed was positively correlated with MRT on the Go/No-Go, r = 0.41, p < 0.05, although it was unrelated to mu, r = 0.10, p = 0.57. Variability in sequential finger movements was positively correlated with SDRT on the Go/No-Go task, r = 0.34, p > 0.05, although it was not significantly correlated with sigma or tau, r = −0.02, p = 0.91 and r = 0.27, p = 0.11, respectively. Partial correlations were also conducted controlling for age and the results did not change in a meaningful way. The significant relationships among measures of speed and variability across tasks for both groups are illustrated in Fig. 3.
Associations among speed (a) and variability (b) across the Go/No-Go and finger sequencing tasks for the subsample of participants that completed both tasks (n = 18 per group). Tap speed was positively correlated with mean reaction time (MRT) on the Go/No-Go task, r = 0.41, p < 0.05, such that children with fast reaction times on the Go/No-Go task also displayed a shorter interval between index finger taps. Variability in sequential finger movements was positively correlated with standard deviation of reaction time (SDRT) on the Go/No-Go task, r = 0.34, p < 0.05
Associations with ADHD Symptoms
To examine whether speed or variability in sequential finger movements or Go/No-Go reaction times uniquely predicted parent-reported ADHD symptoms of hyperactivity/impulsivity and inattention, a series of linear regressions were conducted (see Table 2). For these analyses, traditional RT measures were used (MRT and SDRT) because they were shown to be associated with finger sequencing speed and variability and are on a similar metric.
Finger sequencing speed and variability were significant predictors of ADHD hyperactive/impulsive symptoms, β = 0.29, p < 0.05 and β = 0.40, p < 0.005, respectively. Reaction time speed (MRT) on the Go/No-Go marginally predicted ADHD hyperactive/impulsive symptoms, β = 0.32, p = 0.06, whereas reaction time variability (SDRT) was a significant predictor of hyperactive/impulsive symptoms, β = 0.47, p < 0.005. When controlling for age, finger sequencing speed no longer predicted ADHD hyperactive/impulsive symptoms, β = 0.25, p = 0.11, and finger sequencing variability marginally predicted ADHD hyperactive/impulsive symptoms, β = 0.31, p = 0.07. Similarly, when controlling for age, Go/No-Go MRT no longer predicted ADHD hyperactive/impulsive symptoms, β = 0.25, p = 0.11, whereas SDRT was a significant predictor, β = 0.40, p < 0.05. When all four measures of response speed and variability were included in the same regression model predicting hyperactive/impulsive symptoms, 41 % of the variance in ADHD hyperactive/impulsive symptoms was accounted for by this model, F (4, 31) = 5.4, p < 0.005. In addition, the only unique predictor was finger sequencing variability, β = 0.40, p < 0.05. This pattern was consistent after controlling for age, although tap variability only marginally predicted ADHD hyperactive/impulsive symptoms, β = 0.34, p < 0.10.
For inattentive symptoms, neither finger sequencing speed, β = 0.27, p = 0.06, nor variability, β = 0.23, p = 0.10, was a significant predictor when tested in individual regression models. In contrast, reaction time speed and variability on the Go/No-Go were significant predictors of ADHD inattentive symptoms when tested in individual regression models, β = 0.35, p < 0.05, and β = 0.48, p < 0.005, respectively. These results were similar when controlling for age. When all four measures of response speed and variability were included in the same regression model predicting inattentive symptoms, 29 % of the variance in ADHD inattentive symptoms was accounted for by these variables, F (4, 31) = 3.2, p < 0.05, although none of the variables uniquely predicted inattentive symptoms, ps > 0.10.
Discussion
In this study, response control was examined during a finger sequencing task and a Go/No-Go task in boys with ADHD compared to typically developing boys, providing measures of speed and variability in motor and cognitive control. Consistent with the literature (Epstein et al. 2011), boys with ADHD exhibited slower and more variable reaction times along with greater positive skew in their RT distribution as reflected by increased tau on the Go/No-Go task. Our prediction that boys with ADHD would also complete the self-generated finger sequencing task more slowly and display greater variability in the amount of time it took them to complete the finger sequence was also supported. In addition, the speed and variability of the interval between index finger taps changed over the course of the task differentially for ADHD and control participants. Also consistent with prior research, intrasubject variability was positively correlated across the two tasks, suggesting there may be a common factor underlying ISV across tasks. Furthermore, exploratory analyses indicated that the only unique predictor of parent ratings of hyperactive-impulsive symptoms was variability in intertap interval during finger sequencing, whereas inattentive symptoms were only predicted by reaction time variability on the Go/No-Go task. Therefore, ISV in motor control may be specifically related to hyperactive/impulsive symptoms of ADHD, even after controlling for ISV in cognitive control.
Our finding that boys with ADHD showed slower and more variable finger tapping during a finger sequencing task contributes to the growing literature on motor control deficits implicated in ADHD (e.g., Egeland et al. 2012; Klotz et al. 2011; Rommelse et al. 2008) and increased intrasubject variability across domains in ADHD (Epstein et al. 2011; Frazier-Wood et al. 2012). These findings also provide further evidence of the multiple parallels between neural systems critical for development of behavioral and motor control. Moreover, this is the first study to examine changes in motor control over the course of a finger sequencing task in boys with and without ADHD. The results of this analysis suggested that typically developing boys showed improved motor efficiency with their non-dominant hand from block one to block two and their performance steadily improved over the course of the task with each hand after the first block. In contrast, boys with ADHD failed to show faster finger sequencing speed until later in the task, suggesting they were slower to improve the efficiency of their motor control with repeated practice. For tap variability, the ADHD group became more variable over the course of the task, whereas the control group became less variable. This pattern of results emphasizes the importance of examining the performance of children with ADHD over the course of a task.
The differential change in ISV over the course of the finger sequencing task suggests that learning and automaticity of motor control may be altered in children with ADHD. It is also possible that boys with ADHD have more difficulty consistently exerting the effort needed to complete the finger sequence at a regular rhythm, producing increased variability as the task progressed. This may partially be due to the contribution of energetic factors (e.g., effort and arousal) to motor output (Sergeant 2000; Sergeant et al. 1999) consistent with the finding that energetic factors mediated impaired motor control in children with ADHD (Egeland et al. 2012). It may also be that children with ADHD require additional cognitive control to compensate for motor deficits (Suskauer, Simmonds, Caffo, et al. 2008) and they failed to exert this control either due to deficient in cognitive control, a lack of awareness that additional control was required, or a lack of motivation to exert sufficient control to perform the task efficiently. In addition, a vigilance decrement likely occurred over the course of the task, perhaps to a greater extent for children with ADHD (Tucha et al. 2009), and as the attentional system fatigued performance may have become more variable.
The associations among measures of speed and variability across the tasks are consistent with prior research (Klein et al. 2006) and provides further support for the parallel neural systems involved in motor and cognitive control. Of note, previous studies that have used a simple finger tapping task have not consistently shown impaired performance in children with ADHD (Frazier-Wood et al. 2012; Rommelse et al. 2008), suggesting that deficient motor control may be greatest during non-rote motor tasks involving selection and sequencing of motor actions, such as the one used in this study. The characteristics of our sample may have also contributed to these results because motor problems tend to be more prevalent in boys with ADHD (Larson et al. 2007) and may be more strongly related to symptoms of hyperactivity and impulsivity, as the exploratory analyses suggest.
Additional evidence for subtype differences in motor control has been provided in previous studies (Egeland et al. 2012) and difficulties with motor control have also been shown to be most strongly related to hyperactive/impulsive symptoms of ADHD (MacNeil et al. 2011). This might explain why girls with ADHD, who tend to display fewer symptoms of hyperactivity or impulsivity, are less likely to display motor impairments. The differential relationship between hyperactive/impulsive symptoms and variability in motor control and between inattention symptoms and variability in cognitive control suggests the underlying processes contributing to these behavioral symptoms may differ. These findings have implications for the identification of biologically based subtypes of ADHD and ultimately the development of effective treatments for ADHD.
In addition, including age as a covariate impacted some of the motor findings in this study, despite our limited age range of 4 years (8–12). The slight change in diagnostic effects after accounting for age suggests that motor deficits in boys with ADHD may be greatest in younger children and diminish with age. Moreover, this motor delay may be associated with ADHD symptom presentation which changes over development, with a reduction in hyperactive/impulsive symptoms during the transition from childhood to adolescence (Willoughby 2003).
Several limitations in this study are worth consideration. Variability during the finger sequencing task was measured as the time between the index finger taps rather than the time between each individual finger tap (i.e., index, middle, ring, and pinky fingers). Development of methods to record the timing of each individual finger tap, as opposed to the index and ring fingers only, may provide additional information about nature of the variability observed in boys with ADHD. It may also be useful to conduct a power frequency analysis to characterize the dysrhythmia during finger sequencing to identify possible oscillations in the finger tapping rate (e.g., Ben-Pazi et al. 2006). Extending these results to examine motor function in girls with ADHD as well as younger children will elucidate our understanding of the developmental course of motor function and its relation to ADHD.
Our study provides evidence that increased response variability in boys with ADHD during a self-generated motor sequencing task is related to symptoms of hyperactivity and impulsivity but not inattention. Improving our understanding of motor function in children with ADHD may be particularly informative for elucidating the underlying mechanisms of intrasubject variability and hyperactive and impulsive behavior. Therefore, continued study of motor function in ADHD is crucial for providing insight into the neuropathophysiology of this disorder and for identifying potential biomarkers relevant for the expression of the behavioral symptoms associated with this chronic and heterogeneous disorder.
References
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington: American Psychiatric Association.
Anstey, K. J., Mack, H. A., Christensen, H., Li, S. C., Reglade-Meslin, C., Maller, J., et al. (2007). Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia, 45, 1911–1920. doi:10.1016/j.neuropsychologia.2006.11.020.
Bellgrove, M. A., Hester, R., & Garavan, H. (2004). The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia, 42, 1910–1916. doi:10.1016/j.neuropsychologia.2004.05.007.
Ben-Pazi, H., Shalev, R. S., Gross-Tsur, V., & Bergman, H. (2006). Age and medication effects on rhythmic responses in ADHD: possible oscillatory mechanisms? Neuropsychologia, 44, 412–416. doi:10.1016/j.neuropsychologia.2005.05.022.
Brossard-Racine, M., Shevell, M., Snider, L., Belanger, S. A., & Majnemer, A. (2012). Motor skills of children newly diagnosed with Attention Deficit Hyperactivity Disorder prior to and following treatment with stimulant medication. Research in Developmental Disabilities, 33, 2080–2087. doi:10.1016/j.ridd.2012.06.003.
Castellanos, F. X., Sonuga-Barke, E. J., Scheres, A., Di Martino, A., Hyde, C., & Walters, J. R. (2005). Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry, 57, 1416–1423.
Conners, C. K. (2002). Conners’ rating scales- revised. Toronto: Multi-Health Systems, Inc.
D'Agati, E., Casarelli, L., Pitzianti, M. B., & Pasini, A. (2010). Overflow movements and white matter abnormalities in ADHD. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 34, 441–445. doi:10.1016/j.pnpbp.2010.01.013.
Denckla, M. B. (1985). Revised neurological examination for subtle signs. Psychopharmacology Bulletin, 21, 773–800.
Denckla, M. B., & Rudel, R. G. (1978). Anomalies of motor development in hyperactive boys. Annals of Neurology, 3, 231–233. doi:10.1002/ana.410030308.
Douglas, V. I. (1999). Cognitive control processes in attention-deficit/hyperactivity disorder. In H. C. Quay & A. E. Hogan (Eds.), Handbook of disruptive behavior disorders (pp. 105–138). New York: Kluwer Academic/Plenum Publishing.
DuPaul, G. J., Power, T. J., Anastopoulos, A. D., & Reid, R. (1998). ADHD rating scale—IV. New York: Guilford Press.
Egeland, J., Ueland, T., & Johansen, S. (2012). Central processing energetic factors mediate impaired motor control in ADHD combined subtype but not in ADHD inattentive subtype. Journal of Learning Disabilities, 45, 361–370. doi:10.1177/0022219411407922.
Epstein, J. N., Langberg, J. M., Rosen, P. J., Graham, A., Narad, M. E., Antonini, T. N., et al. (2011). Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. [Research Support, N.I.H., Extramural]. Neuropsychology, 25, 427–441. doi:10.1037/a0022155.
Fliers, E., Rommelse, N., Vermeulen, S. H., Altink, M., Buschgens, C. J., Faraone, S. V., et al. (2008). Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: effects of age and gender. Journal of Neural Transmission, 115, 211–220. doi:10.1007/s00702-007-0827-0.
Frazier-Wood, A. C., Bralten, J., Arias-Vasquez, A., Luman, M., Ooterlaan, J., Sergeant, J., et al. (2012). Neuropsychological intra-individual variability explains unique genetic variance of ADHD and shows suggestive linkage to chromosomes 12, 13, and 17. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 159B, 131–140. doi:10.1002/ajmg.b.32018.
Gilbert, D. L., Isaacs, K. M., Augusta, M., Macneil, L. K., & Mostofsky, S. H. (2011). Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology, 76, 615–621. doi:10.1212/WNL.0b013e31820c2ebd.
Heathcote, A., Popiel, S. J., & Mewhort, D. J. K. (1991). Analysis of response-time distributions, an example using the Stroop Task. Psychological Bulletin, 109, 340–347.
Izawa, J., Pekny, S. E., Marko, M. K., Haswell, C., Shadmehr, R., & Mostofsky, S. H. (2012). Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Research, 5, 124–136.
Klein, C., Wendling, K., Huettner, P., Ruder, H., & Peper, M. (2006). Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry, 60, 1088–1097. doi:10.1016/j.biopsych.2006.04.003.
Klotz, J. M., Johnson, M. D., Wu, S. W., Isaacs, K. M., & Gilbert, D. L. (2011). Relationship between reaction time variability and motor skill development in ADHD. Child Neuropsychology, 18, 576–585. doi:10.1080/09297049.2011.625356.
Kuntsi, J., & Klein, C. (2012). Intraindividual variability in ADHD and its implications for research of causal links. Current Topics in Behavioral Neuroscience, 9, 67–91. doi:10.1007/7854_2011_145.
Lahey, B. B., Miller, T. I., Gordon, R. A., & Riley, A. W. (1999). Developmental epidemiology of the disruptive behavioral disorders. In H. C. Quay & A. E. Hogan (Eds.), Handbook of disruptive behavior disorders (pp. 23–48). New York: Kluwer Academic/Plenum.
Larson, J. C. G., Mostofsky, S. H., Goldberg, M. C., Cutting, L. E., Denckla, M. B., & Mahone, E. M. (2007). Effects of gender and age on motor exam in typically developing children. Developmental Neuropsychology, 32, 543–562.
Leth-Steensen, C., Elbaz, Z. K., & Douglas, V. I. (2000). Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica, 104, 167–190.
MacDonald, S. W., Li, S. C., & Backman, L. (2009). Neural underpinnings of within-person variability in cognitive functioning. Psychology and Aging, 24, 792–808. doi:10.1037/a0017798.
MacNeil, L. K., Xavier, P., Garvey, M. A., Gilbert, D. L., Ranta, M. E., Denckla, M. B., et al. (2011). Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology, 76, 622–628. doi:10.1212/WNL.0b013e31820c3052.
Mostofsky, S. H., & Simmonds, D. J. (2008). Response inhibition and response selection: two sides of the same coin. Journal of Cognitive Neuroscience, 20, 751–761. doi:10.1162/jocn.2008.20500.
Mostofsky, S. H., Schafer, J. G., Abrams, M. T., Goldberg, M. C., Flower, A. A., Boyce, A., et al. (2003). fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research, 17, 419–430.
Mostofsky, S. H., Rimrodt, S. L., Schafer, J. G., Boyce, A., Goldberg, M. C., Pekar, J. J., et al. (2006). Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry, 59, 48–56. doi:10.1016/j.biopsych.2005.06.011.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113.
Reich, W., Welner, Z., & Herjanic, B. (1997). The diagnostic interview for children and adolescents-IV. North Tonawanda: Multi-Health Systems.
Rommelse, N. N., Altink, M. E., Oosterlaan, J., Beem, L., Buschgens, C. J., Buitelaar, J., et al. (2008). Speed, variability, and timing of motor output in ADHD: which measures are useful for endophenotypic research? Behavior Genetics, 38, 121–132. doi:10.1007/s10519-007-9186-8.
Russell, V. A., Oades, R. D., Tannock, R., Killeen, P. R., Auerbach, J. G., Johansen, E. B., et al. (2006). Response variability in attention-deficit/hyperactivity disorder: a neuronal and glial energetics hypothesis. Behavioral and Brain Functions, 2, 30.
Sergeant, J. A. (2000). The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews, 24, 7–12.
Sergeant, J. A., Oosterlaan, J., & van der Meere, J. (1999). Information processing and energetic factors in attention deficit/hyperactivity disorder. In H. Quay & A. Hogan (Eds.), Handbook of disruptive behavior disorders (pp. 75–104). New York: Kluwer.
Sergeant, J. A., Geurts, H., Huijbregts, S., Scheres, A., & Oosterlaan, J. (2003). The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews, 27, 583–592. doi:10.1016/j.neubiorev.2003.08.004.
Shaw, P., Eckstrand, K., Sharp, W., Blumenthal, J., Lerch, J. P., Greenstein, D., et al. (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America, 104, 19649–19654. doi:10.1073/pnas.0707741104.
Simmonds, D. J., Fotedar, S. G., Suskauer, S. J., Pekar, J. J., Denckla, M. B., & Mostofsky, S. H. (2007). Functional brain correlates of response time variability in children. Neuropsychologia, 45, 2147–2157. doi:10.1016/j.neuropsychologia.2007.01.013.
Sonuga-Barke, E. J. (2005). Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry, 57, 1231–1238.
Sonuga-Barke, E. J., & Castellanos, F. X. (2007). Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Reviews, 31, 977–986. doi:10.1016/j.neubiorev.2007.02.005.
Stuss, D. T., Murphy, K. J., Binns, M. A., & Alexander, M. P. (2003). Staying on the job: the frontal lobes control individual performance variability. Brain, 126, 2363–2380. doi:10.1093/brain/awg237.
Suskauer, S. J., Simmonds, D. J., Caffo, B. S., Denckla, M. B., Pekar, J. J., & Mostofsky, S. H. (2008). fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 1141–1150. doi:10.1097/CHI.0b013e3181825b1f.
Suskauer, S. J., Simmonds, D. J., Fotedar, S., Blankner, J. G., Pekar, J. J., Denckla, M. B., et al. (2008). Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. Journal of Cognitive Neuroscience, 20, 478–493. doi:10.1162/jocn.2008.20032.
Tucha, L., Tucha, O., Walitza, S., Sontag, T. A., Laufkotter, R., Linder, M., et al. (2009). Vigilance and sustained attention in children and adults with ADHD. Journal of Attention Disorders, 12, 410–421. doi:10.1177/1087054708315065.
van Meel, C., Oosterlaan, J., Heslenfeld, D., & Sergeant, J. (2005). Motivational effects on motor timing in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 44, 451–460. doi:10.1097/01.chi.0000155326.22394.e6.
Vaurio, R. G., Simmonds, D. J., & Mostofsky, S. H. (2009). Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia, 47, 2389–2396. doi:10.1016/j.neuropsychologia.2009.01.022.
Wang, H. Y., Huang, T. H., & Lo, S. K. (2011). Motor ability and adaptive function in children with attention deficit hyperactivity disorder. The Kaohsiung Journal of Medical Sciences, 27, 446–452. doi:10.1016/j.kjms.2011.06.004.
Wechsler, D. L. (2002). Wechsler individual achievement test - second edition (WIAT-II). San Antonio: The Psychological Corporation.
Wechsler, D. L. (2003). Wechsler intelligence scale for children - fourth edition (WISC-IV). San Antonio: The Psychological Corporation.
Willcutt, E. G., Sonuga-Barke, E. J., Nigg, J. T., & Sergeant, J. A. (2008). Recent developments in neuropsychological models of childhood psychiatric disorders. In T. Banaschewski & L. A. Rohde (Eds.), Biological child psychiatry: recent trends and developments (Vol. 24) (pp. 194–226). Basel: Karger.
Willoughby, M. T. (2003). Developmental course of ADHD symptomatology during the transition from childhood to adolescence: a review with recommendations. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44, 88–106.
Wodka, E. L., Mahone, E. M., Blankner, J. G., Larson, J. C., Fotedar, S., Denckla, M. B., et al. (2007). Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology, 29, 345–356. doi:10.1080/13803390600678046.
Wu, S. W., Gilbert, D. L., Shahana, N., Huddleston, D. A., & Mostofsky, S. H. (2012). Transcranial magnetic stimulation measures in attention-deficit/hyperactivity disorder. Pediatric Neurology, 47, 177–185. doi:10.1016/j.pediatrneurol.2012.06.003.
Zelaznik, H. N., Vaughn, A. J., Green, J. T., Smith, A. L., Hoza, B., & Linnea, K. (2012). Motor timing deficits in children with Attention-Deficit/Hyperactivity disorder. [Research Support, N.I.H., Extramural]. Hum Mov Sci, 31, 255–265. doi:10.1016/j.humov.2011.05.003.
Acknowledgments
This research was supported in part by NIH grants R01 MH078160 and R01 MH085328 and Johns Hopkins University School of Medicine Institute for Clinical and Translational Research (NIH/National Center for Research Resources Clinical and Translational Science Award program, UL1 RR025005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiels Rosch, K., Dirlikov, B. & Mostofsky, S.H. Increased Intrasubject Variability in Boys with ADHD Across Tests of Motor and Cognitive Control. J Abnorm Child Psychol 41, 485–495 (2013). https://doi.org/10.1007/s10802-012-9690-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-012-9690-z